Introduction
Gastric cancer (GC) ranks as the second leading
cause of cancer-associated mortality worldwide, accounting for
~1,000,000 deaths worldwide per year and ~10% of newly diagnosed
cancer incidence (1). Common features
of GC include invasive progression and a high frequency of
metastasis to lymph nodes (2–4). The malignant potential of cancer is
manifested in the ability of tumor cells to form metastases in
distant organs (5). Various steps in
the complex metastatic process are associated with the
mitogen-activated protein kinase (MAPK) pathways, which are
activated by mitogens and have been found to be upregulated in
human GC (6,7). MAPK8IP1 is able to interact with dual
leucine zipper-bearing kinase/mixed-lineage kinases, MKK7 and c-Jun
N-terminal kinases MAPKs, act as a negative regulator of MAPK
activity (8,9).
Data from several studies have shown that various
genetic alterations induce tumorigenesis and the progression of GC
(10). MicroRNA (miRNA) are
endogenous non-coding RNAs that suppress gene expression at the
transcriptional, post-transcriptional or translational level by
targeting the 3′-UTRs of specific mRNAs (11,12).
Aberrant miRNA expression has frequently been reported in various
tumors, including lung cancer (13,14),
breast cancer (15), colon cancer
(16) and leukemia (17), indicating that they have emerged as
important regulators of human malignancy.
miR-10a has been reported to be aberrantly
overexpressed in human tumors (18,19). For
example, miR-10a is overexpressed in human pancreatic cancer and is
associated with its invasiveness, which occurs, in part, via the
suppression of the HOXA1 gene (20).
Retinoic acid receptor antagonists inhibit miR-10a expression and
block metastatic behavior of pancreatic cancer (21). Moreover, therapeutic silencing of
miR-10b has been shown to inhibit metastasis in a mouse mammary
tumor model (22). In GC, miR-10a has
an important role in metastasis from primary GC to the lymph nodes
(23). However, the role and relevant
pathways of miR-10a in GC metastasis remain unknown.
The present study was performed using 41 GC and 20
normal gastric mucosa tissues. Reverse transcription quantitative
polymerase chain reaction (RT-qPCR) analysis was used to assess the
expression levels of MAPK8IP1 in GC tissue, the results revealed
significantly downregulated MAPK8IP1 in GC tissue. Furthermore, a
statistically significant inverse correlation was observed between
miR-10a and MAPK8IP1 mRNA expression levels in GC specimens.
Luciferase reporter assay and qPCR results suggested that MAPK8IP1
was a direct target of miR-10a in GC cells. Matrigel invasion assay
and wound-healing assay findings showed that MAPK8IP1
overexpression rescued the increased migration ability of miR-10a
effectors in MKN45 cells. Finally, the underlying mechanism of
miR-10a functions in GC were explored.
Materials and methods
Ethics statement
For the use of these clinical materials for research
purposes, prior written informed consent was obtained from all of
the patients and ethical approval was granted by the Ethics
Committees of Gaozhou People's Hospital (Gaozhou, China).
Cell culture and tissue
collection
MKN45 and HNE-1 human GC cell lines (Cancer Research
Institute, Southern Medical University, Guangzhou, China) were
cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented
with 10% fetal calf serum (both from Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) at 37°C in a 5% CO2
incubator. In total, 41 GC specimens and 20 non-cancerous control
normal gastric mucosa tissues were obtained at the time of
diagnosis before any therapy was administered at Gaozhou People's
Hospital. In 41 cases, there were 33 males and 8 females with ages
ranging from 38 to 78 years (mean age, 56.7 years). All specimens
had a confirmed pathological diagnosis and were staged according to
the 2009 UICC-TNM Classification of Malignant Tumors (24).
RNA extraction and RT-qPCR
Total RNA was extracted from MKN45 cells with TRIzol
reagent (Sigma-Aldrich; Merck Millipore, Darmstadt, Germany). DNase
(1 U/µg RNA) was applied with thermal denaturation at 75°C for 5
min to remove genomic DNA. RNA (3 µg) was reverse transcribed into
cDNA using a High-Capacity cDNA Reverse Transcription kit (Applied
Biosystems; Thermo Fisher Scientific, Inc.). The RT mixture
contained 2X RT Master Mix in a final volume of 10 µl, including
2.0 µl 10X RT Buffer, 0.8 µl 25X dNTP Mix (100 mM), 2.0 µl 10X RT
Random Primers, 1.0 µl MultiScribe™ Reverse Transcriptase, 1.0 µl
RNase inhibitor and 3.2 µl nuclease-free H2O. Total RNA
was added to the 2X RT Master Mix to create a 1X mix, and RT was
performed in a thermal cycler (25°C for 10 min, 37°C for 120 min,
and 85°C for 5 min). RT-qPCR analysis of mRNAs of MAPK8IP1,
β-catenin, Snail, fibronectin, vimentin, phosphatase and tensin
homolog (PTEN), Akt, N-cadherin, E-cadherin and GAPDH (internal
standard) were performed on a StepOne™ Real-time PCR system using
Power SYBR® Green PCR Master mix (both Applied
Biosystems; Thermo Fisher Scientific, Inc.) in a final volume of 20
µl, comprising 100 ng cDNA, 10 µl master mix, 1 µl ROX and 0.4
pmol/µl of each primer. Thermal cycling conditions included an
initial denaturation for 1 min at 95°C, followed by 40 cycles
consisting of an annealing step at 95°C for 5 sec and an extension
step at 60°C for 20 sec. Each sample was analyzed in triplicate.
The primer sequences used for PCR are shown in Table I. The relative expression levels of
the genes were calculated according to the 2−ΔΔCq
method. Results were analyzed using Applied Biosystems 7500 system
software v1.4.0.
 | Table I.Summary of the primers used in the
reverse transcription-quantitative polymerase chain reaction. |
Table I.
Summary of the primers used in the
reverse transcription-quantitative polymerase chain reaction.
| Gene | Forward primer
(5′-3′) | Reverse primer
(5′-3′) |
|---|
| MAPK8IP1 |
ATCGCTTCGCCTCCCAATTT |
ATCTCCGAGAGGTCTTCATCC |
| β-catenin |
AAAGCGGCTGTTAGTCACTGG |
CGAGTCATTGCATACTGTCCAT |
| Snail |
TCGGAAGCCTAACTACAGCGA |
AGATGAGCATTGGCAGCGAG |
| Fibronectin |
CGGTGGCTGTCAGTCAAAG |
AAACCTCGGCTTCCTCCATAA |
| Vimentin |
GACGCCATCAACACCGAGTT |
CTTTGTCGTTGGTTAGCTGGT |
| PTEN |
TGGATTCGACTTAGACTTGACCT |
GGTGGGTTATGGTCTTCAAAAGG |
| Akt |
AGCGACGTGGCTATTGTGAAG |
GCCATCATTCTTGAGGAGGAAGT |
| N-cadherin |
AGCCAACCTTAACTGAGGAGT |
GGCAAGTTGATTGGAGGGATG |
| E-cadherin |
CGAGAGCTACACGTTCACGG |
GGGTGTCGAGGGAAAAATAGG |
| GAPDH |
GGAGCGAGATCCCTCCAAAAT |
GGCTGTTGTCATACTTCTCATGG |
Wound-healing assay
Cell migration was assessed using scratch-healing
assays. Briefly, MKN45 cells stably transfected with miR10a-5p and
NC were cultured in 6-well plates (5×105 per well). When
the cells grew to 90% confluence, three scratch wounds across each
well were formed using a P-200 pipette tip. Fresh DMEM supplemented
with reduced (5%) fetal bovine serum (FBS) was added, and the
wound-closing procedure was observed for 24 h. Images were captured
at 0, 12 and 24 h, respectively.
Invasion assay
For the invasion assay, 1×105 cells were
seeded in 100 ml DMEM on the top of polyethylene terephthalate
membranes coated with Matrigel (1.5 mg/ml; BD Biosciences Inc., San
Jose, CA, USA) within Transwell cell culture inserts (24-well
inserts; pore size, 8 mm; Corning Life Sciences, Corning, NY, USA).
The bottom chamber was filled with 600 ml DMEM supplemented with
20% FBS. Cells were incubated for 12 h at 37°C in an atmosphere
containing 5% CO2. Subsequently, the cells were fixed in
2.5% (v/v) glutaraldehyde and stained with crystal violet. The
invasive cells on the gel bottom were visualized under a microscope
and quantified by counting the number of cells in three randomly
chosen fields at 200-fold magnification.
Vector construction and lentivirus
production
Lentiviral (GV209; H1-MCS-CMV-EGFP) particles
carrying a miR-10a-5p precursor and its franking control sequence
were constructed by GeneChem Co., Ltd., (Shanghai, China).
Lentiviral vector for cDNA delivery of MAPK8IP1 was amplified from
human cDNA library and cloned into pLVTHM-GFP lentiviral vector
(genome.ucsc.edu/). The packaged lentiviruses were
named LVmiR10a and LV-MAPK8IP1, respectively. The empty lentiviral
vector LV-con was used as a control.
Statistical analysis
SPSS 20.0 software (IBM SPSS., Armonk NY, USA) was
used for statistical analysis. Data were presented as the mean ±
standard error of the mean of at least three independent
experiments. Two-tailed Student's t-test was used for comparisons
of two independent groups. The relationship between MAPK8IP1 and
miR-10a expression was explored using Spearman's correlation
analysis. P<0.05 was considered to indicate a statistically
significant difference.
Results
MAPK8IP1 was downregulated in GC
specimens and inversely correlated with miR-10a levels
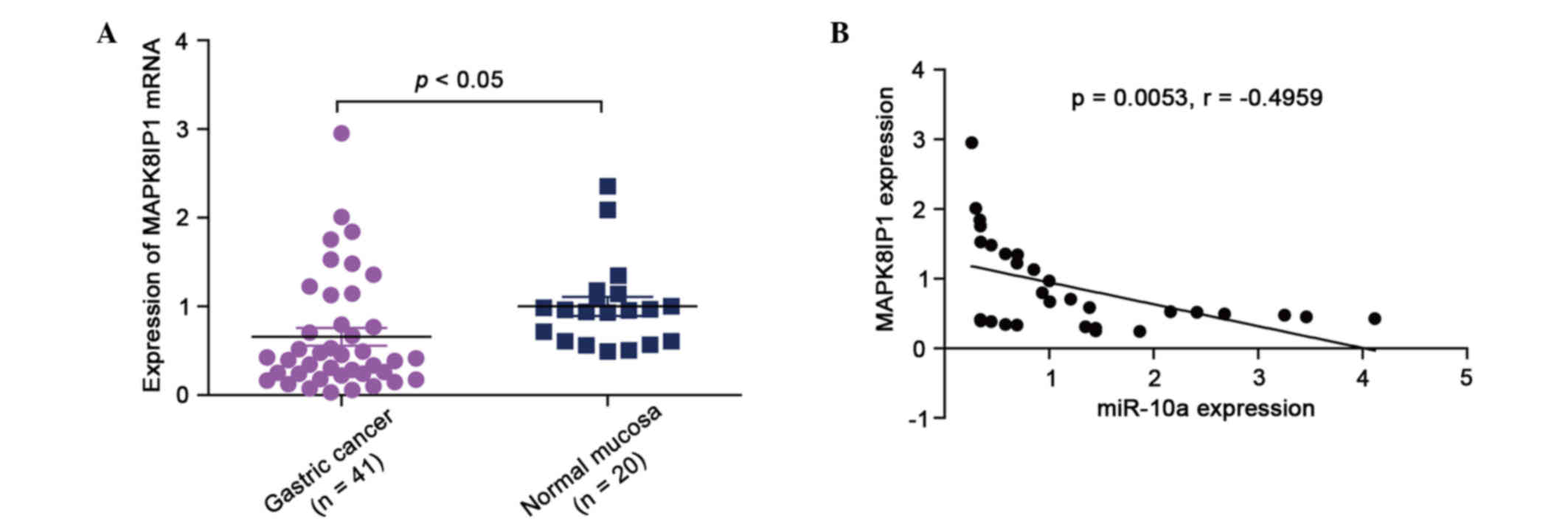
mRNA levels of MAPK8IP1 in GC specimens and normal
gastric mucosa tissues were analyzed by qPCR. The results showed
that the mean expression levels of MAPK8IP1 were significantly
higher in normal gastric mucosa tissues than in GC specimens
(Fig. 1A; P<0.05). Subsequently,
MAPK8IP1 was found to correlate with miR-10a expression in the same
GC specimens. As shown in Fig. 1B, a
significant inverse correlation was observed (2-tailed Spearman's
correlation, r=−0.4959; P=0.0053).
MAPK8IP1 is a direct target of miR-10a
in GC cells
In GC, miR-10a has an important role in metastasis
from primary GC to lymph nodes (23).
However, the role and relevant pathways of miR-10a in GC metastasis
remain largely unknown. To explore the mechanism of GC cells
metastasis induced by miR-10a, whether miR-10a was able to regulate
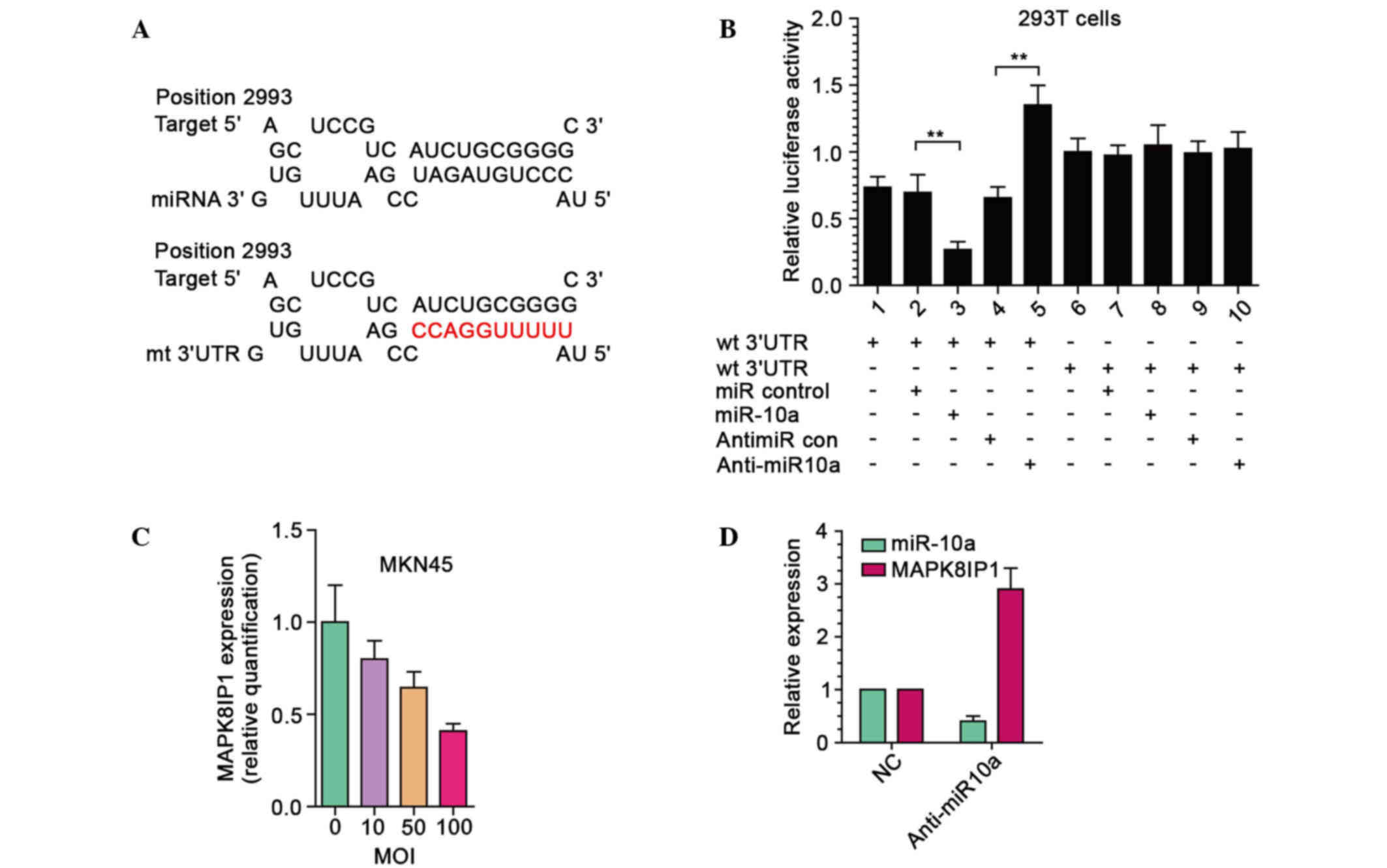
MAPK8IP1 expression in GC cells was investigated. Firstly, a
luciferase reporter assay was performed to determine whether
miR-10a was able to directly target the 3′ UTR of MAPK8IP1 in GC
cells. The target sequence of MAPK8IP1 3′-UTR [wild type (wt)
3′-UTR] or the mutant sequence [mutant (mt) 3′-UTR] was cloned into
a luciferase reporter vector (Fig.
2A). MKN45 cells were subsequently transfected with wt or mt
3′-UTR vector and miR-10a mimic. The results showed a significant
decrease of luciferase activity when compared with miR control
(Fig. 2B, lanes 2 and 3; P<0.01).
Cotransfection with anti-miR-10a and wt 3′-UTR vector in MKN45
cells led to a 2.2-fold increase in luciferase activity (Fig. 2B, lanes 4 and 5; P<0.01). Moreover,
the activity of mt 3′-UTR vector was unaffected by a simultaneous
transfection with miR-10a (Fig. 2B,
lanes 7 and 8).
Following this, MKN45 cells were transduced with
LV-miR10a at four different multiplicity of infections (MOIs) of 0,
10, 50, and 100 and the MAPK8IP1 mRNA expression levels were
examined. As shown in Fig. 2C,
exogenic expression of miR-10a led to a dose-dependent decrease in
MAPK8IP1 mRNA expression levels. At MOI 100, the mRNA expression
level of MAPK8IP1 decreased by 60–70%. Moreover, inhibition of
endogenous miR-10a by anti-miR-10a resulted in upregulated
expression of MAPK8IP1 in MKN45 cells (Fig. 2D). These results suggested that
MAPK8IP1 was a direct target of miR-10a in GC cells.
Targeting of MAPK8IP1 by miR-10a is a
major mechanism for gastric cancer metastasis
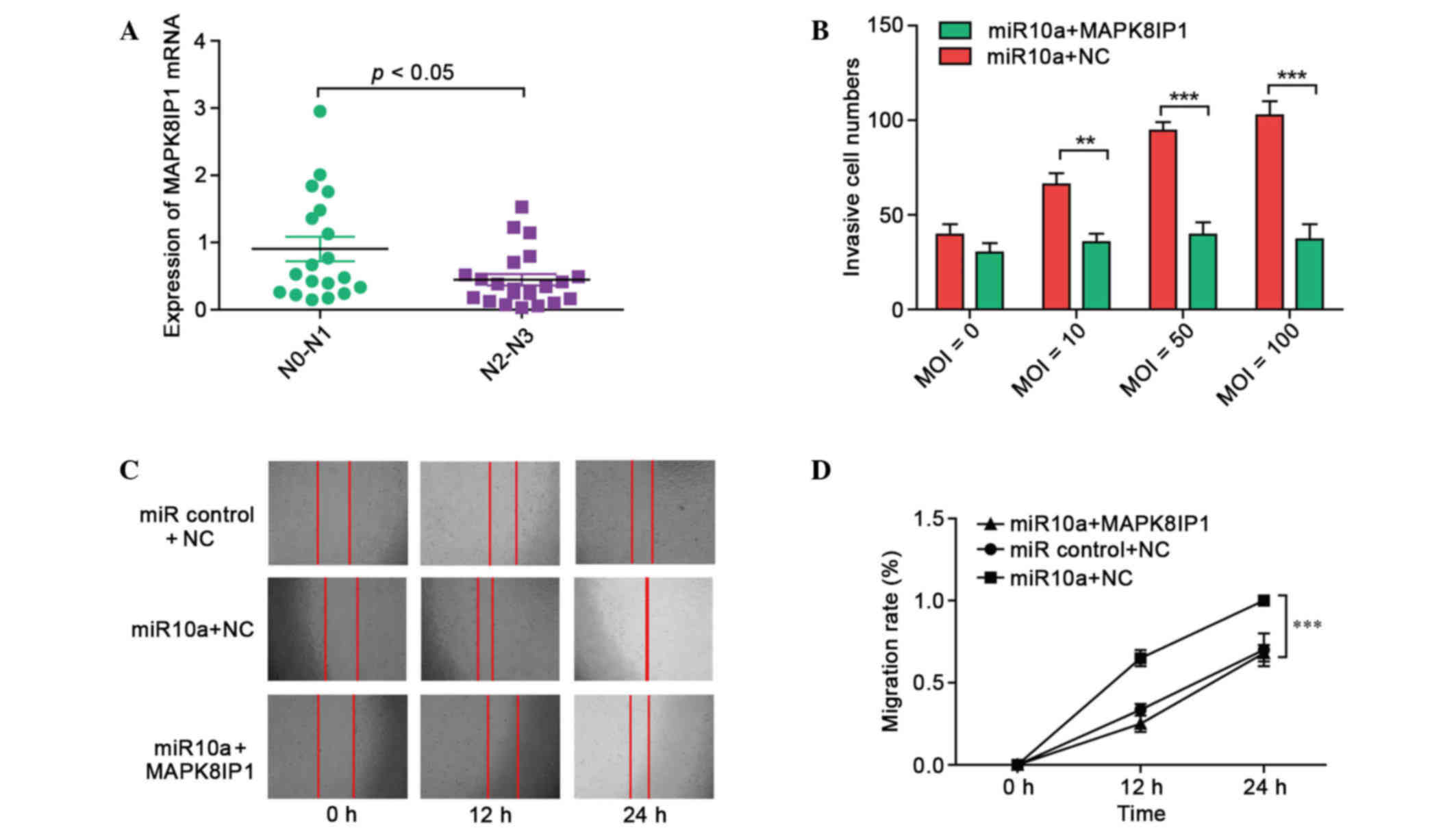
In 41 patients with GC, a positive correlation
between MAPK8IP1 expression and the degree of spread to regional
lymph nodes (N stage) of GC was observed (Fig. 3A), suggesting the relevance of
MAPK8IP1 to GC metastasis. Subsequently, MKN45 cells were
transduced with LV-miR10a at four different MOIs (0, 10, 50, and
100) and a Matrigel invasion assay was used to examine the invasion
ability. As shown in Fig. 3B,
exogenic expression of miR-10a led to increased invasion ability in
MKN45 cells. Furthermore, MAPK8IP1 overexpression rescued the
increased invasion of miR-10a effectors in MKN45 cells.
In a wound-healing assay, overexpression of miR-10a
markedly accelerated cell migration at the edges of the scratch
wound of MKN45 cells (Fig. 3C).
Quantitative analyses at 24 h indicated a significant increment in
wound closure in miR-10a overexpressing cells compared with control
cells (Fig. 3D; P<0.001). However,
MAPK8IP1 overexpression rescued the increased migration ability of
miR-10a effectors in MKN45 cells. These results suggested that
targeting of MAPK8IP1 by miR-10a may be a major mechanism for
gastric cancer metastasis.
Cell migration regulators contribute
to the metastasis effect induced by miR-10a
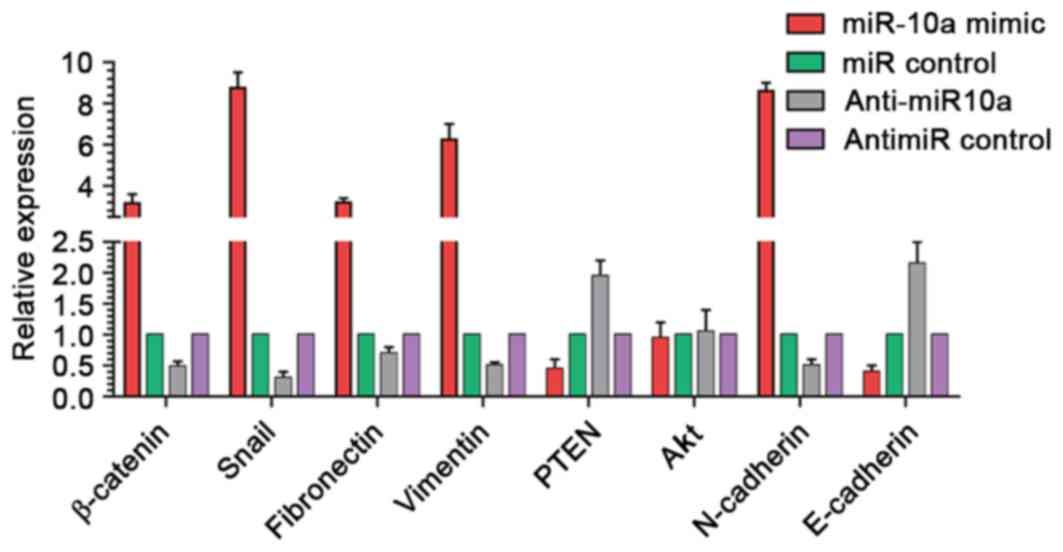
Cell-cell junction and PI3K/Akt pathways are
involved in the regulation of cell migration progression and are
frequently over activated in GC (25,26). To
elucidate whether these regulators were involved in the metastasis
effect of miR-10a, the mRNA levels of eight cell migration
regulators were anayzed in MKN45 cells by qPCR, including
β-catenin, Snail, fibronectin, Vimentin, PTEN, Akt, N-cadherin and
E-cadherin. The results demonstrated that the levels of β-catenin,
Snail, fibronectin, vimentin and N-cadherin were increased by
>2.5-fold, whereas the mRNA levels of PTEN and E-cadherin were
decreased after miR-10a overexpression. By contrast, anti-miR-10a
treatment produced contrasting results (Fig. 4).
Discussion
As demonstrated in previous studies, miRNA are
associated with numerous cellular processes, including
proliferation, differentiation and apoptosis, and they have an
important role in the pathogenesis of cancer (27,28). For
example, a previous study by our group demonstrated that
EBV-miRBART7-3p was a viral oncomir that is highly overexpressed in
NPC tissues, which promotes NPC tumorigenesis (29). Gastric cancer is caused by the
interaction of environmental and genetic factors (30). It has been reported that miR-10a is
overexpressed in human pancreatic cancer and involved in its
invasiveness; retinoic acid receptor antagonists inhibit miR-10a
expression and block metastatic behavior of pancreatic cancer
(20,21). In a mouse mammary tumor model,
therapeutic silencing of miR-10b inhibited metastasis (22). In GC, miR-10a has an important role in
metastasis from primary GC to lymph nodes (23). However, the role and relevant pathways
of miR-10a in GC metastasis remain largely unknown.
MAPK signaling pathway activation correlates with
human cancer progression and metastasis (7). MAPK8IP1 acts as a negative regulator of
MAPK activity (31). To explore the
mechanism of GC cells metastasis induced by miR-10a, the present
study investigated whether miR-10a was able to regulate MAPK8IP1
expression in GC cells. The present findings found that the mean
expression level of MAPK8IP1 was significantly elevated in normal
gastric mucosa tissues, as compared with GC specimens. Furthermore,
it was determined that MAPK8IP1 correlated with miR-10a expression
levels in the same GC specimens. Inverse correlation was observed
between MAPK8IP1 and miR-10a mRNA expression levels. These results
suggested that miR-10a may regulate MAPK8IP1 expression in GC
cells.
Luciferase reporter assay and qPCR results suggested
that MAPK8IP1 was a direct target of miR-10a in GC cells. In 41
patients with GC, a positive correlation was observed between
MAPK8IP1 expression and the degree of spread to regional lymph
nodes (N stage) of GC, suggesting the relevance of MAPK8IP1 to GC
metastasis. Exogenic expression of miR-10a led to increased
invasion ability of MKN45 cells. The present results also showed
that MAPK8IP1 overexpression rescued the increased invasion ability
of miR-10a effectors in MKN45 cells. In a wound-healing assay,
overexpression of miR-10a markedly accelerated cell migration at
the edges of the scratch wound of MKN45 cells. However, MAPK8IP1
overexpression rescued the increased migration ability of miR-10a
effectors in MKN45 cells. Taken together, these results suggested
that targeting of MAPK8IP1 by miR-10a may be a major mechanism for
gastric cancer metastasis.
It is well known that a typical miRNA has ~100
target sites and forms a regulatory network (32). PTEN loss and MAPK activation cooperate
to promote epithelial-to-mesenchymal transition and metastasis in
prostate cancer stem cells (7).
Cell-cell junction and PI3K/Akt pathways are involved in the
regulation of cell migration progression, and are frequently
overactivated in GC (33–35). To elucidate whether these regulators
were associated with the metastasis effect of miR-10a, the mRNA
levels of eight cell migration regulators were examined in MKN45
cells. The present results demonstrated that the levels of
β-catenin, Snail, fibronectin, vimentin and N-cadherin were
increased by >2.5-fold, whereas the mRNA expression levels of
PTEN and E-cadherin were decreased after miR-10a overexpression. By
contrast, anti-miR-10a treatment produced contrasting results.
In conclusion, the present study indicated that
miR-10a-5p directly targets MAPK8IP1, which is a negative regulator
of MAPK activity, and thus may be a major mechanism for gastric
cancer metastasis. These findings suggested that miR-10a may be a
potential target for the treatment of GC in the future.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wakamatsu Y, Sakamoto N, Oo HZ, Naito Y,
Uraoka N, Anami K, Sentani K, Oue N and Yasui W: Expression of
cancer stem cell markers ALDH1, CD44 and CD133 in primary tumor and
lymph node metastasis of gastric cancer. Pathol Int. 62:112–119.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hsu KW, Hsieh RH, Huang KH, Fen-Yau Li A,
Chi CW, Wang TY, Tseng MJ, Wu KJ and Yeh TS: Activation of the
Notch1/STAT3/Twist signaling axis promotes gastric cancer
progression. Carcinogenesis. 33:1459–1467. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Takano Y, Kato Y, Masuda M, Ohshima Y and
Okayasu I: Cyclin D2, but not cyclin D1, overexpression closely
correlates with gastric cancer progression and prognosis. J Pathol.
189:194–200. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Maruyama K, Gunvén P, Okabayashi K, Sasako
M and Kinoshita T: Lymph node metastases of gastric cancer. General
pattern in 1931 patients. Ann Surg. 210:596–602. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Qu JL, Qu XJ, Zhao MF, Teng YE, Zhang Y,
Hou KZ, Jiang YH, Yang XH and Liu YP: Gastric cancer exosomes
promote tumour cell proliferation through PI3K/Akt and MAPK/ERK
activation. Dig Liver Dis. 41:875–880. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mulholland DJ, Kobayashi N, Ruscetti M,
Zhi A, Tran LM, Huang J, Gleave M and Wu H: Pten loss and RAS/MAPK
activation cooperate to promote EMT and metastasis initiated from
prostate cancer stem/progenitor cells. Cancer Res. 72:1878–1889.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Davis RJ: Signal transduction by the JNK
group of MAP kinases. Cell. 103:239–252. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Thompson NA, Haefliger JA, Senn A,
Tawadros T, Magara F, Ledermann B, Nicod P and Waeber G:
Islet-brain1/JNK-interacting protein-1 is required for early
embryogenesis in mice. J Biol Chem. 276:27745–27748. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Crew KD and Neugut AI: Epidemiology of
gastric cancer. World J Gastroenterol. 12:354–362. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bartel DP: MicroRNAs: Target recognition
and regulatory functions. Cell. 136:215–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Esquela-Kerscher A and Slack FJ:
Oncomirs-microRNAs with a role in cancer. Nat Rev Cancer.
6:259–269. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cao M, Seike M, Soeno C, Mizutani H,
Kitamura K, Minegishi Y, Noro R, Yoshimura A, Cai L and Gemma A:
MiR-23a regulates TGF-β-induced epithelial-mesenchymal transition
by targeting E-cadherin in lung cancer cells. Int J Oncol.
41:869–875. 2012.PubMed/NCBI
|
|
14
|
Pacurari M, Addison JB, Bondalapati N, Wan
YW, Luo D, Qian Y, Castranova V, Ivanov AV and Guo NL: The
microRNA-200 family targets multiple non-small cell lung cancer
prognostic markers in H1299 cells and BEAS-2B cells. Int J Oncol.
43:548–560. 2013.PubMed/NCBI
|
|
15
|
Verdoodt B, Neid M, Vogt M, Kuhn V,
Liffers ST, Palisaar RJ, Noldus J, Tannapfel A and
Mirmohammadsadegh A: MicroRNA-205, a novel regulator of the
anti-apoptotic protein Bcl2, is downregulated in prostate cancer.
Int J Oncol. 43:307–314. 2013.PubMed/NCBI
|
|
16
|
Wang P, Zou F, Zhang X, Li H, Dulak A,
Tomko RJ Jr, Lazo JS, Wang Z, Zhang L and Yu J: microRNA-21
negatively regulates Cdc25A and cell cycle progression in colon
cancer cells. Cancer Res. 69:8157–8165. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Calin GA, Ferracin M, Cimmino A, Di Leva
G, Shimizu M, Wojcik SE, Iorio MV, Visone R, Sever NI, Fabbri M, et
al: A MicroRNA signature associated with prognosis and progression
in chronic lymphocytic leukemia. N Engl J Med. 353:1793–1801. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Veerla S, Lindgren D, Kvist A, Frigyesi A,
Staaf J, Persson H, Liedberg F, Chebil G, Gudjonsson S, Borg A, et
al: MiRNA expression in urothelial carcinomas: Important roles of
miR-10a, miR-222, miR-125b, miR-7 and miR-452 for tumor stage and
metastasis, and frequent homozygous losses of miR-31. Int J Cancer.
124:2236–2242. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ujifuku K, Mitsutake N, Takakura S,
Matsuse M, Saenko V, Suzuki K, Hayashi K, Matsuo T, Kamada K,
Nagata I and Yamashita S: miR-195, miR-455-3p and miR-10a (*) are
implicated in acquired temozolomide resistance in glioblastoma
multiforme cells. Cancer Lett. 296:241–248. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ohuchida K, Mizumoto K, Lin C, Yamaguchi
H, Ohtsuka T, Sato N, Toma H, Nakamura M, Nagai E, Hashizume M and
Tanaka M: MicroRNA-10a is overexpressed in human pancreatic cancer
and involved in its invasiveness partially via suppression of the
HOXA1 gene. Ann Surg Oncol. 19:2394–2402. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Weiss FU, Marques IJ, Woltering JM,
Vlecken DH, Aghdassi A, Partecke LI, Heidecke CD, Lerch MM and
Bagowski CP: Retinoic acid receptor antagonists inhibit miR-10a
expression and block metastatic behavior of pancreatic cancer.
Gastroenterology. 137:2136–2145.e1-7. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ma L, Reinhardt F, Pan E, Soutschek J,
Bhat B, Marcusson EG, Teruya-Feldstein J, Bell GW and Weinberg RA:
Therapeutic silencing of miR-10b inhibits metastasis in a mouse
mammary tumor model. Nat Biotechnol. 28:341–347. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen W, Tang Z, Sun Y, Zhang Y, Wang X,
Shen Z, Liu F and Qin X: miRNA expression profile in primary
gastric cancers and paired lymph node metastases indicates that
miR-10a plays a role in metastasis from primary gastric cancer to
lymph nodes. Exp Ther Med. 3:351–356. 2012.PubMed/NCBI
|
|
24
|
Novák J and Fabian P: Comments on the TNM
classification of malignant tumours - 7th edition. Klin Onkol.
24:149–150. 2011.(In Czech). PubMed/NCBI
|
|
25
|
Brabletz T, Jung A, Spaderna S, Hlubek F
and Kirchner T: Opinion: migrating cancer stem cells - an
integrated concept of malignant tumour progression. Nat Rev Cancer.
5:744–749. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu J, Zhang Y, Xu R, Du J, Hu Z, Yang L,
Chen Y, Zhu Y and Gu L: PI3K/Akt-dependent phosphorylation of GSK3β
and activation of RhoA regulate Wnt5a-induced gastric cancer cell
migration. Cell Signal. 25:447–456. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang Z, Liu S, Shi R and Zhao G: miR-27
promotes human gastric cancer cell metastasis by inducing
epithelial-to-mesenchymal transition. Cancer Genet. 204:486–491.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li X, Zhang Y, Zhang H, Liu X, Gong T, Li
M, Sun L, Ji G, Shi Y, Han Z, et al: miRNA-223 promotes gastric
cancer invasion and metastasis by targeting tumor suppressor
EPB41L3. Mol Cancer Res. 9:824–833. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cai L, Li J, Zhang X, Lu Y, Wang J, Lyu X,
Chen Y, Liu J, Cai H, Wang Y and Li X: Gold nano-particles (AuNPs)
carrying anti-EBV-miR-BART7-3p inhibit growth of EBV-positive
nasopharyngeal carcinoma. Oncotarget. 6:7838–7850. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Doll R and Peto R: The causes of cancer:
Quantitative estimates of avoidable risks of cancer in the United
States today. J Natl Cancer Inst. 66:1191–1308. 1981.PubMed/NCBI
|
|
31
|
Harding TC, Xue L, Bienemann A, Haywood D,
Dickens M, Tolkovsky AM and Uney JB: Inhibition of JNK by
overexpression of the JNL binding domain of MAPK8IP1 prevents
apoptosis in sympathetic neurons. J Biol Chem. 276:4531–4534. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Saunders MA, Liang H and Li WH: Human
polymorphism at microRNAs and microRNA target sites. Proc Natl Acad
Sci USA. 104:3300–3305. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li D, Qu X, Hou K, Zhang Y, Dong Q, Teng
Y, Zhang J and Liu Y: PI3K/Akt is involved in bufalin-induced
apoptosis in gastric cancer cells. Anticancer Drugs. 20:59–64.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Xie X, Tang B, Zhou J, Gao Q and Zhang P:
Inhibition of the PI3K/Akt pathway increases the chemosensitivity
of gastric cancer to vincristine. Oncol Rep. 30:773–782.
2013.PubMed/NCBI
|
|
35
|
Liu J, Liu Q, Wan Y, Zhao Z, Yu H, Luo H
and Tang Z: Osteopontin promotes the progression of gastric cancer
through the NF-κB pathway regulated by the MAPK and PI3K. Int J
Oncol. 45:282–290. 2014.PubMed/NCBI
|