Introduction
Radiotherapy is one of the most important treatments
for solid tumors; however, hypoxia occurs in a wide range of solid
tumors, and is strongly associated with radio-resistance of
malignant tumors (1,2). Hypoxia can trigger the angiogenic switch
by activating the transcription of hypoxia-inducible factor
(HIF)-1α (3,4). Aberrant microvessels, which exhibit
severe structural alterations (often a dilated, tortuous, elongated
and even saccular morphology) and dysfunction serve critical roles
in the development of hypoxia (5,6).
HIF-1, as the crucial mediator of the adaptive
response of cells to hypoxia, is a HIF-1α/HIF-1β heterodimer
(7). The expression of HIF-1α
increases under hypoxic conditions, since HIF-1α can protect
proteins from ubiquitination and proteasomal degradation (8). HIF-1 can induce the expression of
>200 functional genes that participate in cell survival by
binding to the hypoxia response element in the target promoters
(9). HIF-1α, the oxygen sensitive
subunit of HIF-1, is stabilized and forms a dimer with HIF-1β in
the nucleus during hypoxia, which can release angiogenesis
promoters such as vascular endothelial growth factor (VEGF)
(10,11). This process involves a series of genes
associated with tumor progression, angiogenesis, glycolysis,
cellular growth, invasion and apoptosis (11,12).
VEGF, a downstream target of HIF-1α, regulates the
cell response to hypoxia, and serves a significant role in tumor
angiogenesis (13,14). As the key angiogenic promoter, the
majority of anti-angiogenic strategies that can selectively inhibit
the abnormal vasculature commonly present in solid tumors have been
developed to block the VEGF signaling pathway (6,15).
Preclinical data have demonstrated that recombinant human
endostatin can improve radio-sensitivity in a nude mouse model
bearing nasopharyngeal carcinomas by reducing the expression of
VEGF (16).
Endostatin is an endogenous 20-kDa COOH-terminal
fragment of collagen XVIII, and is activated by proteolytic
processing (17,18). It was first identified in murine
hemangioendothelioma cells, and was subsequently demonstrated to be
a potent angiogenesis inhibitor (19). However, there are limited reports on
the improvement of radio-resistance by endostatin. Genetic
radiotherapy, a new paradigm for cancer treatment, is a combination
of gene therapy and radiotherapy (20), and may facilitate the use of
endostatin in cancer treatment. Our previous study successfully
combined gene therapy with irradiation mediated by the pEgr-1
promoter (21). Further studies to
investigate the effect of pEgr-1-endostatin in combination with
ionizing radiation (IR) on improving radio-resistance in hypoxic
conditions should be performed.
In the present study, a nude mouse xenograft model
bearing SKOV3 cells was established, which successfully expressed
pEgr-1-endostatin upon exposure to IR. The changes in endostatin,
HIF-1α, VEGF and microvessels were explored in the control,
IR-treated and pEgr-1-endostatin-IR-treated groups. Our results
suggested that the combination of pEgr-1-endostatin with IR could
improve radio-resistance in hypoxic conditions.
Materials and methods
Cell culture and plasmid
The human ovarian cancer cell line SKOV3 was
purchased from the Cell Bank of the Chinese Academy of Sciences
(Shanghai, China). SKOV3 cells were routinely maintained in
Dulbecco's modified Eagle medium (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal
bovine serum (Invitrogen; Thermo Fisher Scientific, Inc.), 100 U/ml
penicillin G and 100 µg/ml streptomycin, in an incubator with 5%
CO2 at 37°C under humidified conditions. The
pEgr-1-endostatin plasmid (provided by the Central Laboratory of
The Affiliated Hospital of Qingdao University, Qingdao, China) and
Lipofectamine 2000 (Gibco; Thermo Fisher Scientific, Inc.) were
mixed 1:1 to a final volume of 100 µl, which contained 50 µg
plasmid.
Nude mouse xenograft model
The procedures for animal experiments were all
approved by the Committee on the Use and Care of Animals of The
Affiliated Hospital of Qingdao University (Qingdao, China), and
were performed in accordance with ethical standards. A total of 80
BALB/c nude mice (female; age, 4–5 week-old; weight, 10–13 g) were
acquired from Beijing Vital River Laboratory Animal Technology Co.
Ltd. [Beijing, China; approval number, SCXK (Beijing) 2006–0010]
and were housed under pathogen-free conditions at 27±1°C, with a 10
h light and 14 h dark cycle. Viable cells were quantified using a
cell counting chamber to adjust the total cell concentration to
3×108 cells/ml. The mice skin was disinfected at the
point of injection. A total 6×107 cells in suspension
was injected subcutaneously in the right hind leg of each mouse.
Tumor size (in mm) was measured directly by pathologists as the
largest diameter of the tumor mass.
Group division and administration of
IR
When the inoculated primary tumor reached 20.0 mm in
diameter, the 24 mice were randomly divided into three groups with
equal number (n=8). The mice in the control group were not
subjected to IR. The IR-treated group was locally exposed to 6 MV
X-ray with a Clinac 23EX (Varian Medical Systems, Palo Alto, CA,
USA), at a dose rate of 300 cGy/min and source skin distance of 100
cm. The dosage for each mouse was 300 cGy. The mice in the
endostatin-IR-treated group were also locally exposed to Varian
Clinac 23EX 6 MV X-ray under the same conditions and dosage after
injection of plasmid for 12 h. The injection of plasmid was
performed in five different sites on average. Mice were sacrificed
using cervical translocation after 48 h irradiation, and the intact
tumor masses were removed immediately. Samples of the tumors were
fixed in formalin for 8 h, and then embedded with paraffin. The
remaining tumor tissues were frozen at −80°C in liquid nitrogen for
further study.
Reverse transcription-polymerase chain
reaction (RT-PCR)
Searching the GenBank database (National Center for
Biotechnology Information, Bethesda, MD, USA) enabled the
identification of the primer sequences used in PCR amplification,
which are listed in Table I. All
primers used in the present study were designed specifically using
Primer Express® Software v3.0.1 (Thermo Fisher
Scientific, Inc.). Total RNA was extracted from frozen tumor
samples using TRIzol reagent (Gibco; Thermo Fisher Scientific,
Inc.). RNA concentration and purity were determined by absorbance
(A) 260 and A280 measurements using a NanoDrop® ND-1000
spectrophotometer (Thermo Fisher Scientific, Inc., Pittsburgh, PA,
USA). RT-PCR was performed using PCR amplification equipment (Roche
Diagnostics, Basel, Switzerland). The PCR reaction mixture
consisted of 12.5 µl buffer, 10 µl RNA, 0.8 µl each of the forward
and reverse primers for the target genes and for GAPDH, 0.5 µl
avian myeloblastosis virus (AVM) reverse transcriptase (Takara Bio,
Inc., Otsu, Japan), 0.5 µl AVM2 Taq polymerase (Takara Bio, Inc.),
4.9 µl purified water and 2 µl complementary DNA, up to a final
volume of 30 µl. PCR was performed under the following conditions:
60°C for 30 min and 95°C for 3 min for 1 cycle, followed by 95°C
for 5 sec and 60°C for 20 sec for 40 cycles. Upon amplification,
the products were loaded onto a 2% agarose gel in Tris-borate-EDTA
buffer for agarose gel electrophoresis at 120 V for 20 min. The
specific bands were visualized with ethidium bromide and
photographed under ultraviolet light. A gel scanner system (EP
0241904 B1; Teledyne Isco, Inc., Lincoln, NE, USA) was used for
densitometric analysis. The intensity of HIF-1α, VEGF and
endostatin PCR products were normalized to that of GAPDH.
 | Table I.Primers for polymerase chain reaction
amplification. |
Table I.
Primers for polymerase chain reaction
amplification.
| Gene | Primer sequence | Amplicon (bp) |
|---|
| HIF-1α | Forward: 5′-CTT CTG
GAT GCT GGT GAT TTG-3′ | 245 |
|
| Reverse: 5′-TAT ACG
TGA ATG TGG CCT GTG-3′ |
|
| VEGF | Forward: 5′-AGG AGG
GCA GAA TCA TCA CG-3′ | 418 |
|
| Reverse: 5′-TAT GTG
CTG GCC TTG GTG AG-3′ |
|
| Endostatin | Forward: 5′-GGA ATT
CAT GCA CAG CCA CCG CGA CTT C-3′ | 422 |
|
| Reverse: 5′-CGG GAT
CCT ACT TGG AGG CAG TCA TG-3′ |
|
| GAPDH | Forward: 5′-TGA AGG
TCG GTG TGA ACG GAT TTG GC-3′ | 450 |
|
| Reverse: 5′-CAT GTA
GGC CAT GAG GTC CAC CAC-3′ |
|
ELISA for endostatin
Eye-blood samples from the mice were collected in
vivo prior to sacrifice, and the serum was separated by
centrifugation (1,000 × g) for 5 min at 22–25°C. In two
indepent experiments, a 100-µl sample was analyzed using an
endostatin ELISA kit (Jingmei BioTech Co., Ltd., Beijing, China).
The assay was carried out according to the manufacturer's protocol.
The absorbance was measured at 450 nm by an ELISA instrument
(BIOBASE2000; Jinan Biobase Biotech Co., Ltd., Jinan, China). The
endostatin concentrations were quantified by comparison with a
series of endostatin standard samples included in the assay kit.
All samples were analyzed in duplicate independently, and readings
were obtained in triplicate.
HIF-1α immunohistochemistry
HIF-1α immunohistochemistry for hypoxic tumor cells
was performed on 4-µm-thick sections of formalin-fixed,
paraffin-embedded tissues. HIF1-α was detected with a rabbit
anti-mouse monoclonal antibody (1:300 dilution; #AH339-1; Abcam,
Cambridge, UK) following overnight incubation at 4°C. The
procedures followed a semi-quantitative criteria: Slides were first
scanned at ×100 magnification, and 10 cellular fields were randomly
selected, from which, 200 cells and the number of HIF-1α-positive
cancer cells were counted. The results were interpreted by two
investigators without knowledge of the corresponding
clinicopathological data. Immunohistochemical staining was assessed
semi-quantitatively by measuring both the intensity of the staining
(0 for non-staining; 1 for yellow staining; 2 for brown-yellow
staining; and 3 for brown staining) and the quantity of the
staining (0, 0–5%; 1, 5–25%; 2, 25–50%; 3, 50–75%; and 4, 75–100%).
Raw data were converted to immunohistochemical score (IHS) by
combining the quantity score (0–4) with the staining intensity
score (0–3). The final scores could range from 0 to 7. An IHS of
6–7 was considered strong immunoreactivity; 3–5, moderate; 1–2,
weak; and 0, negative (22). The
final scoring was achieved by comparing the scores between the
observers, and any discrepancies were resolved by consensus. In
case of disagreement, the slides were re-examined, and consensus
was reached by the observers.
Western blotting
Tissues were lysed using radioimmunoprecipitation
assay buffer (Sigma-Aldrich; Merck Millipore, Darmstadt, Germany).
The soluble protein concentration was determined by the Bradford
method. Total protein (10 µg) was resolved on 10–15% SDS-PAGE and
electro-transferred onto polyvinylidene difluoride membranes. The
membranes were blocked with PBS containing 1% Tween 20 at 4°C
overnight, washed and incubated overnight at 4°C with anti-HIF-1α
(#AH339-2) and anti-endostatin (#BAF1098) primary antibodies
(1:1,000 dilution in TBS-T; Bioworld Technology, Inc., St. Louis
Park, MN, USA). Upon being washed, membranes were incubated for 1 h
at 22–25°C with the corresponding secondary antibodies (1:1,200
dilution in TBS-T; #A00131-1; Abcam) for 1 h at room temperature.
The immunoblots were detected using an electrochemiluminescence kit
(Pierce Biotechnology, Inc., Rockford, IL, USA) and exposed to the
Fusion FX5 automatic gel imaging analysis system (Vilber Lourmat,
Marne-La-Vallée, France).
Cluster of differentiation (CD)31
immunohistochemistry and microvessel density (MVD) assessment
CD31 immunohistochemistry for tumor blood vessels
was performed on 4-µm-thick sections of formalin-fixed,
paraffin-embedded tissues. CD31 was detected with a rabbit
anti-mouse monoclonal antibody (anti-CD31; 1:200 dilution;
#31-1006-00; Abcam). Following deparaffinization and rehydration,
tissue sections were treated with 3% H2O2 for
20 min to block the endogenous peroxidase activity. Sections were
washed three times in PBS for 5 min, and incubated for 20 min at
room temperature with a protein-blocking solution consisting of PBS
with 10% goat serum (#16210064; Thermo Fisher Scientific, Inc.).
Excess blocking solution was drained, and the anti-CD31 primary
antibody reaction was carried out at 4°C overnight. Next, sections
were washed three times with PBS for 5 min, and subsequently
incubated with biotin-labeled anti-rabbit immunoglobulin G (1:50
dilution; #A21068; Abcam) for 30 min at 37°C. Following three
washes, horseradish peroxidase-conjugated streptavidin (Thermo
Fisher Scientific, Inc.) was added to the sections, incubated at
37°C for 30 min and washed three times with PBS (5 min each).
3,3′-Diaminobenzidine was used to detect antigen-antibody binding.
Counterstaining was performed with hematoxylin, and following
dehydration, slides were mounted with glycerogelatin. In order to
evaluate the MVD on the CD31-stained tumor and corresponding
non-tumor tissue sections, the hot-spots method was utilized, as
previously described (11). The
average count of microvessels in 10 fields was calculated at ×200
magnification using the Chalkley counting method of vessel hot
spots (11).
Statistics analysis
SPSS 19.0 software (IBM SPSS, Armonk, NY, USA) and
GraphPad Prism 5.0 software (GraphPad Software, Inc., La Jolla, CA,
USA) were used for data analysis. The levels of endostatin, CD31,
HIF-1α and VEGF transcription were presented as the mean ± standard
deviation. One-way analysis of variance was performed for
comparison among the different groups, and subsequently, the
Student's t-test was applied for statistical analysis between the
groups. P<0.05 was considered to indicate a statistically
significant difference.
Results
Expression of endostatin in the three
groups
The expression level of endostatin messenger (m) RNA
was detected using RT-PCR after 48 h of irradiation. All groups
exhibited certain expression of endostatin (Fig. 1A and B). The relative mRNA expression
for the three groups was 0.309±0.0165, 0.295±0.0293 and
0.813±0.495, respectively. The level of endostatin in the
endostatin-IR-treated group was significantly higher than that in
the control and the IR-treated groups (F=380.078, P<0.001). No
significant differences were observed between the control group and
the IR-treated group (t=0.705, P=0.492). The expression of
endostatin was further detected using western blotting. The gray
scale of the stained area was determined under identical conditions
in the different groups. The results demonstrated that the
expression of endostatin was significantly increased in the
endostatin-IR-treated group compared with the control and
IR-treated groups (P=0.039) (Fig.
1C). ELISA was used to determine the plasma endostatin level,
and confirmed the aforementioned results of endostatin expression
in the endostatin-IR-treated, IR-treated and endostatin-treated
groups (Fig. 1D). The results
obtained were as follows: Control group, 11.73±0.65 ng/ml
endostatin; IR-treated group, 10.85±1.15 ng/ml endostatin; and
endostatin-IR-treated group, 49.07±2.27 ng/ml endostatin. There was
no significant difference between the control group and the
IR-treated group (t=1.885, P=0.0804). The expression of
endostatin in the endostatin-IR-treated group was higher than that
in the control group (t=44.770, P<0.001) and the
IR-treated group (t=42.480, P<0.001). Our results
indicated that the endostatin level in the endostatin-IR-treated
group was successfully increased at both the mRNA and protein
level.
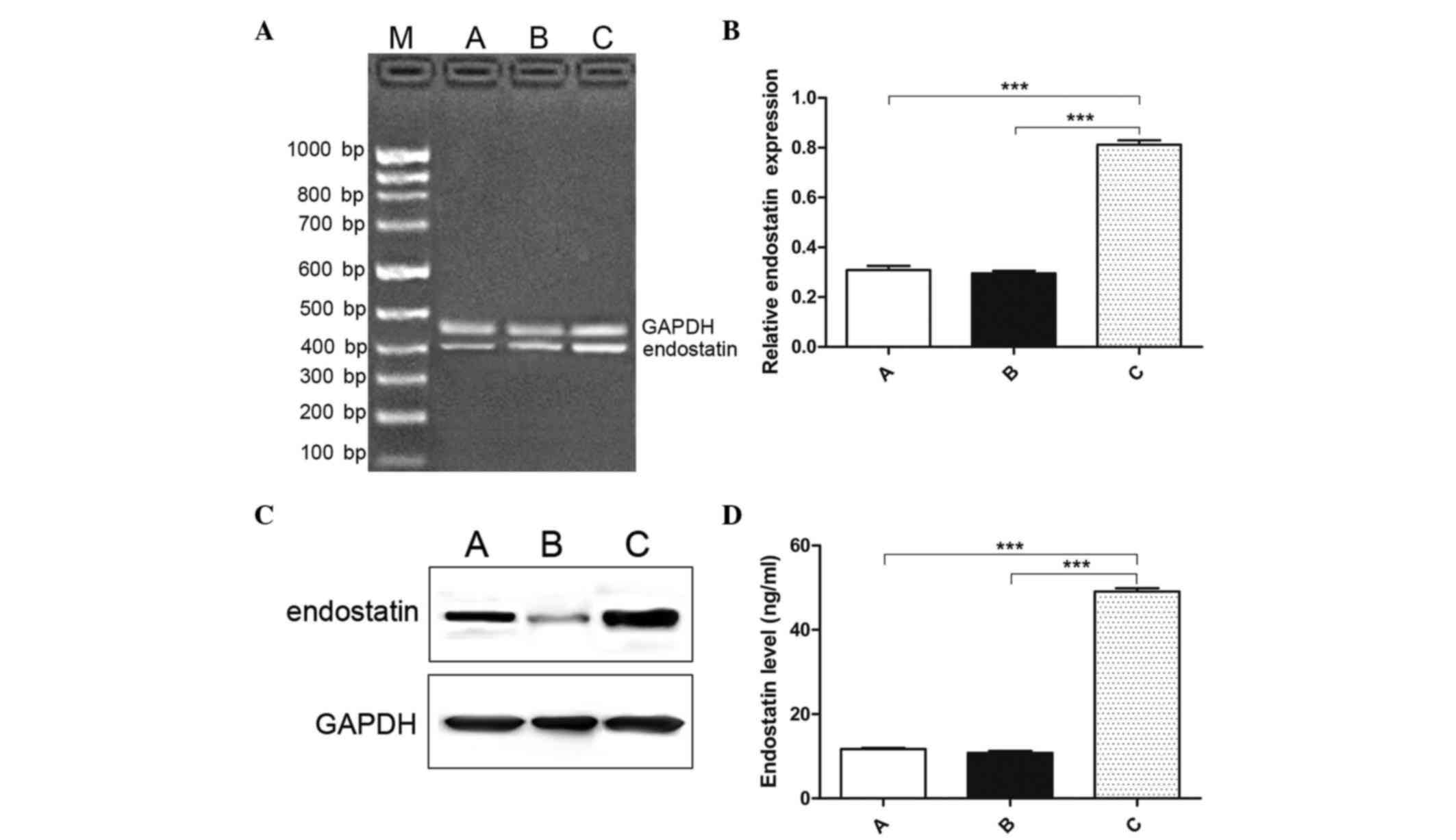 | Figure 1.Expression of endostatin in the three
groups. (A) Agarose gel electrophoresis results of endostatin mRNA
expression, as detected by reverse transcription-polymerase chain
reaction in the different groups. (B) The relative mRNA level of
endostatin was examined after group C had been injected with
pEgr-1-endostatin plasmid for 12 h and exposed to 300 cGy/min X-ray
for 48 h. Endostatin was successfully increased in the group C
compared with that in groups A and B (F=380.078, P<0.001). (C)
The protein level of endostatin was evaluated by western blotting.
The results indicated that the expression of endostatin was
significantly increased in group C compared with groups A and B
(P=0.039). (D) ELISA was used to determine the plasma endostatin
level, and the expression of endostatin in group C was observed to
be higher than that in groups A (t=44.770, P<0.001) and B
(t=42.480, P<0.001). A, control group; B, IR-treated group; C,
endostatin-IR-treated group; IR, ionizing radiation; M, marker;
mRNA, messenger RNA. ***P<0.001. |
MVD
Tumor microvessels were labeled by CD31 and assessed
by counting MVD (Fig. 2A and B). The
MVD counts for each group were as follows: Control group,
15.890±1.476 microvessels; IR-treated group, 20.970±1.410
microvessels; and endostatin-IR-treated group, 6.366±0.155
microvessels. The MVD levels were significantly different among the
different groups (F=22.660, P<0.001) (Fig. 2A). An obvious increase in MVD was
observed in the IR-treated group compared with that in the control
group (t=7.040, P<0.001), and a significant decrease was
observed in the endostatin-IR-treated group compared with that in
the control (t=18.153, P<0.001) and IR-treated groups
(t=29.120, P<0.001). By comparing the morphology of the
tumor vasculature in the three groups it was observed that the
microvessels in the endostatin-IR-treated group were more regularly
distributed and had fewer giant branches than those in the
IR-treated group (Fig. 2B).
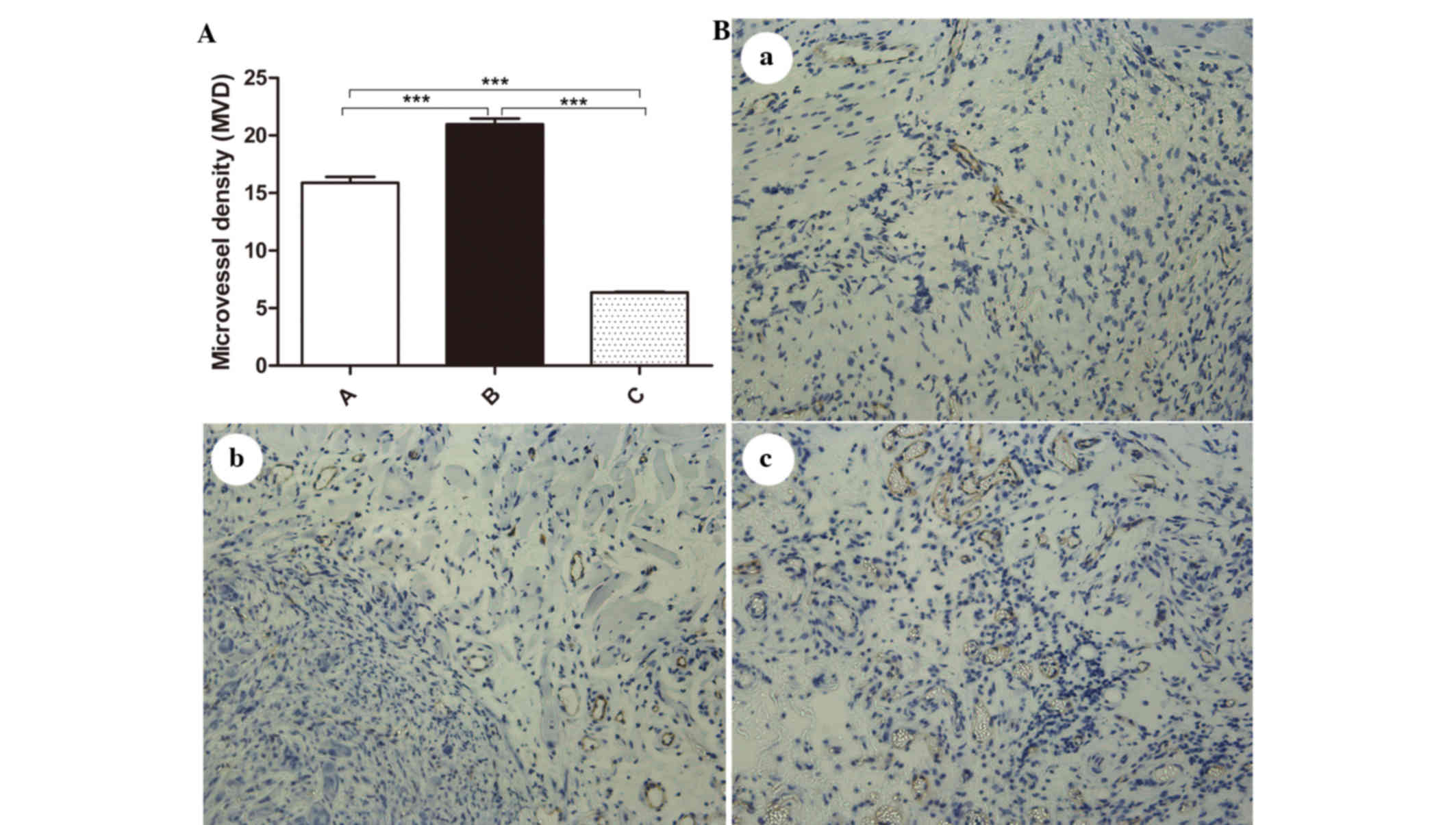 | Figure 2.MVD in tumor tissues. (A) The MVD
counts for each group were significantly different among the
different groups (F=22.660, P<0.001). An obvious increase in MVD
was observed in group B (20.970±1.410) compared with that in group
A (15.890±1.476, t=7.040, P<0.001), while a significant decrease
in MVD was observed in group C (6.366±0.155) compared with that in
groups A (t=18.153, P<0.001) and B (t=29.120, P<0.001). (B)
Tumor microvessels were identified by cluster of differentiation 31
immunohistochemistry (magnification, ×200). Comparison of the
morphology of the tumor vasculature in the three groups revealed
that the microvessels in the (a) endostatin-IR-treated group were
more regularly distributed and had fewer giant branches than those
in the (b) control and (c) IR-treated groups. A, control group; B,
IR-treated group; C, endostatin-IR-treated group; IR, ionizing
radiation; MVD, microvessel density. ***P<0.001. |
Expression of HIF-1α in the three
groups
The mRNA expression levels of HIF-1α in the three
groups were 0.513±0.042, 0.734±0.056 and 0.388±0.051, respectively.
The difference was statistically significant in the three groups
(F=98.410, P<0.001) (Fig. 3A and
B). Compared with the control group, the expression of HIF-1α
increased significantly in the IR-treated group (t=8.961,
P<0.001), while it decreased significantly in the
endostatin-IR-treated group (t=5.339, P=0.001). A
significant difference was noted between the IR-treated group and
the endostatin-IR-treated group (t=12.930, P<0.001).
Western blotting was used to analyze the expression of HIF-1α at
the protein level. A higher average optical density for HIF-1α was
observed in the IR-treated group compared with that in the control
and the endostatin-IR-treated groups (Fig. 3C). The difference was statistically
significant (P=0.046). Immunohistochemistry assessment revealed
various expression levels for HIF-1α, with moderate staining in the
control group, strong staining in the IR-treated group and weak
staining in the endostatin-IR-treated group (Fig. 3D).
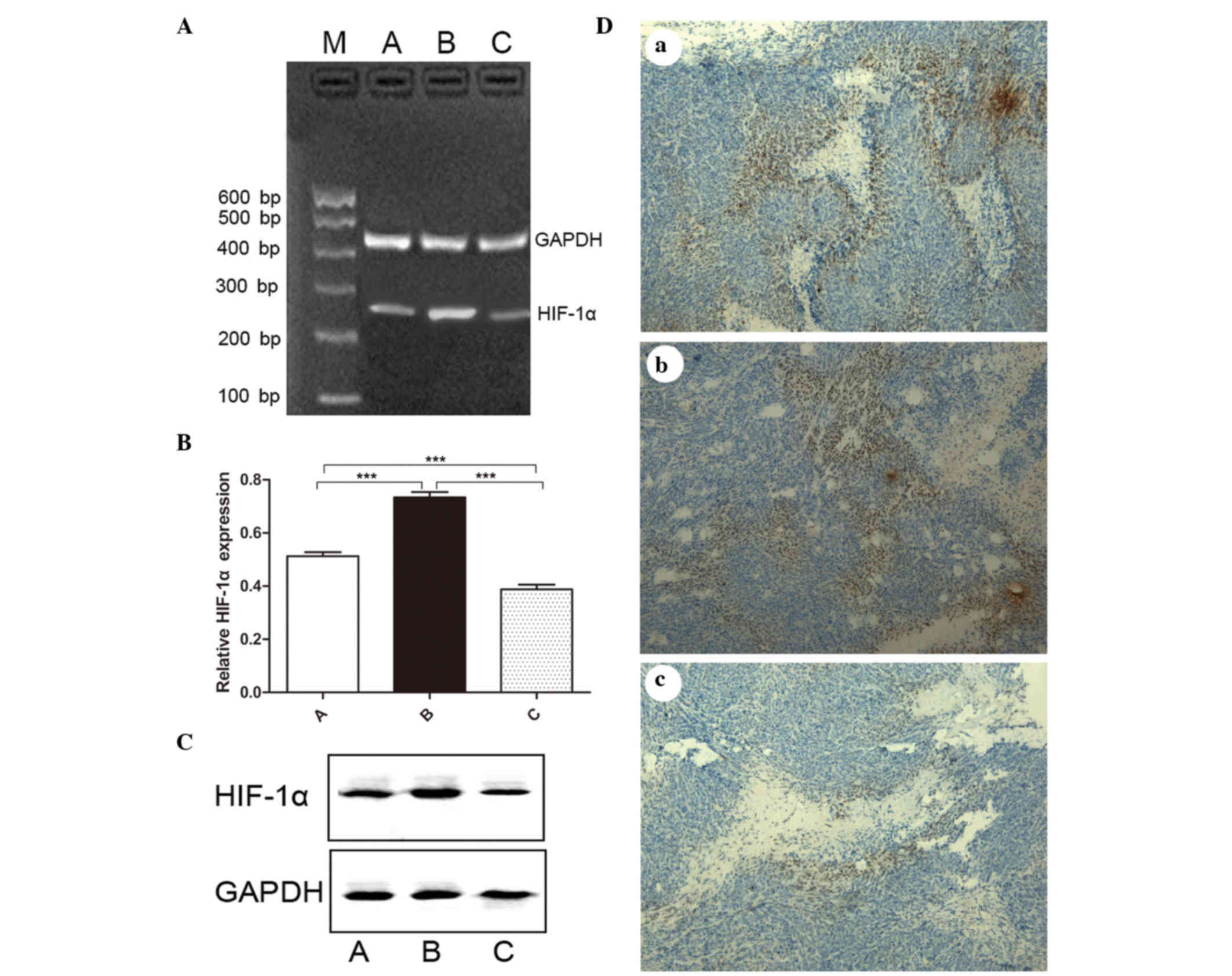 | Figure 3.Expression of HIF-1α in tumor tissues.
(A) Agarose gel electrophoresis results of HIF-1α mRNA expression,
as evaluated by reverse transcription-polymerase chain reaction in
different groups. (B) Relative levels of HIF-1α mRNA in different
groups. The difference was statistically significant in the three
groups: Groups A and B (t=8.961, P<0.001); groups A and C
(t=5.339, P=0.001); groups B and C (t=12.930, P<0.001). (C) The
protein level of HIF-1α was analyzed by western blotting. A higher
average optical density value for HIF-1α was observed in group B
compared with that in groups A and C. The difference was
statistically significant (P=0.046). (D) Immunohistochemistry
assessment indicated that the expression of HIF-1α was (a) moderate
in the control group; (b) strong in the IR-treated group; and (c)
weak in the endostatin-IR-treated group (magnification, ×200). A,
control group; B, IR-treated group; C, endostatin-IR-treated group;
IR, ionizing radiation; M, marker; mRNA, messenger RNA; HIF,
hypoxia-inducible factor. ***P<0.001. |
Expression of VEGF in the three
groups
VEGF is a downstream target of HIF-1α, and both VEGF
and HIF-1α are major regulators of angiogenesis in numerous types
of cancer (23,24). The present study detected VEGF mRNA
expression using RT-PCR, and a significant difference was observed
in the three groups (F=119.691, P<0.001) (Fig. 4A and B). The relative VEGF mRNA
expression was increased in the IR-treated group (0.653±0.073,
t=4.069, P=0.0011) but it was decreased in the
endostatin-IR-treated group (0.250±0.036, t=13.880,
P<0.001), compared with that in the control group (0.530±0.044).
Compared with the IR-treated group, the low expression of VEGF was
significantly different in the endostatin-IR-treated group
(t=14.050, P<0.001).
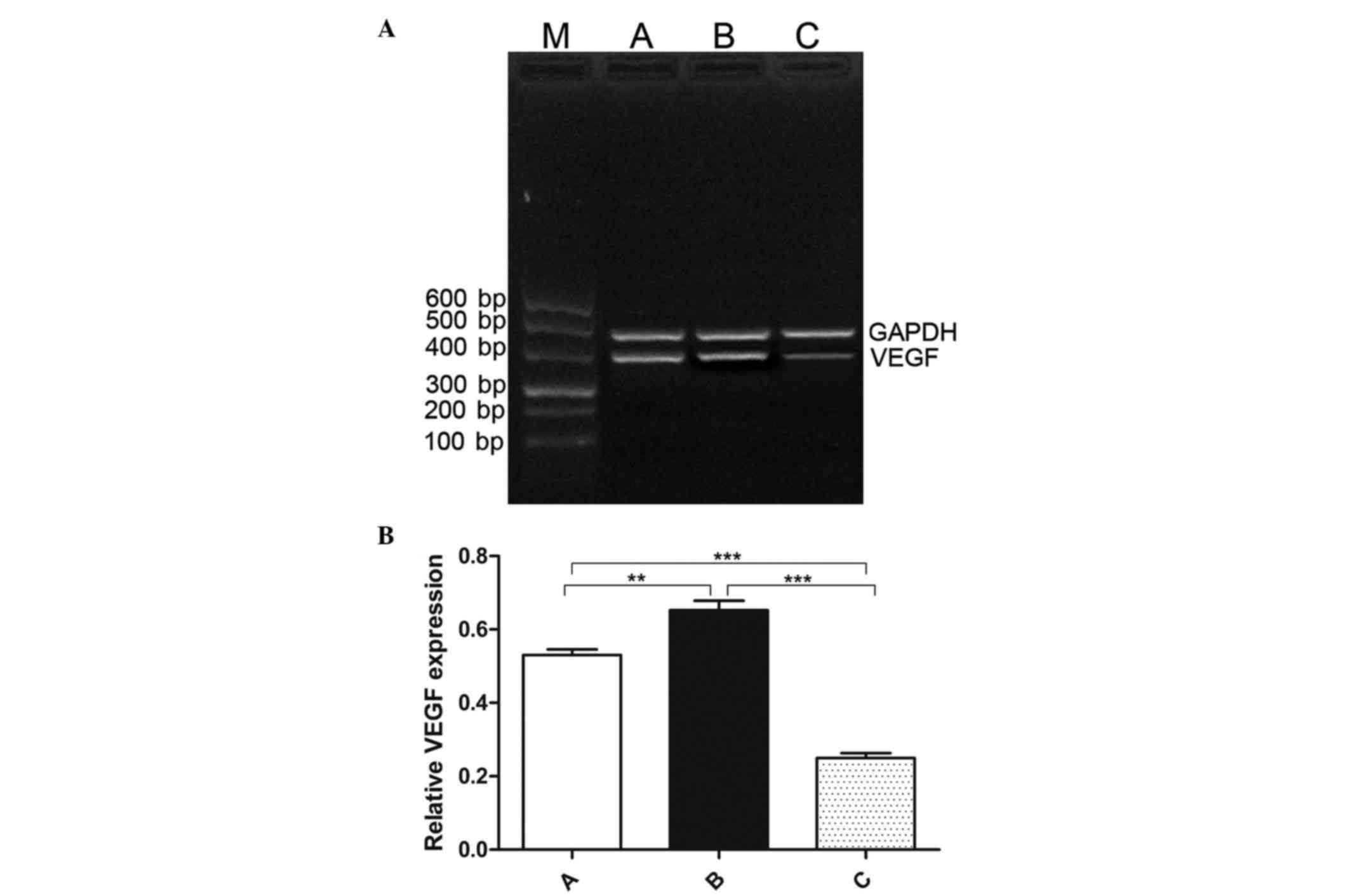 | Figure 4.Expression of VEGF in tumor tissues.
(A) Agarose gel electrophoresis results of VEGF mRNA expression in
tumor tissues, as detected by reverse transcription-polymerase
chain reaction. (B) A significant difference was observed in the
three groups (F=119.691, P<0.001). Compared with group A
(0.530±0.0444), the relative VEGF mRNA expression was increased in
group B (0.653±0.0727, P=0.001) but decreased in group C
(0.250±0.0359, P<0.001). The expression of VEGF in group C was
significantly lower than that in group B (t=14.050, P<0.001). A,
control group; B, IR-treated group; C, endostatin-IR-treated group;
VEGF, vascular endothelial growth factor; IR, ionizing radiation;
mRNA, messenger RNA. **P≥0.001 and <0.05, ***P<0.001. |
Discussion
Tumor hypoxia, which occurs mainly as a result of
inadequate tissue perfusion in solid tumors, is a well-known
challenge for successful radiotherapy (25). Various strategies to overcome
hypoxia-related radio-resistance in solid tumors have been
developed, including hypoxic sensitizers, hyperbaric oxygen and
angiogenesis inhibitors (26,27). Endostatin is a potentially effective
angiogenesis inhibitor, and has been modified for clinical
application (28). However, reports
on the combination of pEgr-1-endostatin with IR for the treatment
of angiogenesis and radio-resistance in solid tumors are
limited.
Angiogenesis has an essential role in the formation
of a new vascular network to supply nutrients and oxygen and to
remove waste products (29). Newly
formed microvessels in hypoxic conditions do not present a normal
morphology, which results in radio-resistance in the majority of
solid tumors during radiotherapy (30). In order to explore the effect of
endostatin in preventing radio-resistance during IR treatment, a
nude mouse model bearing SKOV3 ovarian carcinoma was established in
the present study, and the mice were divided into three groups.
Following injection of pEgr-1-endostatin plasmid for 12 h, the mice
in the endostatin-IR-treated group were exposed to IR for 48 h, and
the IR-treated group was exposed to the same condition. Our results
indicated that endostatin was successfully increased in the
endostatin-IR-treated group compared with that in the control and
the IR-treated groups. This is consistent with our previous study's
finding that the anti-tumor effects of pEgr-1-endostatin-tumor
necrosis factor-α recombinant plasmid expression were successfully
induced by IR (18). Administration
of endostatin in vivo led to a strong suppression of
tumor-induced angiogenesis (17). In
our study, it was observed that tumor MVD was decreased
significantly following administration of IR in the
endostatin-IR-treated group, and the morphology of the tumor
vasculature revealed that the microvessels were regularly
distributed and had less giant branches than those in the
IR-treated group. Our findings indicated that the expression of
pEgr-1-endostatin was successfully induced by IR, and suggested
that endostatin may improve radio-resistance by normalizing the
tumor vasculature.
Hypoxia is a major pro-angiogenic phenomenon in
solid tumors that promotes angiogenesis via the HIF-1 transcription
factor complex (31). VEGF gene
expression is upregulated in hypoxia via the oxygen sensor HIF-1α,
and both VEGF and HIF-1α are the main regulators of angiogenesis
(22,23,32). In
the process of angiogenesis, the endostatin plays a facilitating
role in the apoptosis of endothelial cells; meanwhile, it plays a
counteracting role in the proliferation of endothelial cells
(33,34). In the present study, the expression of
HIF-1α and VEGF at the mRNA and protein level were further explored
in the three groups. It was observed that both HIF-1α and VEGF were
significantly increased in the IR-treated group, while they were
decreased in the endostatin-IR-treated group, compared with those
in the control group. These findings suggested that IR treatment
for solid tumors may result in hypoxia by increasing irregular
microvessels, due to aberrant expression of HIF-1α and VEGF. In
addition, our results indicated that endostatin combined with IR
can downregulate the expression of HIF-1α and VEGF, which
ultimately decreases the number of abnormal microvessels. Thus, it
may be concluded that endostatin can improve IR-induced hypoxia,
which, combined with IR, may be a new treatment for solid tumors.
These results are consistent with the study of Mauceri et
al, who reported that the cytotoxic effect of the combination
of an angiogenic inhibitor with IR on endothelial cells was more
effective than that of single IR treatment (32). The HIF-1/VEGF signaling pathway may be
the possible mechanism for the endostatin-mediated normalizing
effects on vessels. Increasing evidences also indicated that
endostatin may suppress endothelial cell proliferation and preclude
the subsequent recruitment of new vasculature by activating
downstream apoptotic signals or by blocking the activation and
catalytic activity of matrix metalloproteinases (33,34). Thus,
further experiments should be performed in order to clarify these
observations.
In conclusion, IR could induce angiogenesis, which
resulted in hypoxia, and endostatin decreased the number of
microvessels via the HIF-1/VEGF signaling pathway. The present
results also indicated that pEgr-1-endostatin combined with IR may
be a new strategy for treating solid tumors. However, the
limitation of our study is that the mechanism of endostatin in
preventing radio-resistance was not explored in depth. Future in
vitro and in vivo studies are required on this combined
treatment for solid tumors.
Acknowledgements
The present study was supported by a program from
Qingdao Municipal Science and Technology Commission [Qingdao,
China; grant no. 2012-1-3-2-(13)-nsh].
References
|
1
|
Gray LH, Conger AD, Ebert M, Hornsey S and
Scott OC: The concentration of oxygen dissolved in tissues at the
time of irradiation as a factor in radiotherapy. Br J Radiol.
26:638–648. 1953. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Moulder JE and Rockwell S: Tumor hypoxia:
Its impact on cancer therapy. Cancer Metastasis Rev. 5:313–341.
1987. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Holzer LA, Cör A, Pfandlsteiner G and
Holzer G: Expression of VEGF, its receptors and HIF-1α in
Dupuytren's disease. Acta Orthop. 84:420–425. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Brahimi-Horn MC, Chiche J and Pouyssegur
J: Hypoxia and cancer. J Mol Med (Berl). 85:1301–1307. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Vaupel P, Hockel M and Mayer A: Detection
and characterization of tumor hypoxia using pO2 histography.
Antioxid Redox Signal. 9:1221–1235. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Multhoff G, Radons J and Vaupel P:
Critical role of aberrant angiogenesis in the development of tumor
hypoxia and associated radioresistance. Cancers (Basel). 6:813–828.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ikeda E: Cellular response to tissue
hypoxia and its involvement in disease progression. Pathol Int.
55:603–610. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Salceda S and Caro J: Hypoxia-inducible
factor 1alpha (HIF-1alpha) protein is rapidly degraded by the
ubiquitin-proteasome system under normoxic conditions. Its
stabilization by hypoxia depends on redox-induced changes. J Biol
Chem. 272:22642–22647. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Semenza GL: Targeting HIF-1 for cancer
therapy. Nat Rev Cancer. 3:721–732. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Arjamaa O, Nikinmaa M, Salminen A and
Kaarniranta K: Regulatory role of HIF-1alpha in the pathogenesis of
age-related macular degeneration (AMD). Ageing Res Rev. 8:349–358.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang P, Zhang X, Hao X, Wang Y, Hui Y,
Wang H, Hu D and Zhou J: Rac1 activates HIF-1 in retinal pigment
epithelium cells under hypoxia. Graefes Arch Clin Exp Ophthalmol.
247:633–639. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Semenza GL: Regulation of mammalian O2
homeostasis by hypoxia-inducible factor 1. Annu Rev Cell Dev Biol.
15:551–578. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tammela T, Zarkada G, Nurmi H, Jakobsson
L, Heinolainen K, Tvorogov D, Zheng W, Franco CA, Murtomäki A,
Aranda E, et al: VEGFR-3 controls tip to stalk conversion at vessel
fusion sites by reinforcing Notch signalling. Nat Cell Biol.
13:1202–1213. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Semenza GL: HIF-1 and human disease: One
highly involved factor. Genes Dev. 14:1983–1991. 2000.PubMed/NCBI
|
|
15
|
Jain RK: Normalization of tumor
vasculature: An emerging concept in antiangiogenic therapy.
Science. 307:58–62. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ke QH, Zhou SQ, Huang M, Lei Y, Du W and
Yang JY: Early efficacy of Endostar combined with chemoradiotherapy
for advanced cervical cancers. Asian Pac J Cancer Prev. 13:923–926.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
O'Reilly MS, Boehm T, Shing Y, Fukai N,
Vasios G, Lane WS, Flynn E, Birkhead JR, Olsen BR and Folkman J:
Endostatin: An endogenous inhibitor of angiogenesis and tumor
growth. Cell. 88:277–285. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Qi X, Liu Y, Wei W, Huang X and Zuo Y:
Effects of the C-terminal of endostatin on the tumorigenic
potential of H22 cells. Biomed Rep. 1:761–765. 2013.PubMed/NCBI
|
|
19
|
Huang RP and Adamson ED: A biological role
for Egr-1 in cell survival following ultra-violet irradiation.
Oncogene. 10:467–475. 1995.PubMed/NCBI
|
|
20
|
Zhang Y, Qiu W, Liang J, Yu Z and Yue L:
Anti-tumor effects of pEgr-1-endostatin-TNF-α recombinant plasmid
expression induced by ionizing radiation. Asian Pac J Cancer Prev.
12:2933–2937. 2011.PubMed/NCBI
|
|
21
|
El-Gendi S and Abdel-Hadi M: Lymphatic
vessel density as prognostic factor in breast carcinoma: Relation
to clinicopathologic parameters. J Egypt Natl Canc Inst.
21:139–149. 2009.PubMed/NCBI
|
|
22
|
Ferrara N and Davis-Smyth T: The biology
of vascular endothelial growth factor. Endocr Rev. 18:4–25. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cao D, Hou M, Guan YS, Jiang M, Yang Y and
Gou HF: Expression of HIF-1alpha and VEGF in colorectal cancer:
Association with clinical outcomes and prognostic implications. BMC
Cancer. 9:4322009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wilson WR and Hay MP: Targeting hypoxia in
cancer therapy. Nat Rev Cancer. 11:393–410. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li L, Huang JL, Liu QC, Wu PH, Liu RY,
Zeng YX and Huang WL: Endostatin gene therapy for liver cancer by a
recombinant adenovirus delivery. World J Gastroenterol.
10:1867–1871. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yoshimura M, Itasaka S, Harada H and
Hiraoka M: Microenvironment and radiation therapy. Biomed Res Int.
2013:6853082013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang J, Sun Y, Liu Y, Yu Q, Zhang Y, Li K,
Zhu Y, Zhou Q, Hou M, Guan Z, et al: Results of randomized,
multicenter, double-blind phase III trial of rh-endostatin (YH-16)
in treatment of advanced non-small cell lung cancer patients.
Zhongguo Fei Ai Za Zhi. 8:283–290. 2005.(In Chinese). PubMed/NCBI
|
|
28
|
Folkman J: Tumor angiogenesis: Therapeutic
implications. N Engl J Med. 285:1182–1186. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Forsythe JA, Jiang BH, Iyer NV, Agani F,
Leung SW, Koos RD and Semenza GL: Activation of vascular
endothelial growth factor gene transcription by hypoxia-inducible
factor 1. Mol Cell Biol. 16:4604–4613. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Brizel DM, Scully SP, Harrelson JM,
Layfield LJ, Bean JM, Prosnitz LR and Dewhirst MW: Tumor
oxygenation predicts for the likelihood of distant metastases in
human soft tissue sarcoma. Cancer Res. 56:941–943. 1996.PubMed/NCBI
|
|
31
|
Lohela M, Bry M, Tammela T and Alitalo K:
VEGFs and receptors involved in angiogenesis versus
lymphangiogenesis. Curr Opin Cell Biol. 21:154–165. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mauceri HJ, Beckett MA, Liang H, Sutton
HG, Pitroda S, Galka E, Efimova E, Darga T, Khodarev NN, King CR,
et al: Translational strategies exploiting TNF-alpha that sensitize
tumors to radiation therapy. Cancer Gene Ther. 16:373–381. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kim YM, Jang JW, Lee OH, Yeon J, Choi EY,
Kim KW, Lee ST and Kwon YG: Endostatin inhibits endothelial and
tumor cellular invasion by blocking the activation and catalytic
activity of matrix metalloproteinase. Cancer Res. 60:5410–5413.
2000.PubMed/NCBI
|
|
34
|
Rehn M, Veikkola T, Kukk-Valdre E,
Nakamura H, Ilmonen M, Lombardo C, Pihlajaniemi T, Alitalo K and
Vuori K: Interaction of endostatin with integrins implicated in
angiogenesis. Proc Natl Acad Sci USA. 98:1024–1029. 2001.
View Article : Google Scholar : PubMed/NCBI
|


















