Introduction
The liver is a target organ implicated in a number
of primary and metastatic types of cancer. Primary liver cancer
types include hepatocellular carcinoma and cholangiocarcinoma; the
most frequent original sites of metastasis in liver cancer are the
stomach, pancreas and colon (1).
Surgery is the most effective curative treatment for primary and
metastatic liver cancer (2,3); however, if surgery is not a viable
option, chemotherapy or molecular target therapy are considered for
further treatment (4–6). The agents used in chemotherapy and
molecular targeted therapy frequently cause hepatotoxicity, which
limits the efficacy of this treatment (7,8).
Therefore, the development of therapeutic approaches with a lower
risk of hepatotoxicity is required.
Metabolism in cancer cells is characterized by
increased aerobic glycolysis and lactate production, even under a
sufficient oxygen supply (the Warburg effect), and cancer cells
require more glucose compared with the surrounding normal tissues
(9). This phenomenon is applied to
positron emission tomography, in which 18-fluorodeoxyglucose, an
analogue of glucose, is taken up by cancer cells (10). Unlike glucose, 18-fluorodeoxyglucose
is not metabolized and accumulates in cancer cells; therefore, a
positive signal may be detected from cancerous tissues (11). Glucose is an important source of
energy that is essential for cell survival (12,13).
Galactose enters glycolysis as a substrate for the enzyme
galactokinase (GALK), which is expressed in the liver and kidney
(14,15). Arginine is an essential amino acid
produced from ornithine by ornithine carbamoyltransferase (OTC) in
the urea cycle, which is exclusive to hepatocytes (16). Normal cells produce arginine de
novo, whereas cancer cells take up arginine from extracellular
tissues (17).
Hepatocytes express GALK and OTC, and therefore, may
be expected to survive in a medium lacking glucose and arginine,
but supplemented with galactose and ornithine (18,19). Our
previous study developed a hepatocyte selection medium (HSM), which
lacks glucose and arginine but contains galactose and ornithine
(20). Primary human hepatocytes are
able to survive in HSM, and this medium purifies primary human
hepatocytes from co-culture with human-induced pluripotent stem
cells (21).
Therefore, the present study analyzed the
suppression of the proliferation and motility of hepatocellular
carcinoma cells, pancreatic cancer cells and gastric cancer cells
in HSM.
Materials and methods
Cell culture
Human hepatocellular carcinoma HLF and PLC/PRF/5
cells, human pancreatic cancer MIA-Paca2 and PANC-1 cells and human
gastric cancer MKN45 and MKN74 cells were purchased from the Riken
Cell Bank (Cell Engineering Division, Riken Biosource Center,
Tsukuba, Japan). HLF cells, PLC/PRF/5 cells and MIA-Paca2 cells
were cultured in Dulbecco's modified Eagle's medium (DMEM;
Sigma-Aldrich; Merck Millipore, Darmstadt, Germany) supplemented
with 10% fetal bovine serum (FBS; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA). PANC-1, MKN45 and MKN74 cells were cultured in
RPMI-1640 (Sigma-Aldrich; Merck Millipore) supplemented with 10%
FBS. Cell lines were cultured with 5% carbon dioxide at 37°C in a
humidified chamber, and passaged twice a week.
HSM
HSM was prepared from amino acid powders, using the
formulation method of Leibovitz-15 medium (Thermo Fisher
Scientific, Inc.), but omitting arginine, tyrosine, glucose and
sodium pyruvate, and adding galactose (900 mg/l; Wako Pure Chemical
Industries, Ltd., Osaka, Japan), ornithine (1 mM; Wako Pure
Chemical Industries, Ltd.), glycerol (5 mM; Wako Pure Chemical
Industries, Ltd.) and proline (260 mM; Wako Pure Chemical
Industries, Ltd.). Proline is necessary for DNA synthesis, and
therefore, it was included in the medium (30 mg/l) (22). Knockout serum replacement (KSR; Thermo
Fisher Scientific, Inc.) was used at a final concentration of 10%
in place of FBS in order to establish defined xeno-free conditions
in HSM.
Cell proliferation analysis
HLF, PLC/PRF/5, MIA-Paca2, PANC-1, MKN45 and MKN74
cells were trypsinized, harvested and seeded onto 96-well
flat-bottomed plates at a density of 1,000 cells/well, then
incubated at 37°C for 24 h in DMEM or RPMI-1640 supplemented with
10% FBS. Subsequent to changing the medium to HSM, the cells were
cultured at 37°C for a further 72 h, and subjected to an MTS assay
(Promega Corporation, Madison, WI, USA), according to the
manufacturer's protocol. MTS is reduced by the cells into a colored
formazan product that reduces the absorbance at 490 nm; the
absorbance at 490 nm was evaluated using an iMark Microplate
Absorbance Reader (Bio-Rad Laboratories, Inc., Hercules, CA,
USA).
Hematoxylin and eosin staining
HLF, PLC/PRF/5, MIA-Paca2, PANC-1, MKN45 and MKN74
cells were cultured in chamber slides (Matsunami, Kishiwada, Japan)
in a 5% CO2 atmosphere at 37°C in a humidified chamber,
and subjected to hematoxylin and eosin staining (Muto Glass, Tokyo,
Japan). The cells were fixed in 10% formalin at room temperature
for 10 min. The cells were stained with hematoxylin, and incubated
at room temperature for 4 min. The slides were incubated in water
at 25°C for 30 min. The cells were dehydrated in 100% ethanol for
10 min three times, and were stained with eosin for 4 min. The
cells were dehydrated in 100% ethanol for 10 min three times, and
mounted under cover slips. The slides were observed under ×200
magnification using an AX80 microscope (Olympus Corporation, Tokyo,
Japan). Five different fields of view were observed.
TUNEL staining
Cells were cultured in chamber slides with 5% carbon
dioxide at 37°C in a humidified chamber and apoptotic cells were
detected using the Wako Apoptosis In Situ Detection kit
(Wako Pure Chemical Industries, Ltd.), following the manufacturer's
protocol. Apoptotic cells were analyzed using a TUNEL assay, which
consists of the addition of TdT to the 3′-terminus of apoptotically
fragmented DNA, followed by immunochemical detection using an
anti-fluorescein antibody conjugated with horseradish peroxidase
and diaminobenzidine (DAB) as the substrate, following the
manufacturer's protocol. The stained slides were observed under
×100 magnification using an AX80 microscope.
Scratch assay
HLF, PLC/PRF/5, MIA-Paca2, PANC-1, MKN45 and MKN74
cells were plated on 4-well chamber slides, and upon reaching 100%
confluency, the cell monolayer was scratched with a sterile razor,
incubated at 37°C for 48 h and stained with hematoxylin and eosin.
The stained slides were observed under ×200 magnification using an
AX80 microscope. The distance between the scratched and growing
edges of the cells was evaluated at five different fields.
Statistical analysis
Data were presented as the mean ± standard
deviation. Cell proliferation, TUNEL assay and scratch assay data
were analyzed by one-way analysis of variance. Statistical analysis
was performed using JMP 5.0J software (SAS Institute, Inc., Cary,
NC, USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
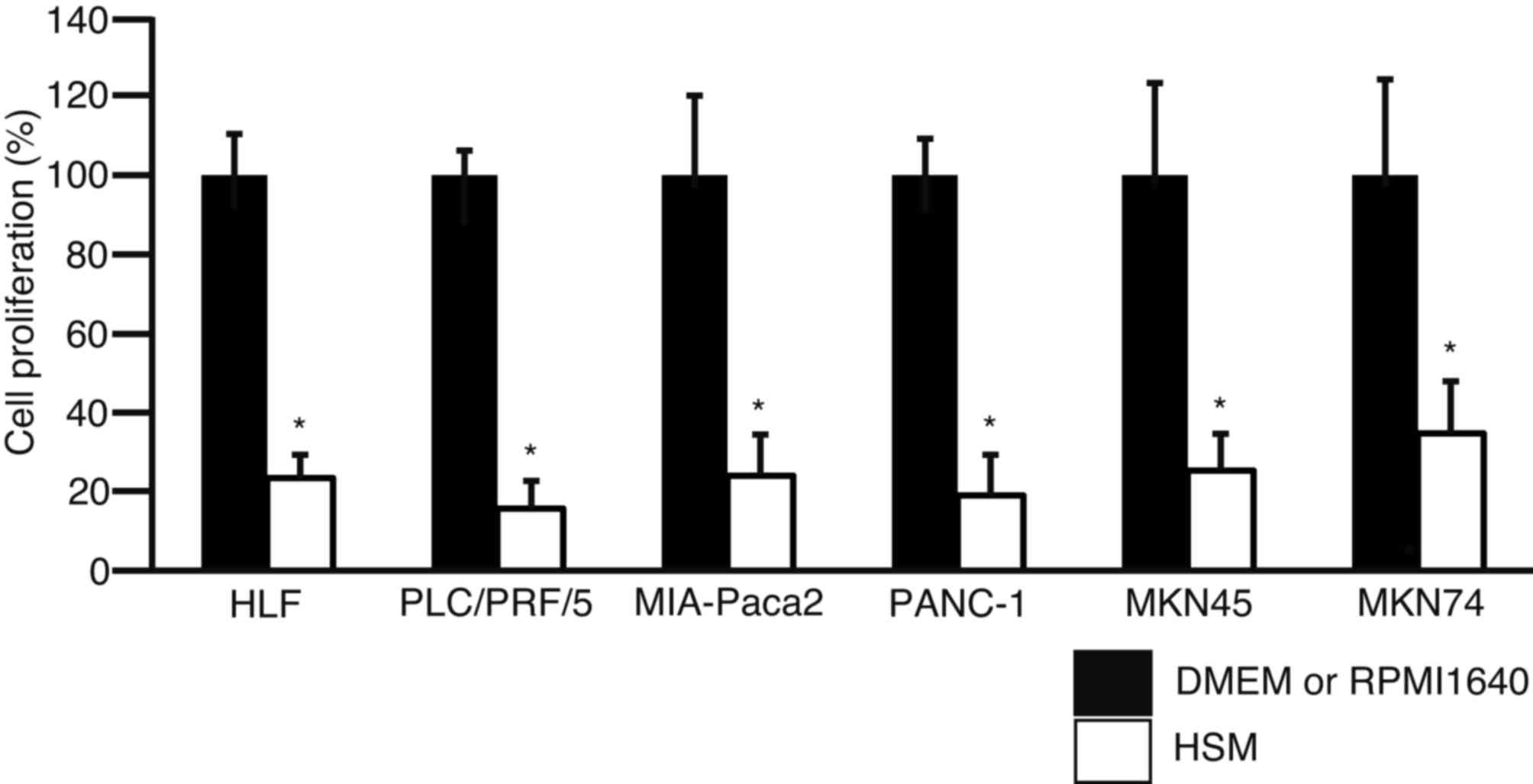
To examine the effect of culture in HSM on cell
proliferation, an MTS assay was performed following three days of
culture in DMEM, RPMI-1640 or HSM (Fig.
1). Proliferation of HLF, PLC/PRF/5, MIA-Paca2, PANC-1, MKN45
and MKN74 cells cultured in HSM decreased to 23.4±5.9, 15.7±7.0,
24.0±10.4, 19.0±10.3, 25.3±9.3 and 34.7±13.2%, respectively. The
results revealed that cell proliferation was significantly
suppressed in HSM for all cell lines (P<0.01).
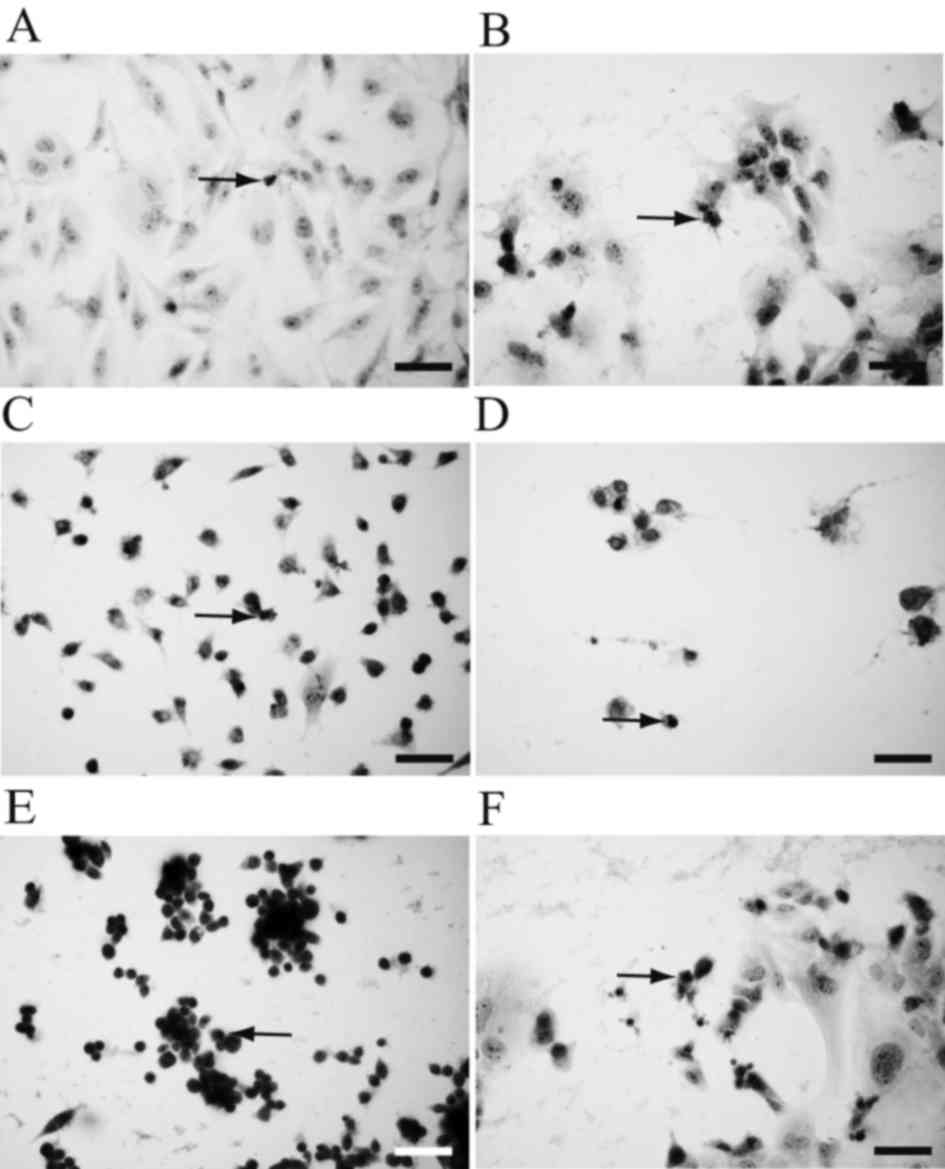
To observe morphological changes, hematoxylin and
eosin staining was performed following two days of culture in HSM
(Fig. 2). HLF cells (Fig. 2A), PLC/PRF/5 cells (Fig. 2B), MIA-Paca2 cells (Fig. 2C), PANC-1 cells (Fig. 2D), MKN45 cells (Fig. 2E) and MKN74 cells (Fig. 2F) all exhibited pyknotic nuclei,
suggesting that the cells had undergone apoptosis.
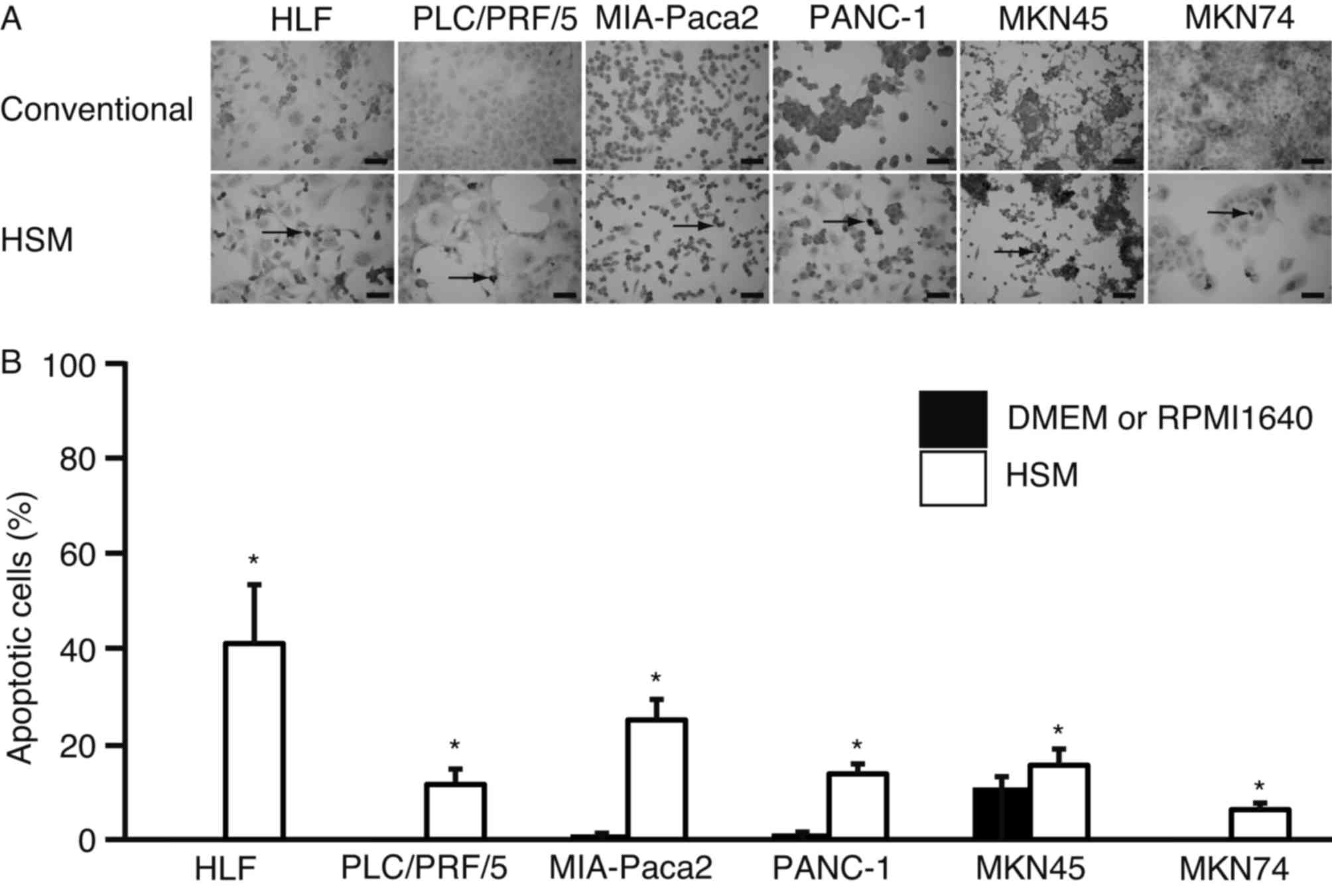
To further examine whether HSM-induced apoptosis had
occurred, a TUNEL assay was performed (Fig. 3). TUNEL-positive cells were observed
in all cell lines (Fig. 3A). The
percentage of apoptotic cells in HLF, PLC/PRF/5, MIA-Paca2, MKN45
and MKN74 cells cultured in DMEM or RPMI-1640 was 0.1±0.0, 0.2±0.0,
1.1±0.0, 1.3±0.0, 10.9±2.1 and 0.1±0.0%, respectively. The
percentage of cells in HLF, PLC/PRF/5, MIA-Paca2, PANC-1, MKN45 and
MKN74 cells cultured in HSM increased to 40.0±12.3, 11.5±3.2,
25.0±4.3, 13.7±2.3, 15.5±3.4 and 6.3±1.4%, respectively. The number
of TUNEL-positive cells was significantly higher in cells cultured
in HSM (P<0.01 in HLF, PLC/PRF/5, MIA-Paca2, PANC-1 and MKN74
cells; P=0.02 in MKN45 cells) (Fig.
3B).
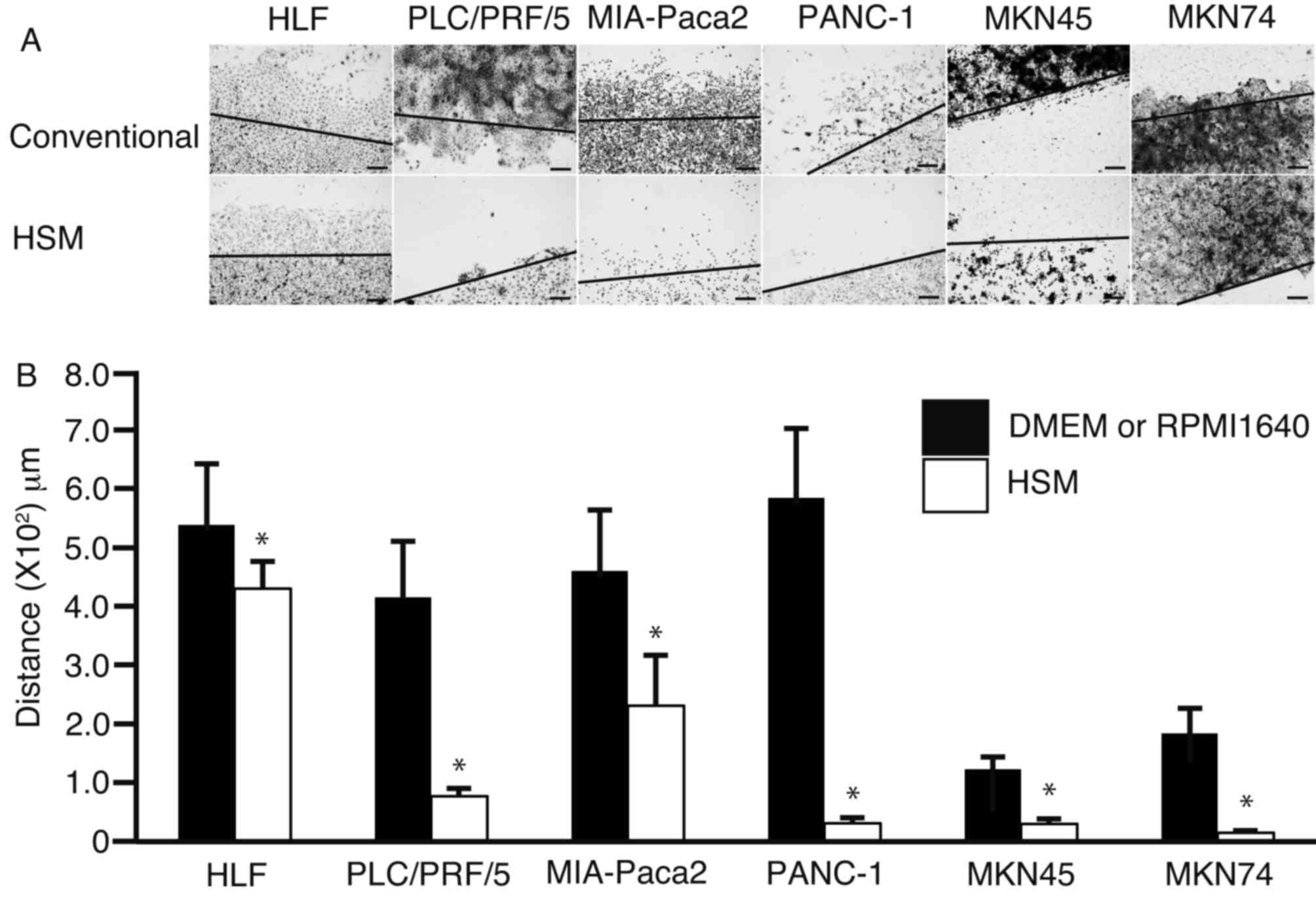
To investigate whether HSM suppresses cell motility,
a scratch assay was performed (Fig.
4). Each cell monolayer was scratched and cultured in DMEM,
RPMI-1640 or HSM for two days (Fig.
4A). The distances between the growing edge and the scratched
edge were 5.4±1.0×102, 4.2±0.9×102,
4.8±1.0×102, 5.8±1.2×102,
1.2±0.2×102 and 1.8±0.4×102 mm, respectively,
in HLF, PLC/PRF/5, MIA-Paca2, PANC-1, MKN45 and MKN74 cells
cultured in DMEM or RPMI-1640. The distances between the growing
edge and the scratched edge were 4.3±0.5×102,
7.7±1.3×10, 2.3±0.9×102, 3.1±0.9×10, 3.0±0.8×10 and
1.5±0.3×102 mm, respectively, in HLF, PLC/PRF/5,
MIA-Paca2, PANC-1, MKN45 and MKN74 cells cultured in HSM. The
distance between the growing edge and the scratched edge was
observed as significantly decreased (P=0.03 in HLF cells; P<0.01
in PLC/PRF/5, MIA-Paca2, PANC1, MKN45 and MKN74 cells) in
HSM-cultured cells compared with cells cultured in DMEM or
RPMI-1640 (Fig. 4B).
Discussion
In the present study, cell proliferation was
significantly suppressed when cells were cultured in HSM,
concordant with previous findings of reduced cell proliferation in
a medium lacking glucose (23). The
suppression of cell proliferation occurs in part due to cell cycle
arrest (23).
Morphological analysis using hematoxylin and eosin
staining suggested that the all the cell cultured in HSM had
undergone apoptosis in the current study, which was further
supported by the results of the TUNEL assay. Cancer cells primarily
depend on glycolysis for energy (9);
therefore, if glucose is lacking in the medium, cancer cells are
unable to proliferate and undergo apoptosis (24). HSM does not contain glucose, and
therefore, it is hypothesized that cancer cells grown in HSM
undergo apoptosis due to glucose deprivation (25–27). In
combination with previous findings, the results of the current
study suggest that glucose metabolism may present a novel target
for cancer treatment (28).
Arginine deprivation has also been demonstrated to
trigger apoptosis in cancer cells, and is proposed as a novel
approach for cancer treatment in melanoma and cervical cancer
(29). One example is the use of
arginase, which degrades arginine and induces apoptosis in cancer
cells (30). A previous study
indicated that cancer cells are resistant to arginine deprivation
in a three-dimensional environment (31). The current study used HSM, which does
not contain glucose or arginine, and the results revealed that that
deprivation of glucose and arginine may synergistically suppress
cell proliferation and induce apoptosis. Therefore, targeting the
metabolism of glucose and arginine appears to be a promising
approach for cancer treatment.
In addition, the current study observed that the
motility of cancer cells was suppressed in HSM. The effects of
glucose or arginine deprivation on the motility of cancer cells
remain to be elucidated; however, glucose deprivation is able to
suppress the motility of microglia in the mouse brain (32). Whether the results obtained for
microglia have the same biological significance in cancer cells
requires further study. However, the results of the current study
suggested that the deprivation of glucose and arginine in
combination inhibited the motility of cancer cells.
An advantage of HSM culture is that it allows
hepatocyte survival due to the presence of galactose and ornithine
(21). Therefore, it was hypothesized
in the present study that primary or metastatic liver cancer may be
treated without associated hepatotoxicity by using HSM.
Transcatheter arterial chemoembolization (TACE) is an established
technique, whereas balloon occluding of the intrahepatic artery is
a relatively novel technique that obstructs the artery with a
micro-balloon and immerses the cancer cells in chemotherapeutic
agents during TACE (33). Therefore,
the current study hypothesizes that cancer cells may undergo
apoptosis when cancerous tissues are immersed in HSM using
balloon-occluded TACE.
In conclusion, the proliferation and motility of
hepatocellular carcinoma cells, pancreatic cancer cells and gastric
cancer cells were suppressed in a medium without glucose and
arginine, but supplemented with galactose and ornithine (HSM). HSM
may have potential as a new treatment of hepatocellular
carcinoma.
References
|
1
|
Cong WM, Dong H, Tan L, Sun XX and Wu MC:
Surgicopathological classification of hepatic space-occupying
lesions: A single-center experience with literature review. World J
Gastroenterol. 17:2372–2378. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nakayama H and Takayama T: Role of
surgical resection for hepatocellular carcinoma based on Japanese
clinical guidelines for hepatocellular carcinoma. World J Hepatol.
7:261–269. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Petrelli F, Coinu A, Cabiddu M, Ghilardi
M, Borgonovo K, Lonati V and Barni S: Hepatic resection for gastric
cancer liver metastases: A systematic review and meta-analysis. J
Surg Oncol. 111:1021–1027. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lencioni R, Petruzzi P and Crocetti L:
Chemoembolization of hepatocellular carcinoma. Semin Intervent
Radiol. 30:3–11. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kim HY and Park JW: Clinical trials of
combined molecular targeted therapy and locoregional therapy in
hepatocellular carcinoma: Past, present, and future. Liver Cancer.
3:9–17. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gnoni A, Santini D, Scartozzi M, Russo A,
Licchetta A, Palmieri V, Lupo L, Faloppi L, Palasciano G, Memeo V,
et al: Hepatocellular carcinoma treatment over sorafenib:
Epigenetics, microRNAs and microenvironment. Is there a light at
the end of the tunnel? Expert Opin Ther Targets. 19:1623–1635.
2015. View Article : Google Scholar
|
|
7
|
Raoul JL, Gilabert M and Piana G: How to
define transarterial chemoembolization failure or refractoriness: A
European perspective. Liver Cancer. 3:119–124. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Karczmarek-Borowska B and Sałek-Zan A:
Hepatotoxicity of molecular targeted therapy. Contemp Oncol (Pozn).
19:87–92. 2015.PubMed/NCBI
|
|
9
|
Vaitheesvaran B, Xu J, Yee J, Q-Y L, Go
VL, Xiao GG and Lee WN: The Warburg effect: A balance of flux
analysis. Metabolomics. 11:787–796. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gallamini A, Zwarthoed C and Borra A:
Positron emission tomography (PET) in oncology. Cancers (Basel).
6:1821–1889. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gauthe M, Richard-Molard M, Cacheux W,
Michel P, Jouve JL, Mitry E, Alberini JL and Lièvre A: Fédération
Francophone de Cancérologie Digestive (FFCD): Role of fluorine 18
fluorodeoxyglucose positron emission tomography/computed tomography
in gastrointestinal cancers. Dig Liver Dis. 47:443–454. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Leffert HL and Paul D: Studies on primary
cultures of differentiated fetal liver cells. J Cell Biol.
52:559–568. 1972. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Matsumoto K, Yamada K, Kohmura E,
Kinoshita A and Hayakawa T: Role of pyruvate in ischaemia-like
conditions on cultured neurons. Neurol Res. 16:460–464. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ohira RH, Dipple KM, Zhang YH and McCabe
ER: Human and murine glycerol kinase: Influence of exon 18
alternative splicing on function. Biochem Biophys Res Commun.
331:239–246. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ai Y, Jenkins NA, Copeland NG, Gilbert DH,
Bergsma DJ and Stambolian D: Mouse galactokinase: Isolation,
characterization, and location on chromosome 11. Genome Res.
5:53–59. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wheatley DN, Scott L, Lamb J and Smith S:
Single amino acid (arginine) restriction: Growth and death of
cultured HeLa and human diploid fibroblasts. Cell Physiol Biochem.
10:37–55. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Qiu F, Huang J and Sui M: Targeting
arginine metabolism pathway to treat arginine-dependent cancers.
Cancer Lett. 364:1–7. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Phillips JW, Jones ME and Berry MN:
Implications of the simultaneous occurrence of hepatic glycolysis
from glucose and gluconeogenesis from glycerol. Eur J Biochem.
269:792–797. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sumida KD, Crandall SC, Chadha PL and
Qureshi T: Hepatic gluconeogenic capacity from various precursors
in young versus old rats. Metabolism. 51:876–880. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tomizawa M, Toyama Y, Ito C, Toshimori K,
Iwase K, Takiguchi M, Saisho H and Yokosuka O: Hepatoblast-like
cells enriched from mouse embryonic stem cells in medium without
glucose, pyruvate, arginine, and tyrosine. Cell Tissue Res.
333:17–27. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tomizawa M, Shinozaki F, Sugiyama T,
Yamamoto S, Sueishi M and Yoshida T: Survival of primary human
hepatocytes and death of induced pluripotent stem cells in media
lacking glucose and arginine. PLoS One. 8:e718972013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nakamura T, Teramoto H, Tomita Y and
Ichihara A: L-proline is an essential amino acid for hepatocyte
growth in culture. Biochem Biophys Res Commun. 122:884–891. 1984.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fu P, Tang R, Yu Z, Huang S, Xie M, Luo X
and Wang W: Bumetanide-induced NKCC1 inhibition attenuates
oxygen-glucose deprivation-induced decrease in proliferative
activity and cell cycle progression arrest in cultured OPCs via
P-38 MAPKs. Brain Res. 1613:110–119. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gonzalez CD, Alvarez S, Ropolo A,
Rosenzvit C, Bagnes MF and Vaccaro MI: Autophagy, Warburg, and
Warburg reverse effects in human cancer. Biomed Res Int.
2014:9267292014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Qiu J, Zhou XY, Zhou XG, Li Y, Cheng R and
Liu HY: MicroRNA210 knockdown contributes to apoptosis caused by
oxygen glucose deprivation in PC12 cells. Mol Med Rep. 11:719–723.
2015.PubMed/NCBI
|
|
26
|
Wardi L, Alaaeddine N, Raad I, Sarkis R,
Serhal R, Khalil C and Hilal G: Glucose restriction decreases
telomerase activity and enhances its inhibitor response on breast
cancer cells: Possible extra-telomerase role of BIBR 1532. Cancer
Cell Int. 14:602014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kim HS, Kim MJ, Lim J, Yang Y, Lee MS and
Lim JS: NDRG2 overexpression enhances glucose deprivation-mediated
apoptosis in breast cancer cells via inhibition of the LKB1-AMPK
pathway. Genes Cancer. 5:175–185. 2014.PubMed/NCBI
|
|
28
|
Elf SE and Chen J: Targeting glucose
metabolism in patients with cancer. Cancer. 120:774–780. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Feun LG, Kuo MT and Savaraj N: Arginine
deprivation in cancer therapy. Curr Opin Clin Nutr Metab Care.
18:78–82. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zeng X, Li Y, Fan J, Zhao H, Xian Z, Sun
Y, Wang Z, Wang S, Zhang G and Ju D: Recombinant human arginase
induced caspase-dependent apoptosis and autophagy in non-Hodgkin's
lymphoma cells. Cell Death Dis. 4:e8402013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Vynnytska-Myronovska B, Kurlishchuk Y,
Bobak Y, Dittfeld C, Kunz-Schughart LA and Stasyk O:
Three-dimensional environment renders cancer cells profoundly less
susceptible to a single amino acid starvation. Amino Acids.
45:1221–1230. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Eyo U and Dailey ME: Effects of
oxygen-glucose deprivation on microglial mobility and viability in
developing mouse hippocampal tissues. Glia. 60:1747–1760. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Imai N, Ishigami M, Ishizu Y, Kuzuya T,
Honda T, Hayashi K, Hirooka Y and Goto H: Transarterial
chemoembolization for hepatocellular carcinoma: A review of
techniques. World J Hepatol. 6:844–850. 2014. View Article : Google Scholar : PubMed/NCBI
|