Introduction
The Philadelphia chromosome (Ph) or breakpoint
cluster region-Abelson (BCR-ABL) fusion gene is more common
in patients with chronic myelogenous leukemia (CML) (1) than in those with precursor B-acute
lymphoblastic leukemia (ALL) (2);
however, Ph + acute myeloid leukemia (AML) has also been
reported.
AML has an incidence of 3.7 per 100,000 individuals,
with anemia, bleeding, fever and bone pain as its typical symptoms.
Currently, the standard therapy for AML consists of chemotherapy,
immunotherapy, targeting therapy and hematopoietic stem cell
transplantation (3,4).
Although AML secondary to chemotherapy or
radiotherapy has been previously reported (5), to the best of our knowledge, there are
no reports of concurrent Ph + AML and B cell lymphoma in untreated
patients. The current case reports presents a patient who presented
with leukocytosis and lymphadenopathy and was diagnosed with Ph +
AML concurrent with large B cell lymphoma.
Case report
A 37-year-old Chinese woman complaining of bone pain
and fever was admitted to the West China Hospital of Sichuan
University (Chengdu, China) in June 2011. The patient was
previously healthy, and the complete blood count (CBC) at 3 months
prior to admission was normal. Upon pathological examination,
multiple bone tenderness and a painless 2×1-cm lymph node in the
left axillary fossa were palpable. Laboratory test results
suggested mild leukocytosis (13.6×106/µl; normal range,
3.5–9.5×106/µl) with 10% white blood cells but normal
hemoglobin and platelet counts. Bone marrow smear demonstrated
33.5% myeloid blasts and biopsy revealed diffuse infiltration of
AML-like cells. Flow cytometry (FCM) results demonstrated white
blood cells co-expressing human leukocyte antigen-antigen
D-related, CD34, CD38, CD13, CD33, CD117, cytoplasmic
myeloperoxidase and aberrant CD19. Giemsa-banding discovered
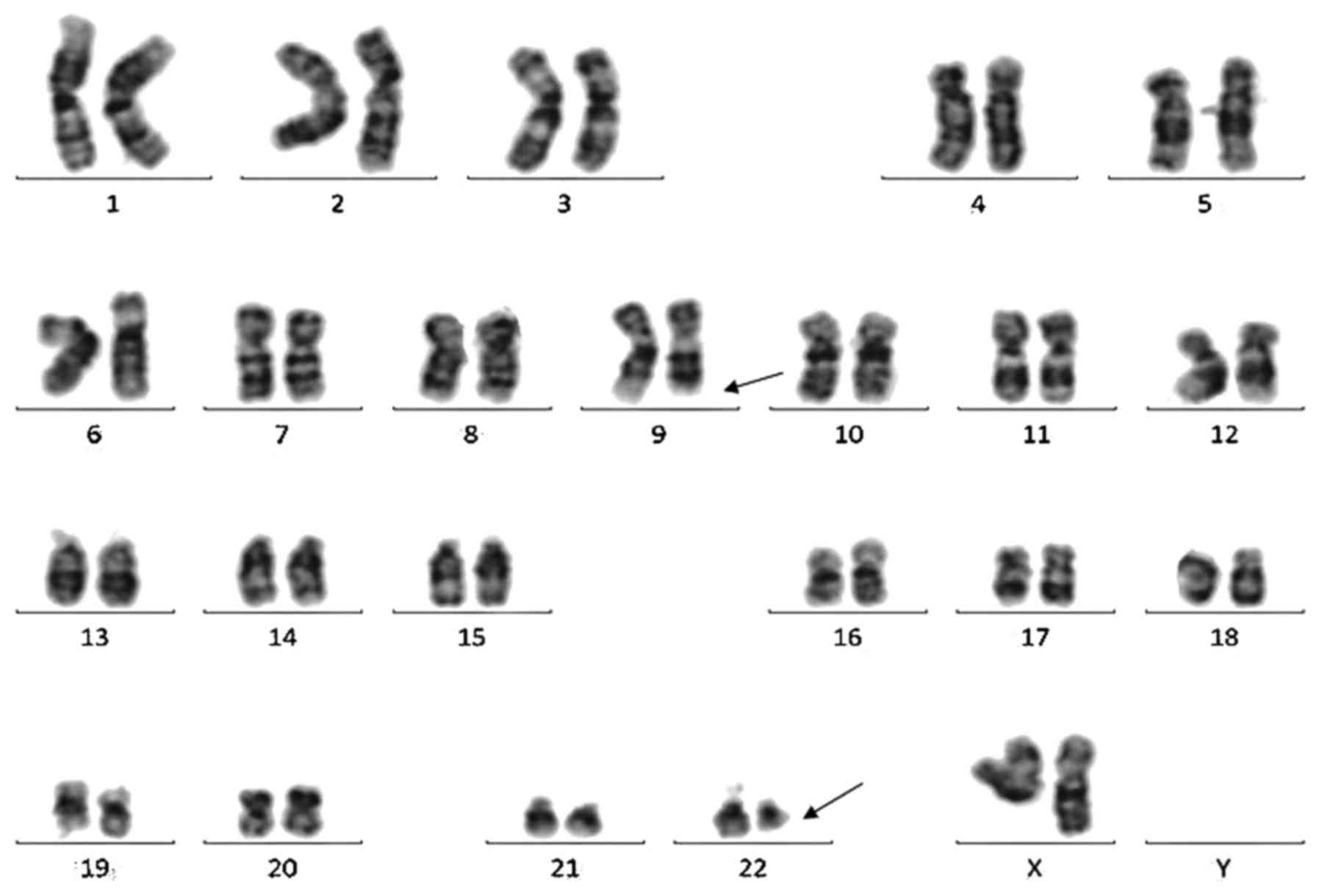
t(9;22)(q34;q11) in 5/20 metaphases (Fig.
1). Quantitative polymerase chain reaction (PCR) detected P210
type BCR-ABL fusion gene and BCR-ABL/ABL ratio
as 5.45×105 copies/ml (5.19%). Bone scan with
99mTc-methylene diphosphonate revealed multiple
abnormalities throughout the body. The BCR-ABL fusion gene RT-PCR
detection kit was purchased from Shanghai Source Biomedical
Technology Co., Ltd. (Shanghai, China). The thermocycling
conditions were as follows: 42°C for 30 min, 94°C for 5 min, 94°C
for 15 sec and 60°C for 60 sec, for a total of 40 cycles. An
1.5×1.5-cm lymph node in the left axillary fossa was revealed by
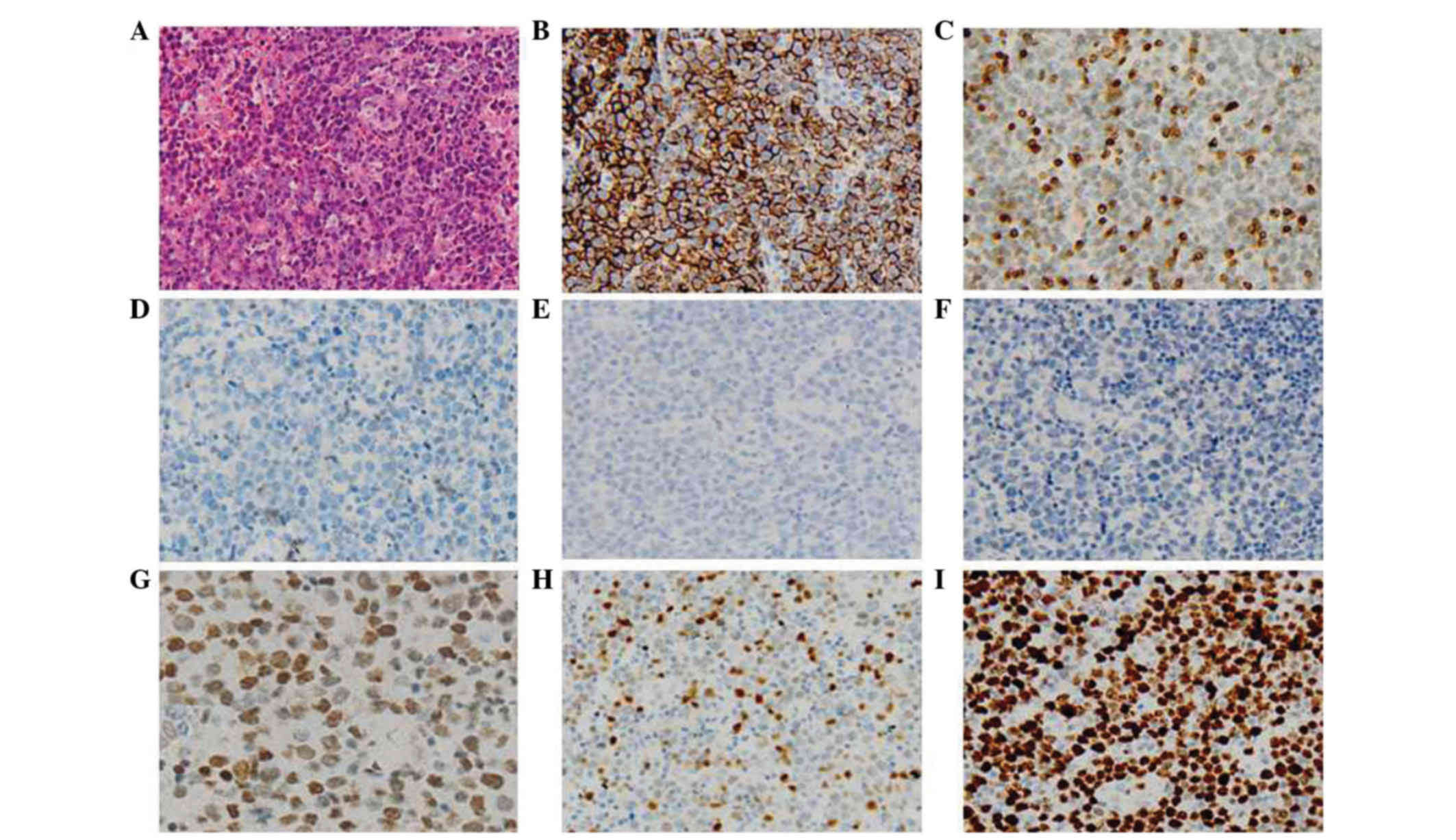
computed tomography (CT) scan. Lymph node biopsy was performed and
the formalin-fixed, paraffin-embedded tissue was subjected to
pathological diagnosis. The results supported the diagnosis of
non-Hodgkin lymphoma (diffuse large B cell lymphoma, aggressive, of
non-germinal center B cell origin) according to the 2008 World
Health Organization Classification (Fig.
2) (4). Considering the leukemic
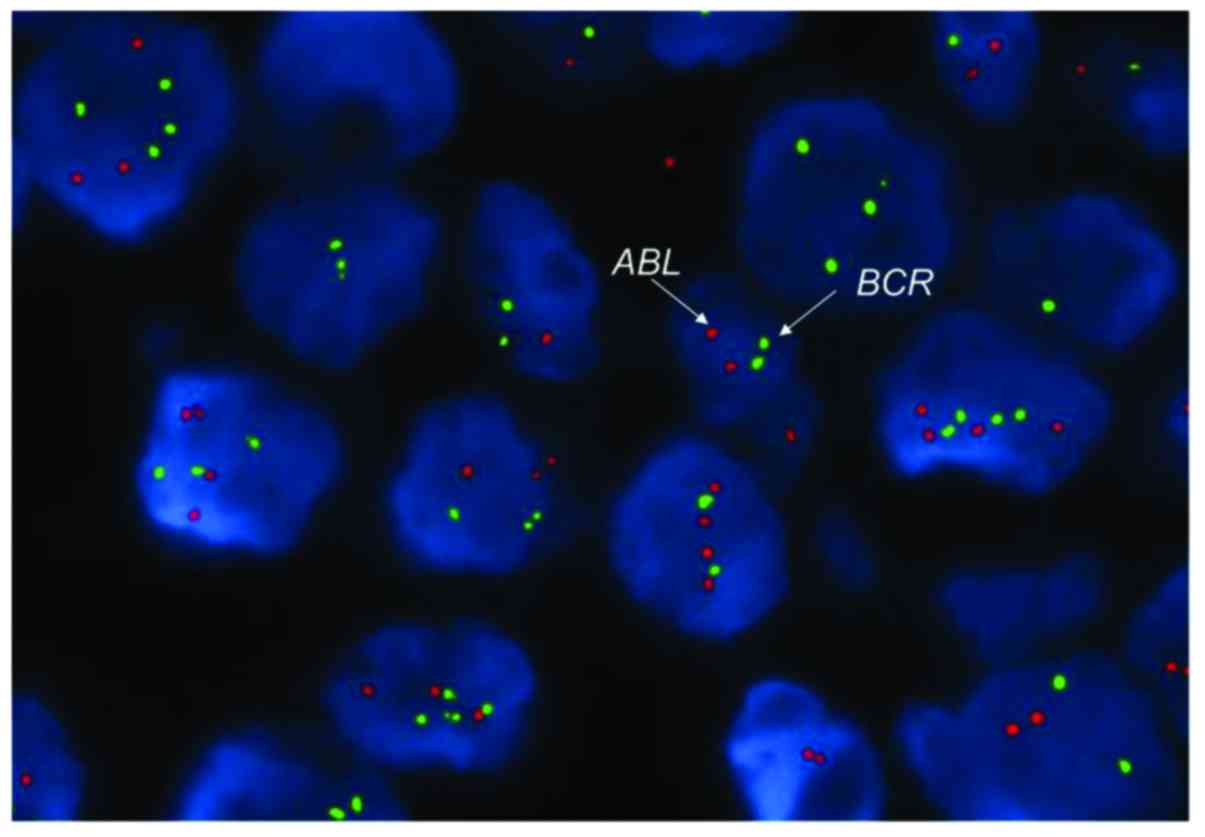
cells harboring the BCR-ABL gene, fluorescence in
situ hybridization (FISH) with BCR-ABL probes was
performed on the lymph node specimen with no fusion signal detected
(Fig. 3). Two days later, the patient
developed a headache on the right side of the head, without nausea
or vomiting. Head CT scan was normal, while T2-weighted magnetic
resonance imaging scan demonstrated a long, contrast
agent-intensified signal on multiple sites of the skull, excluding
the cerebral parenchyma. The results of routine, biochemical and
FCM tests of the cerebrospinal fluid were normal.
Based on the aforementioned findings, Ph + AML with
concurrent diffuse large B cell lymphoma was diagnosed. The bone
pain and headache were considered to indicate leukemia or lymphoma
infiltration, due to no evidence of infection. Treatment with
rituximab (600 mg on day 1), adriamycin (70 mg on day 2),
cyclophosphamide (1,000 mg on day 2), vincristine (2 mg on day 2),
cytarabine (150 mg on days 2–8) and dexamethasone (15 mg on days
2–8) was administered and relieved the symptoms shortly. The lymph
node returned to its normal state and complete hematologic
remission was obtained; however, BCR-ABL transcripts
remained positive. Two courses of a similar regimen followed for
consolidation. CHR was confirmed prior to each consolidation, while
BCR-ABL transcripts remained detectable and increased
(BCR-ABL/ABL, from 0.67 up to 8.8%). Soon after the
second consolidation, the patient developed middle grade fever and
joint pain in the left knee; leukemia relapse was confirmed by bone
marrow smear, FCM and further elevated BCR-ABL transcripts
(BCR-ABL/ABL, 71.73%). Treatment with imatinib
mesylate (IM) (400 mg daily for >2 months) and chemotherapy [10
mg idarubicin (days 1,3 and 5), 2 mg vincristine (day 1), 100 mg
cytarabine (days 1–5) and 30 mg prednisone (days 1–5)] was
administered, alleviating the symptoms from the following day;
however, the reappearing and continuously increasing peripheral
blasts suggested leukemia progression. IM was considered
ineffective and the bone marrow specimen was sent to confirm the
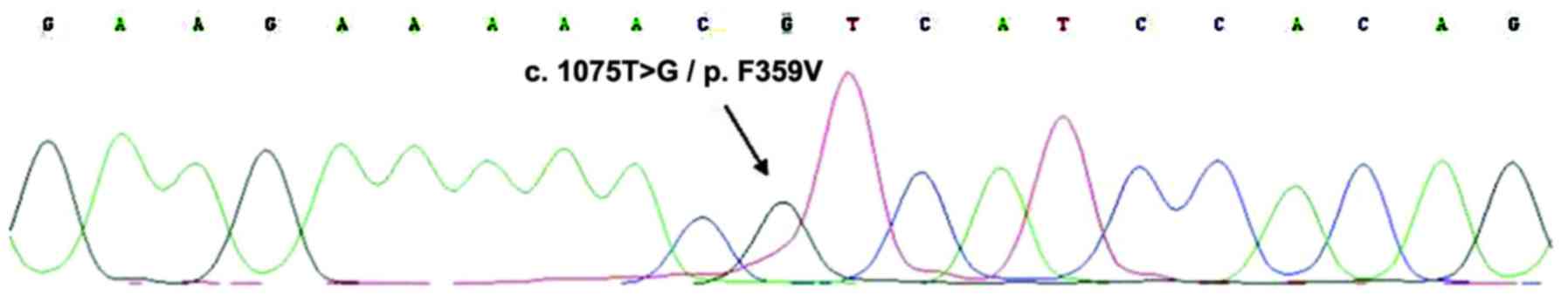
suspected ABL kinase mutation, which demonstrated
F395V mutation. For that purpose, DNA was extracted from the
bone marrow specimen, and PCR and DNA sequencing were used to
assess the presence of the F395V mutation (Fig. 4). In addition, gene mutations common
for AML (CEBPA, DNMT3A, FLT3-ITD, IDH1, IDH2, KIT, KRAS, NPM1,
NRAS, TET2 and WT1) were investigated in smears at
diagnosis and relapse and were all negative. Due to
ineffectiveness, IM was switched for dasatinib (75 mg daily), which
resulted in a mild decrease in BCR-ABL transcripts, but no
hematologic response. Twenty days after relapse, the patient
suddenly developed an intracranial hemorrhage and succumbed shortly
after.
Written informed consent was signed by the husband
of the patient and approved by the Ethics Committee of the West
China Hospital of Sichuan University (Chengdu, China).
Discussion
The Ph chromosome or BCR-ABL fusion gene can
be found in >95% of all CML cases, and 5–30% of adult and 2–5%
of pediatric ALL cases (2); however,
it has also been reported in AML. Keung et al (6) conducted a retrospective study of 148
cases of t(9;22)(q34;q11), and identified 84% as CML chronic phase,
13% as de novo ALL, 1% as de novo AML and 2% as
myelodysplastic syndrome (MDS). The estimated incidence of Ph + AML
was 0.6%. Ph + AMLs were reported with either major or minor BCR
gene rearrangements, similar to those of Ph + ALL, suggesting that
Ph + AML is a distinct disease rather than CML-myeloid blast crisis
(MBC) phase. Furthermore, rare cases of Ph + MDS were also
reported, which imply that Ph + AML is a distinct disease entity
rather than representing blastic transformation from CML (6). Clinically, Ph + AML presents with less
incidence of splenomegaly and significant basophilia (7). Immunophenotypic analysis of Ph + AML
disclosed co-expression of CD34 and multiple myeloid markers (such
as CD13 and CD33), and a common aberrant expression of lymphoid
markers (≥2 in 60% of cases) (8);
additional cytogenetic abnormalities, such as extra copies of Ph or
trisomy 8, were more commonly detected in CML-MBC, compared with Ph
+ AML (59.9 vs. 25%; P=0.008) (9).
The coexistence of normal and Ph+ metaphases at
diagnosis is more characteristic of Ph + AML (5). The induction of chemotherapy usually
causes CBC and karyotype normalization in Ph + AML, but chronic or
accelerated-phase hemogram and persistence of the Ph chromosome in
CML-MBC (7,8). Konoplev et al (10) detected NPM1 and FLT3-ITD
mutations (frequent in AML) in 2/9 and 1/9 of patients with Ph +
AML, respectively, in addition to no mutation in patients with
CML-MBC, and no ABL1 mutation (common in CML-MBC) in any of
the 9 Ph + AML patients. Array comparative genomic hybridization
was performed in Ph + AML, bilineage leukemia, Ph + ALL and CML
(11). Losses of IGH, TRG2, VPREB1
and IGLL1 were detected in Ph + AML, Ph + ALL and CML lymphoid
BC but not in CML-chronic phase, CML-MBC or AML with normal
karyotype, which further verified the difference between Ph + AML
and CML-MBC; however, no single clinical or laboratory test can
definitively distinguish the two diseases. The previous CBC of the
present patient was normal, and no splenomegaly and basophilia were
found at diagnosis. The FCM test result suggested a diagnosis of Ph
+ AML. Following the induction of chemotherapy, the CBC returned to
normal. Taken together, all aforementioned findings suggest that
de novo Ph + AML may have been a more appropriate diagnosis
for this patient.
Due to its rarity, no standard therapies for Ph +
AML have been established, and treatment options derive largely
from reports of similar cases. In the pre-tyrosine kinase inhibitor
(TKI) era, Ph + AML was usually treated by conventional
chemotherapy. Cuneo et al (9)
reported that conventional chemotherapy achieved CR in 4/11
patients (36%), while in the study by Paietta et al, none of
the 6 patients obtained remission (5). Due to its marked effect on CML, IM was
also used in Ph + AML. IM was reported for the treatment of Ph +
AML, achieving sustained cytogenetic response and 1 case of
molecular remission for 15 months (12–14). In a
larger retrospective treatment study, 7/16 patients were treated
with IM, among which, 6 achieved HR and 1 achieved CHR, although
the response durations were short (median, 2.5 months; range, 1–6
months) (9). IM should therefore be
considered front-line therapy (9),
but the optimal dosage, timing and duration remain to be
determined.
IM is usually administered at a dose of 400 mg
daily, when combined with chemotherapy (15,16), or at
a dose of 600 mg daily when administered alone (12–14). Sun
et al (17) reported 2
patients with Ph + AML, who were treated with IM and
daunorubicin-based chemotherapy, followed by allo-hematopoietic
stem cell transplantation (HSCT) and IM maintenance regimen. Both
patients obtained CHR and complete cytogenetic and molecular
responses for 44 and 48 months. Another patient receiving unrelated
allo-HSCT during CR2, and sustained CHR for 70 months (18); therefore, IM combined with
chemotherapy, followed by allo-HSCT and IM maintenance treatment
appears to be an effective treatment option for Ph + AML,
particularly when IM is used early (17).
IM was not initially administered to the present
patient, which may account for the failure of molecular response.
Although IM was added following relapse, it failed to bring
molecular effect due to the F395V mutation. Despite
dasatinib treatment theoretically overcoming this mutation,
desatinib may have been used too late and led only to a decrease in
transcripts but no HR. This finding implies that the BCR-ABL signal
is not the only or not the major pathway to cause Ph + AML. The
initial treatment plan for the present patient was treatment with
allo-HSCT, but it was hindered by the lack of matched donor. In
addition, auto-HSCT could not be considered for a patient without
molecular remission.
Despite the fact that secondary AML or MDS has been
frequently reported in patients receiving chemotherapy and/or
radiotherapy, concomitant lymphoma and AML in previously untreated
patients is extremely rare (19).
Only a few cases of AML concurrent with lymphoma have been reported
(19–23); To the best of our knowledge, this is
the first report of simultaneous Ph + AML and diffuse large B cell
lymphoma. It is known that leukemia may involve extramedullary
sites, including lymph nodes. In the present case, the lymph node
biopsy revealed no leukemic cells, excluding AML infiltration of
lymph nodes. In addition, lymphoma may disseminate to the bone
marrow resembling leukemia; however, no lymphoma cells were
detected by FCM in the bone marrow specimen, excluding lymphoma
dissemination as well. Since CML may progress to both myeloid and
lymphoid leukemia, it has been hypothesized that a single
pluripotent stem cell may develop either myeloid or lymphoid
neoplasms (24). It was therefore
assumed that the leukemia and lymphoma in the present patient may
have had the same origin. The leukemic cells were shown to harbor
the Ph chromosome, but no BCR-ABL fusion signal was observed
on the lymph node specimen, indicating that the two diseases were
unlikely to share the same origin. One hypothesis is that the
single stem cell gained different second hits (mutations) during
differentiation and developed distinct neoplasms. Cauwelier et
al (25) detected monoclonal B
lymphocytes in the blood and marrow of a patient with MDS (with
trisomy 13), but no evidence of lymphoma. FISH was performed on
sorted CD19+ and CD34+ cells for the
detection of trisomy 13. Trisomy 13 was detected in 55% of
CD34+ cells and 5.5% of CD19+ cells, the
latter was considered negative. X-chromosome inactivation showed
that both CD34+ and CD19+ cells were
monoclonal, while their inactivated chromosomes were different,
suggesting that the two populations had different origins.
The etiology of dual tumors such as the one in the
present case were obscure. The patient had worked as a nurse in a
dental clinic for a long period of time. It was unclear whether the
extended exposure to dental repairing materials had played a role
in the development and pathogenesis of the disease. Jaalouk et
al (22) reported a case of
concurrent large B cell lymphoma and MDS, whose treatment with
steroids caused a rapid augmentation of the myeloid clone and
transformation to AML, indicating that the lymphoid clone may
downregulate the myeloid clone. In the present patient, the
enlarged lymph node was normalized soon after chemotherapy
treatment and remained normal thereafter, while the leukemia
deteriorated rapidly, supporting the interclonal inhibition
hypothesis.
Ph + AML is a rare disease associated with poor
prognosis. It possesses different clinical manifestations and
laboratory test results from those of CML-MBC, and should therefore
be regarded as a distinct disease. Appropriate treatment options
include TKI, combined chemotherapy and treatment with allo-HSCT.
Although AML or MDS secondary to chemotherapy, radiotherapy and/or
HSCT have previously been reported, Ph + AML concurrent with large
B cell lymphoma is considerably more rare, and its underlying
pathogenesis remains to be elucidated.
References
|
1
|
Faderl S, Talpaz M, Estrov Z, O'Brien S,
Kurzrock R and Kantarjian HM: The biology of chronic myeloid
leukemia. N Engl J Med. 341:164–172. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fielding AK: How I treat Philadelphia
chromosome-positive acute lymphoblastic leukemia. Blood.
116:3409–3417. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Deschler B and Lübbert M: Acute myeloid
leukemia: Epidemiology and etiology. Cancer. 107:2099–2107. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Vardiman JW, Thiele J, Arber DA, Brunning
RD, Borowitz MJ, Porwit A, Harris NL, Le Beau MM,
Hellström-Lindberg E, Tefferi A and Bloomfield CD: The 2008
revision of the World Health Organization (WHO) classification of
myeloid neoplasms and acute leukemia: Rationale and important
changes. Blood. 114:937–951. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Paietta E, Racevskis J, Bennett JM,
Neuberg D, Cassileth PA, Rowe JM and Wiernik PH: Biologic
heterogeneity in Philadelphia chromosome-positive acute leukemia
with myeloid morphology: The eastern cooperative oncology group
experience. Leukemia. 12:1881–1885. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Keung YK, Beaty M, Powell BL, Molnar I,
Buss D and Pettenati M: Philadelphia chromosome positive
myelodysplastic syndrome and acute myeloid leukemia-retrospective
study and review of literature. Leuk Res. 28:579–586. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Berger R: Differences between blastic
chronic myeloid leukemia and Ph-positive acute leukemia. Leuk
Lymphoma. 11:(Suppl 1). 235–237. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Soupir CP, Vergilio JA, Dal Cin P,
Muzikansky A, Kantarjian H, Jones D and Hasserjian RP: Philadelphia
chromosome-positive acute myeloid leukemia: A rare aggressive
leukemia with clinicopathologic features distinct from chronic
myeloid leukemia in myeloid blast crisis. Am J Clin Pathol.
127:642–650. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cuneo A, Ferrant A, Michaux JL, Demuynck
H, Boogaerts M, Louwagie A, Doyen C, Stul M, Cassiman JJ, Dal Cin
P, et al: Philadelphia chromosome-positive acute myeloid leukemia:
Cytoimmunologic and cytogenetic features. Haematologica.
81:423–427. 1996.PubMed/NCBI
|
|
10
|
Konoplev S, Yin CC, Kornblau SM,
Kantarjian HM, Konopleva M, Andreeff M, Lu G, Zuo Z, Luthra R,
Medeiros LJ and Bueso-Ramos CE: Molecular characterization of De
novo Philadelphia chromosome-positive acute myeloid leukemia. Leuk
Lymphoma. 54:138–144. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nacheva EP, Grace CD, Brazma D, Gancheva
K, Howard-Reeves J, Rai L, Gale RE, Linch DC, Hills RK, Russell N,
et al: Does BCR/ABL1 positive acute myeloid leukaemia exist? Br J
Haematol. 161:541–550. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ito K, Tominaga K, Suzuki T, Jinnai I and
Bessho M: Successful treatment with imatinib mesylate in a case of
minor BCR-ABL-positive acute myelogenous leukemia. Int J Hematol.
81:242–245. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jentsch-Ullrich K, Pelz AF, Braun H,
Koenigsmann M, Mohren M, Wieacker P and Franke A: Complete
molecular remission in a patient with Philadelphia-chromosome
positive acute myeloid leukemia after conventional therapy and
imatinib. Haematologica. 89:ECR152004.PubMed/NCBI
|
|
14
|
Yamaguchi M and Konishi I: Successful
treatment with imatinib mesylate for Philadelphia
chromosome-positive refractory acute myeloid leukemia. Rinsho
Ketsueki. 44:254–256. 2003.PubMed/NCBI
|
|
15
|
Lazarevici V, Golovleva I, Nygren I and
Wahlin A: Induction chemotherapy and post-remission Imatinib
therapy for De novo BCR-ABL positive AML. Am J Hematol. 81:470–471.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kondo T, Tasaka T, Sano F, Matsuda K, Kubo
Y, Matsuhashi Y, Nakanishi H, Sadahira Y, Wada H, Sugihara T and
Tohyama K: Philadelphia chromosome-positive acute myeloid leukemia
(Ph +AML) treated with imatinib mesylate (IM): A report with IM
plasma concentration and bcr-abl transcripts. Leuk Res.
33:e137–e138. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sun J, Wang Z, Luo Y, Tan Y, Allan DS and
Huang H: Prolonged survival with imatinib mesylate combined with
chemotherapy and allogeneic stem cell transplantation in De novo
Ph+ acute myeloid leukemia. Acta Haematol. 127:143–148. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bacher U, Haferlach T, Alpermann T, Zenger
M, Hochhaus A, Beelen DW, Uppenkamp M, Rummel M, Kern W, Schnittger
S and Haferlach C: Subclones with the t(9;22)/BCR-ABL1
rearrangement occur in AML and seem to cooperate with distinct
genetic alterations. Br J Haematol. 152:713–720. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kapadia SB and Kaplan SS: Simultaneous
occurrence of non-Hodgkin's lymphoma and acute myelomonocytic
leukemia. Cancer. 38:2557–2560. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Youness E, Ahearn MJ and Drewinko B:
Simultaneous occurrence of non-Hodgkin's lymphoma and spontaneous
acute granulocytic leukemia. Am J Clin Pathol. 70:415–420. 1978.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ohwada C, Nakaseko C, Tanaka H, Abe D, Oda
K, Ozawa S, Takeuchi M, Shimizu N, Cho R, Saito Y and Nishimura M:
Successful matched unrelated BMT for secondary AML which developed
simultaneously with relapsed Hodgkin's lymphoma. Bone Marrow
Transplant. 39:569–570. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jaalouk G, Avvisati G, Latagliata R,
Pacchiarotti A, Pulsoni A, Mecarocci S and Malagnino F:
Simultaneous occurrence of large B-cell non-Hodgkin lymphoma and
myelodysplastic syndrome rapidly evolving into acute myeloblastic
leukemia. Leuk Lymphoma. 21:339–341. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Montefusco E, Fazi F, Cordone I, Ariola C,
Nanni M, Spadea A, Spiriti MA, Fenu S, Mandelli F and Petti MC:
Molecular remission following high-dose hydroxyurea and fludarabine
plus cytarabine in a patient with simultaneous acute myeloid
leukemia and low-grade lymphoma. Leuk Lymphoma. 40:671–674. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Janossy G, Woodruff RK, Paxton A, Greaves
MF, Capellaro D, Kirk B, Innes EM, Eden OB, Lewis C, Catovsky D and
Hoffbrand AV: Membrane marker and cell separation studies in
Ph1-positive leukemia. Blood. 51:861–877. 1978.PubMed/NCBI
|
|
25
|
Cauwelier B, Nollet F, De Laere E, Van
Leeuwen M, Billiet J, Criel A and Louwagie A: Simultaneous
occurrence of myelodysplastic syndrome and monoclonal B lymphocytes
with a different clonal origin. Leuk Lymphoma. 43:191–193. 2002.
View Article : Google Scholar : PubMed/NCBI
|