Introduction
Ovarian cancer is one of the most dangerous female
malignant tumors (1). There are
several types of ovarian carcinoma, but epithelial ovarian
carcinoma (EOC) is the most frequent one, representing >90% of
all ovarian cancers (2).
Historically, treatment of ovarian cancer has involved surgery
combined with platinum-based chemotherapy (3). The main factors affecting the prognosis
of patients with ovarian cancer are advanced stage at diagnosis and
primary or secondary chemotherapy drug resistance, especially for
those persistent or recurrent ovarian carcinoma. The five-year
survival rate for ovarian cancer patients is currently 30%
(4). The molecular alterations of
cisplatin (DDP)-resistant cancer cells have previously been
researched, however, the underlying mechanisms promoting DDP
resistance in ovarian cancer cells remain to be elucidated
(5). Therefore, research on the
mechanisms of DDP resistance and reversing drug resistance in EOC
are particularly urgent for the patients with current and
refractory EOC.
The endoplasmic reticulum (ER) is an essential
cellular compartment for protein synthesis and maturation. It also
has other functions, including calcium storage and maintenance in
Ca2+ homeostasis, steroid synthesis, and lipid and
glycogen synthesis (6). Various
physiological and pathological conditions may affect ER
homeostasis, ultimately causing ER stress (ERS) (4–9).
Accumulation of misfolded proteins and alterations in
Ca2+ homeostasis in ER results in ERS and activation of
autophagy in carcinoma cells. Typically, autophagy activates
pro-survival mechanisms, as well as cell death programs,
particularly if autophagy activated following ERS is a pro-survival
response to restore the ER homeostasis by clearing the unfolded
aggregates (10). Several studies
have reported that ERS induces autophagy in mammalian cancer cell
lines and mouse embryonic fibroblasts (11,12). Heat
shock proteins function as molecular chaperones that regulate
cellular homeostasis and promote cell survival responses.
Inhibition of autophagy and molecular chaperones may be a suitable
pharmacological target to promote apoptosis in tumor cells. A
previous study revealed that Hsp27 was involved in DDP- and ERS
induced autophagy activation in HCC cells (13). It has also been suggested that
autophagy may be more active in DDP-resistant ovarian cancer cells
(14). The 78-kDa glucose-regulated
protein 78 (GRP78), also known as BiP or HSPA5, is predominantly
localized in the ER as a molecular chaperone, and is associated
with regulating the ERS pathway. Induction of GRP78 has been
recognized as a marker for ERS and the onset of the unfolded
protein response (15). Previous
studies have indicated that the mechanistic target of rapamycin
(mTOR) and Beclin 1 may have important roles in regulating cell
autophagy (16). The present study
aimed to determine the ERS effect on the DDP sensitivity of SKOV3
ovarian cancer cells. On this basis, the mechanism of the drug
resistance of ovarian cancer cells to DDP was studied, which may
contribute to the reversal of DDP resistance in ovarian cancer
cells.
Materials and methods
Cell lines and culture conditions
SKOV3 human ovarian cancer cells were obtained from
the Chinese Academy of Medical Sciences (Beijing, China) and Peking
Union Medical College (Beijing, China). Cell lines were cultured at
37°C in RPMI-1640 culture medium (Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) supplemented with 10% fetal bovine serum
(Invitrogen; Thermo Fisher Scientific, Inc.) in an atmosphere
containing 5% CO2 and 90% air.
Reagents
Human immunodeficiency virus (HIV) proteinase
inhibitor, Saquinavir, was purchased from R&D Systems, Inc.,
(Minneapolis, MN, USA). 3-Methyladenine (3-MA), an autophagy
inhibitor, was purchased from Sigma-Aldrich (Merck Millipore,
Darmstadt, Germany). Protein kinase inhibitor, LY294002, was
obtained from Promega Corp. (Madison, WI, USA). GRP78, mTOR, Beclin
1 and β-actin antibodies were obtained from Santa Cruz
Biotechnology, Inc., (Dallas, TX, USA). First Strand cDNA Synthesis
kit was purchased from Beijing ComWin Biotech Co., Ltd (Beijing,
China). BCA Protein Assay kit was purchased from Beyotime Institute
of Biotechnology (Wuhan, China). Primers for mTOR, Beclin 1 and
β-actin were acquired from Sangon Biotech Co., Ltd., (Shanghai,
China)
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Total RNA was extracted using TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) and complementary DNA
(cDNA) was synthesized using a First Strand cDNA Synthesis kit
according to the manufacturer's instructions. Subsequently, 1 µl
synthesized cDNA was used for each PCR with each primer pair. qPCR
was performed with 2X EasyTaq® PCR SuperMix kit (Beijing
Transgen Biotech Co., Ltd., Beijing, China), and 25 µl of the
reaction mixture was used in a qPCR program. The 25-µl reaction
mixture contained 1 µl each primer, 12.5 µl 2X PCR Mix, 1 µl
template cDNA and ≤9.5 µl double-distilled water (catalog no.
KGDN4500; Nanjing KeyGen Biotech Co., Ltd., Nanjing, China) The
primers were synthesized by Sangon Biotech Co., Ltd., as follows:
mTOR, forward 5′-TTGAGGTTGCTATGACCAGAGAGAA-3′ and reverse
5′-TTACCAGAAAGGACACCAGCCAATG-3′, 566 bp; Beclin 1, forward
5′-CAGTTTGGCACAATCAATAAC-3′ and reverse
5′-CATCCATCCTGTAGGGAAGAC-3′, 352 bp; β-actin, forward
5′-TGTTTGAGACCTTCAACACCC-3′ and reverse 5′-AGCACTGTGTTGGCGTACAG-3′,
340 bp. Protocol parameters were as follows: Initial incubation at
95°C for 10 min, followed by 30 cycles of denaturation at 95°C for
30 sec, annealing (55°C) for 30 sec, elongation at 72°C for 1 min
and a final extension at 72°C for 8 min. The experiment was
repeated three times, and the efficiency of cDNA synthesis from
each sample was estimated using β-actin primers, which served as an
endogenous control. Relative gene expression levels were calculated
and analyzed using Quantity One analysis software (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). Data were analyzed using
the 2−ΔΔCq method (17).
Western blot analysis
Cells were harvested and lysed in
radioimmunoprecipitation assay buffer. Protein concentration was
determined using a BCA Protein Assay kit. Denatured proteins (50
µg) were separated by 12% SDS-PAGE and transferred to
polyvinylidene difluoride membranes using the Bio-Rad
electro-transfer system (Bio-Rad Laboratories, Inc.). The membranes
were incubated with antibodies to visualize the proteins. Following
blocking with 5% w/v non-fat dried milk for 1 h, the membranes were
incubated overnight with primary antibodies against GRP78 (1:500;
bs-1219R), mTOR (1:500; bs-1992R) and Beclin 1 (1:500; bs-1353R)
(all from Beijing Biosynthesis Biotechnology Co., Ltd., Beijing,
China), rinsed with TBS-Tween 20, and subsequently incubated at
37°C for 2 h with anti-rabbit immunoglobulin G secondary antibody
conjugated to horseradish peroxidase (sc-2004; Santa Cruz
Biotechnology, Inc.) at a dilution of 1:1,000. After applying
Enhanced Chemiluminescent Plus detection reagents (EMD Millipore,
Billerica, MA, USA), protein bands were visualized using an X-ray
film (Fujifilm, Tokyo, Japan). β-actin was used as an internal
control for protein loading and analysis. Quantitation of band
intensity was performed by densitometry using Quantity One 1-D
analysis software version 4.6.9 (Bio-Rad Laboratories, Inc.). The
experiment was conducted three times.
MTT assay
MTT assays were used to assess the number of viable
cells. After cells (180-µl solution containing 1.0×105
cells/ml) were cultured for 24 h in 96-well plates
(1.8×104 cells/well), culture medium was removed and
replaced by 200 µl complete culture medium containing DDP. Final
concentrations of DDP were 100, 50, 25, 12.5, 6.25, 3.125, 1.56 and
0.78 µg/ml, respectively. Each treatment was repeated in four
wells. The control group received the same volume of culture medium
with cells, without drugs, whereas blank wells contained the same
volume of culture medium without cells and drugs. Cells were
cultured for 72 h. Cell viability was assessed using the MTT
colorimetric assay. Briefly, 20 µl MTT was added and incubated for
4 h. Subsequently, 150 µl dimethyl sulfoxide was added to dissolve
the formazan crystals. Following shaking for 10 min, the absorbance
values were measured at a wavelength of 570 nm using a microplate
reader (Molecular Devices, Sunnyvale, CA, USA). The experiment was
repeated three times. The DDP half maximal inhibitory concentration
(IC50) in SKOV3 cells was calculated.
Statistical analysis
All values were shown as the mean ± standard
deviation from at least three independent experiments. Data were
analyzed by Student's t-test (for comparing two intergroup results)
or one-way analysis of variance with SPSS 17.0 (SPSS, Inc.,
Chicago, IL, USA). IC50 was analyzed with the linear
regression. P<0.05 was considered to indicate a statistically
significant difference.
Results
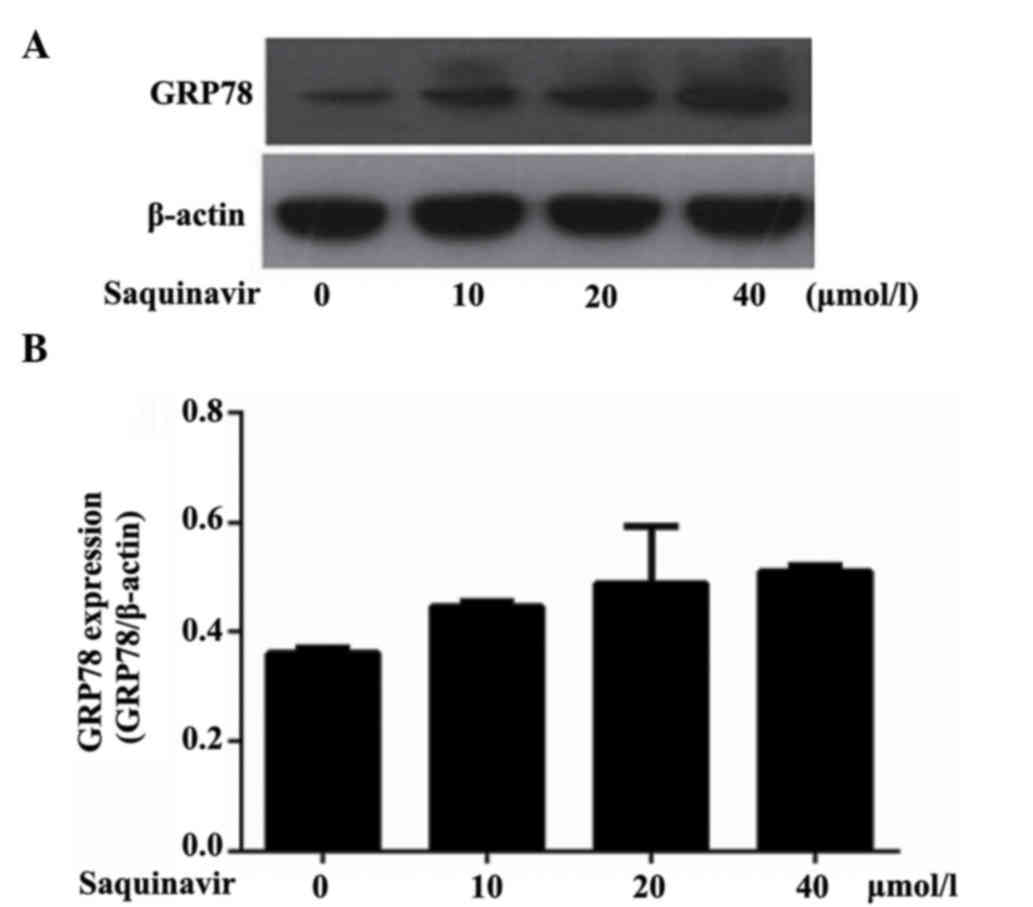
Expression of GRP78 in SKOV3 tumor
cells
As an ER chaperone, GRP78 is commonly used as a
marker of ERS. Western blotting analysis demonstrated that GRP78
protein expression levels were increased when tumor cells SKOV3
were treated with Saquinavir for 24 h. Moreover, as shown in
Table I and Fig. 1, the expression levels of GRP78
significantly increased following treatment with Saquinavir in a
dose-dependent manner (Ρ<0.05). These results were significantly
different when compared with each other (P<0.05). These findings
indicated that Saquinavir may lead to ERS, and that the level of
ERS may be associated with the concentration of Saquinavir.
 | Table I.Relative protein expression levels of
GRP78 following treatment with Saquinavir. |
Table I.
Relative protein expression levels of
GRP78 following treatment with Saquinavir.
| Groups | GRP78 |
|---|
| Control | 0.361±0.009 |
| Saquinavir 10
µmol/l | 0.446±0.010 |
| Saquinavir 20
µmol/l | 0.489±0.105 |
| Saquinavir 40
µmol/l | 0.511±0.010 |
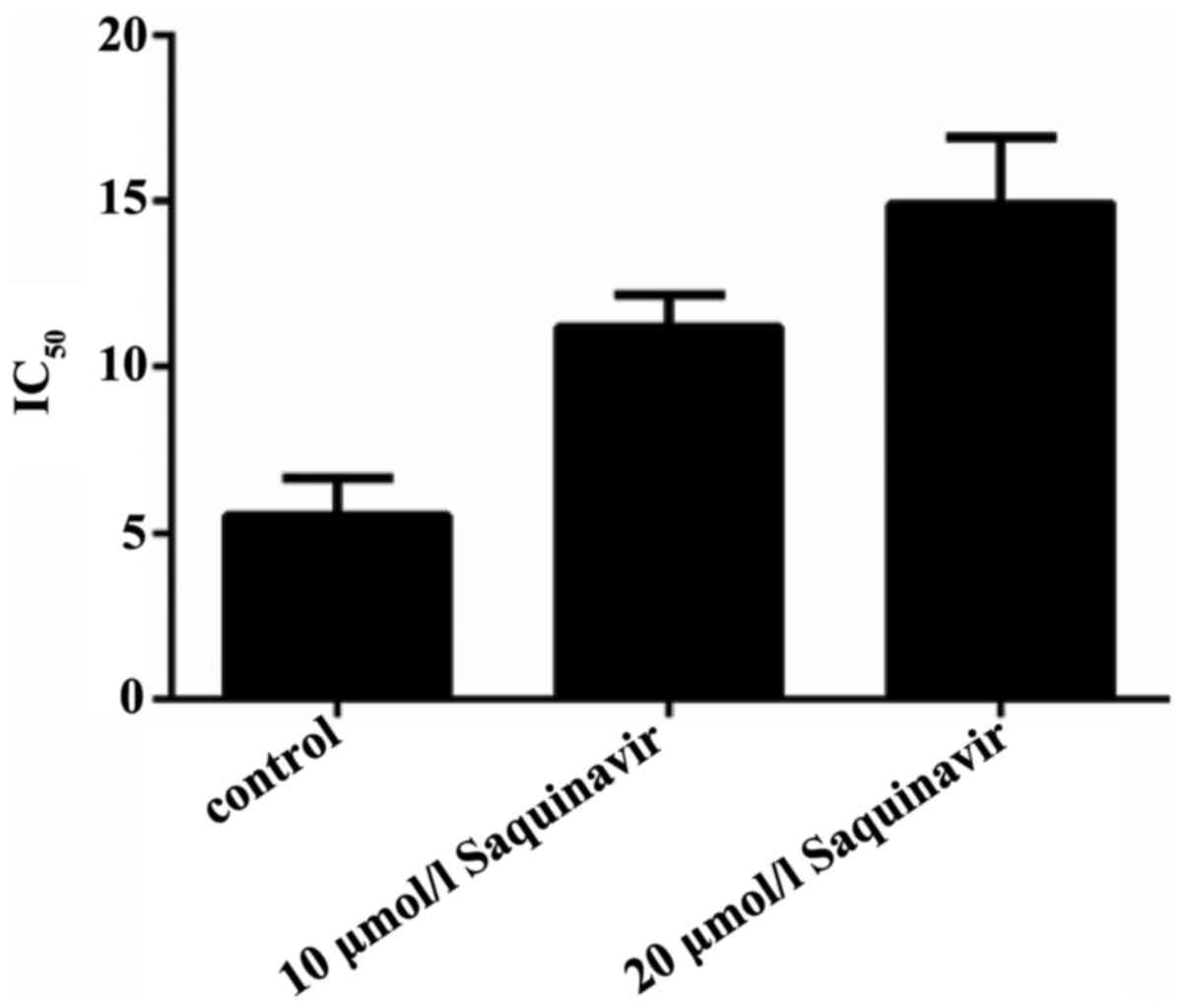
Effect of Saquinavir-induced ERS on
the sensitivity of ovarian cancer cells to DDP
To confirm the effect of ERS on the sensitivity of
SKOV3 ovarian cancer cells to DDP, the cells were treated with
different concentrations of Saquinavir for 24 h. The
IC50 of the SKOV3 cells was 5.490±1.148 µg/l. Following
exposure to 10 and 20 µmol/l Saquinavir, the IC50 of
SKOV3 cells significantly increased to 11.199±0.984 and
14.906±2.015 µg/l, respectively (P=0.003 and P<0.001); however,
no statistically significant difference was detected between the
cells treated with 10 and 20 µmol/l Saquinavir (P=0.21; Fig. 2). These findings suggest that
Saquinavir resulted in ERS in SKOV3 tumor cells, which reduced the
sensitivity of these ovarian cancer cells to DDP.
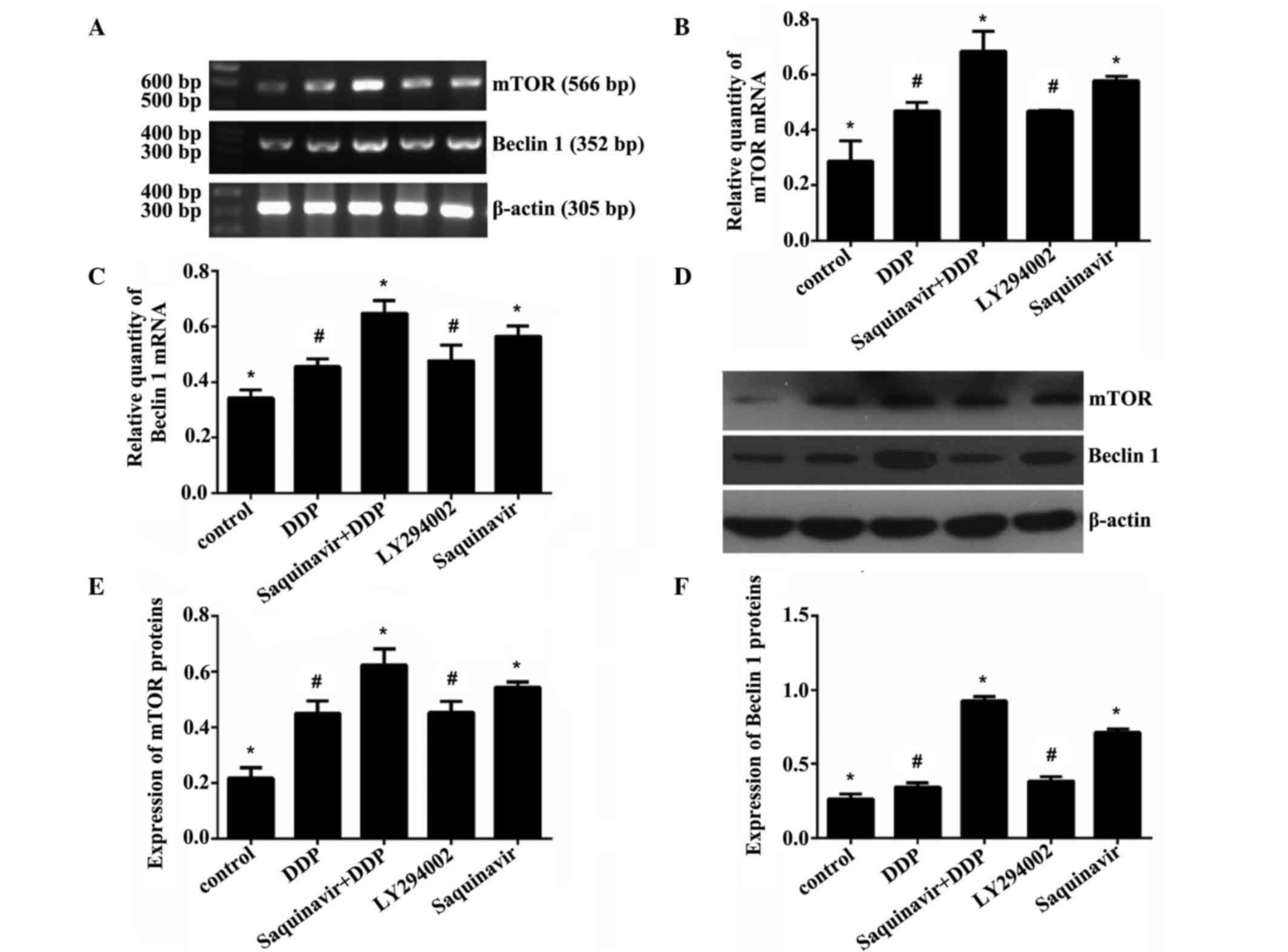
Effect of Saquinavir on protein and
messenger RNA (mRNA) expression levels of mTOR and Beclin 1 in
SKOV3 cells
Saquinavir, LY294002 (PI3K inhibitor) and DDP were
added to the different cell groups, respectively, to final
concentrations of 20, 20 and 5 µg/ml, respectively. The expression
levels of autophagy-related genes mTOR and Beclin 1 were assessed
in SKOV3 ovarian cancer cells by RT-qPCR and western blotting. As
shown in Tables II and III and Fig.
3, when the cells were exposed to Saquinavir for 24 h, the
protein and mRNA expression levels of mTOR and Beclin 1 in SKOV3
cells were increased. The results demonstrated that the protein and
mRNA expression levels of mTOR and Beclin 1 in the Saquinavir + DDP
group were significantly increased in comparison with the other
groups. The Saquinavir group exhibited the next highest expression
levels. Moreover, the protein and mRNA expression levels of mTOR
and Beclin 1 in SKOV3 cells treated with Saquinavir were higher
than those exhibited by cells in the LY294002 group. These results
suggested that the PI3K inhibitor, LY294002, did not inhibit the
mTOR expression completely through the PI3K/AKT/mTOR pathway. The
protein and mRNA expression levels of mTOR and Beclin 1 among the
groups of experiment were demonstrated to be significantly
different (F=23.140, P<0.001 and F=24.389, P<0.001,
respectively). Protein expression levels of mTOR and Beclin 1 among
the groups were also significantly different (F=39.345, P<0.001
and F=261.877, P<0.001, respectively). However, further
comparison indicated that the protein and mRNA expression levels of
mTOR and Beclin 1 were significantly different between any two
groups (control vs. DDP; control vs. Saquinavir + DDP; control vs.
LY294002; control vs. Saquinavir; DDP vs. Saquinavir + DDP; DDP vs.
Saquinavir; Saquinavir + DDP vs. LY294002; Saquinavir + DDP vs.
Saquinavir; and LY294002 vs. Saquinavir), with the exception of the
LY294002 and DDP groups.
 | Table II.Expression of mTOR and Beclin 1
messenger RNA in SKOV3 human ovarian cancer cells. |
Table II.
Expression of mTOR and Beclin 1
messenger RNA in SKOV3 human ovarian cancer cells.
| Groups | mTOR | Beclin 1 |
|---|
| Control | 0.287±0.073 | 0.342±0.029 |
| DDP | 0.468±0.031 | 0.456±0.028 |
| Saquinavir +
DDP | 0.684±0.072 | 0.647±0.047 |
| LY294002 | 0.467±0.005 | 0.477±0.056 |
| Saquinavir | 0.5772±0.016 | 0.565±0.037 |
 | Table III.Expression of mTOR and Beclin 1
proteins in SKOV3 human ovarian cancer cells. |
Table III.
Expression of mTOR and Beclin 1
proteins in SKOV3 human ovarian cancer cells.
| Groups | mTOR | Beclin 1 |
|---|
| Control | 0.218±0.038 | 0.265±0.033 |
| DDP | 0.450±0.045 | 0.345±0.029 |
| Saquinavir +
DDP | 0.624±0.058 | 0.924±0.033 |
| LY294002 | 0.453±0.041 | 0.384±0.030 |
| Saquinavir | 0.544±0.019 | 0.712±0.024 |
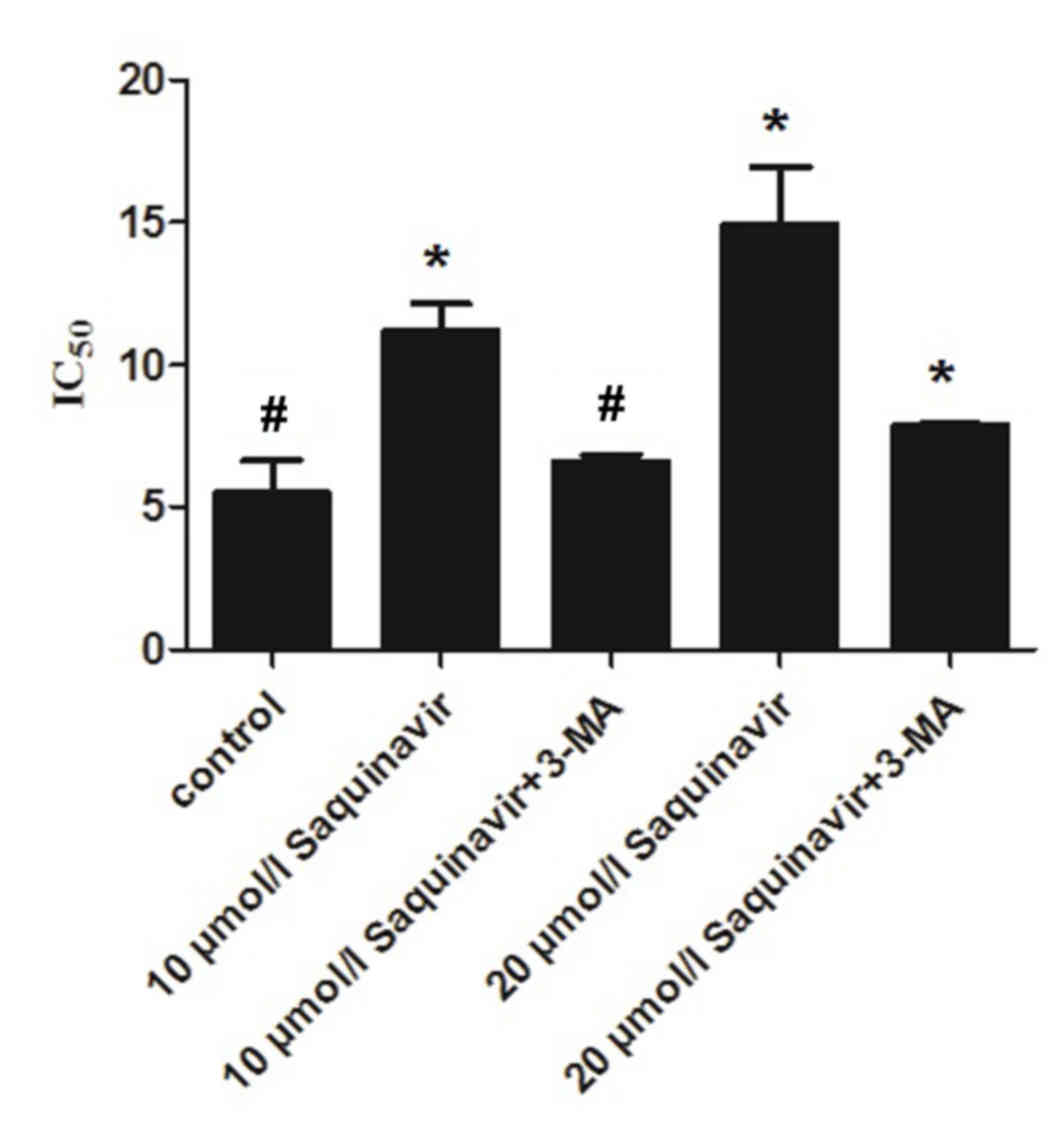
Effect of autophagy suppression on the
sensitivity of ovarian cancer cells to DDP
The present study confirmed that Saquinavir resulted
in ERS in SKOV3 cells, which reduced the sensitivity of ovarian
cancer cells to DDP. Furthermore, it demonstrated that the
sensitivity of ovarian cancer cells to DDP was associated with the
level of ERS. In the present study, SKOV3 cells were exposed to the
autophagy inhibitor, 3-MA, and various concentrations of
Saquinavir. IC50 values in the control, Saquinavir 10
µmol/l, Saquinavir 10 µmol/l + 3-MA, Saquinavir 20 µmol/l and
Saquinavir 20 µmol/l + 3-MA groups were 5.490±1.148, 11.199±0.984,
6.624±0.218, 14.906±2.015 and 7.888±0.086 µg/ml, respectively
(Fig. 4). IC50 of DDP was
decreased in SKOV3 cells after 3-MA combined with Saquinavir
treatment compared with Saquinavir treatment alone. This indicated
that the autophagic response induced by Saquinavir treatment was
inhibited by 3-MA. These results suggest that the sensitivity to
DDP was significantly improved in SKOV3 cells after 3-MA exposure
(F=31.898, P<0.001).
Discussion
ER is an essential intracellular organelle with
multiple roles including the synthesis, folding, assembly and
maturation of nascent proteins, Ca2+ storage,
glycosylation, and the trafficking of newly-synthesized membrane
and secretory proteins (18).
Perturbations of these processes have been demonstrated to
interfere with the proper functioning of ER, thus leading to a
condition defined as ERS (8,9). Previous studies have shown that DDP
resistance in cancer cells was associated with ERS (19,20). DDP
results in the accumulation of unfolded and misfolded proteins in
the lumen of the ER. This ERS subsequently induces autophagy. The
majority of accumulated proteins and soluble proteins were cleared
by autophagy pathways and tumor cells are able to survive (21,22). It
has been demonstrated that tunicamycin, an ERS inducer, augmented
DDP cytotoxicity by upregulating ERS-mediated apoptosis, indicating
that autophagy has an important role in preventing DDP-induced
apoptosis in HeLa cells (23).
Saquinavir, which is a HIV proteinase inhibitor, is able to induce
ERS in various tumors, including NC160 cells (24), non-small cell lung cancer (25) and melanocytoma (26). McLean et al (27) have previously shown that Saquinavir
leads to ERS and cell autophagy.
Altering the tumor microenvironment and growth
patterns could activate ERS. The survival of cells upon undergoing
ERS induces better adaptation to various physiological and
pathological conditions, which is one of the important mechanisms
in tumor cells remaining malignant and promoting drug resistance
(28). GRP78 has been
well-established as an ER chaperone and is widely used as a marker
for ERS (29). Our study confirmed
that Saquinavir leads to ERS in SKOV3 tumor cells, which reduces
the sensitivity of ovarian cancer cells to DDP. Furthermore, the
sensitivity of ovarian cancer cells to DDP appears to be associated
with the level of ERS. 3-MA is a specific inhibitor of the
autophagic pathway. After SKOV3 tumor cells are exposed to the
autophagy inhibitor 3-MA, sensitivity of ovarian cancer cells to
DDP could be effectively reversed. Therefore, it was speculated
that ERS may induce DDP resistance through enhanced autophagy in
SKOV3 cells. This demonstrated that an increase in the level of ERS
had an important role in DDP resistance in ovarian cancer,
particularly secondary DDP resistance associated with continuous
ERS and an increase in the level of autophagy in ovarian cancer
exposed to DDP, a chemotherapeutic agent, periodically.
Accumulating evidence has indicated that ERS is
associated with tumor cell survival, tumor progression and
chemotherapy resistance (29–31); however, its precise mechanism remains
unclear. Previous studies have shown that the PI3K/Akt/mTOR pathway
is involved in ERS-triggered apoptosis, and is also associated with
the regulation of autophagy (32,33);
however, PI3K inhibitor did not inhibit the expression of mTOR
completely. This suggested other signaling pathways may exist,
requiring further investigation (34).
Autophagy is a ‘self-eating’ process by which a cell
digests damaged organelles or misfolded proteins by sequestering
the target cargo in a double membrane and fusing to lysosomes for
degradation, thereby supplementing intermediate metabolism with the
products of digestion (35,36). mTOR has a critical role in the
initiation of the autophagic process. mTOR activation is able to
inhibit autophagy. Beclin 1, which is a mammalian autophagy gene,
was the first protein that was demonstrated to induce autophagy
(37). Studies have identified that
cell autophagy has a critical role in the occurrence and
development of tumor cells, and it has been suggested that
autophagy may have a role in cancer cell chemoresistance (38,39). A
previous study researched co-treatment of DDP with trifluoperazine,
an inducer of autophagy, which sensitized H460/cis DDP-resistant
lung carcinoma cells to DDP, suggesting that the decreased levels
of autophagy may promote DDP resistance in lung cancer (40). Another previous study suggested that
ERS induced apoptosis and led to DDP resistance in human ovarian
cancer cells, but provided limited information on the role of
autophagy in DDP resistance (41).
ERS may regulate autophagy through mTOR and Beclin 1 pathways. The
present study further explored that when ERS was induced by
Saquinavir in tumor cells SKOV3, the expression levels of mTOR and
Beclin 1 were upregulated, decreasing the sensitivity of ovarian
cancer cells to DDP. We hypothesize that ovarian cancer cells
experienced chemotherapy or radiotherapy, and numerous organelles
or proteins were destroyed in ovarian cancer cells. Enhanced cell
autophagy would aid the clearance of these harmful substances to
maintain stabilization of homoeostasis and the cells could prevent
from death. Cells benefit from moderate ERS to alleviate damage,
whereas sustained ERS induces cell death (42). Continuous autophagy in cells leads to
the breakdown of important organelles and proteins and eventual
cell death via autophagy. To prevent this type of cell death, the
PI3K/AKT/mTOR pathway is activated in ovarian cancer cells to
appropriately suppress autophagy. In the present study, it was also
demonstrated that the expression levels of mTOR were upreguated,
which suggested autophagy was inhibited. Our findings have
identified that downregulating autophagy via 3-MA may prevent the
effect of Saquinavir-induced ERS on the sensitivity of ovarian
cancer cells to DDP. Therefore, we hypothesize that an increase in
the level of autophagy had a dominant role in the ERS activation of
ovarian cancer cells and decreased the drug sensitivity of ovarian
cancer to DDP.
In conclusion, mTOR and Beclin 1 may be important in
the regulation of cell autophagy. ERS acts like a double-edged
sword, since it can induce apoptosis and promote cell survival. The
present findings demonstrated that ERS was able to promote cell
survival through regulation of the level of autophagy and have a
role in protecting cell from being destroyed. It was speculated
that ERS and enhanced cell autophagy were important mechanisms
which resulted in DDP resistance in ovarian cancer. Targeting ERS
or inhibiting autophagy may be an encouraging technique to overcome
chemotherapeutic resistance.
Acknowledgements
The authors would like to thank Tianjin Medical
University (Heping, China) and all the teachers in our lab,
particularly Professor Hao Zhang and Professor Zheng Su, for their
assistance during the experiment.
References
|
1
|
Khanra K, Panda K, Mitra AK, Sarkar R,
Bhattacharya C and Bhattacharya N: Exon 8–9 mutations of DNA
polymerase β in ovarian carcinoma patients from Haldia, India.
Asian Pac J Cancer Prev. 13:4183–4186. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ho CM, Chanq SF, Hsiao CC, Chien TY and
Shih DT: Isolation and characterization of stromal progenitor cells
from ascites of patients with epithelial ovarian adenocarcinoma. J
Biomed Sci. 19:232012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Romanidis K, Nagorni EA, Halkia E and
Pitiakoudis M: The role of cytoreductive surgery in advanced
ovarian cancer: The general surgeon's perspective. J BUON.
19:598–604. 2014.PubMed/NCBI
|
|
4
|
Siddik ZH: Cisplatin: Mode of cytotoxic
action and molecular basis of resistance. Oncogene. 22:7265–7279.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Galluzzi L, Senovilla L, Vitale I, Michels
J, Martins I, Kepp O, Castedo M and Kroemer G: Molecular mechanisms
of cisplatin resistance. Oncogene. 31:1869–1883. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pang XL, He G, Liu YB, Wang Y and Zhang B:
Endoplasmic reticulum stress sensitizes human esophageal cancer
cell to radiation. World J Gastroenterol. 19:1736–1748. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Haeri M and Knox BE: Endoplasmic reticulum
stress and unfolded protein response pathways: Potential for
treating age-related retinal degeneration. J Ophthalmic Vis Res.
7:45–59. 2012.PubMed/NCBI
|
|
8
|
Walter P and Ron D: The unfolded protein
response: From stress pathway to homeostatic regulation. Science.
334:1081–1086. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tabas I and Ron D: Integrating the
mechanisms of apoptosis induced by endoplasmic reticulum stress.
Nat Cell Biol. 13:184–190. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Verfaillie T, Salazar M, Velasco G and
Agostinis P: Linking ER stress to autophagy: Potential implications
for cancer therapy. Int J Cell Biol. 2010:9305092010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kang JH, Chang YC and Maurizi MR:
4-O-carboxymethyl ascochlorin causes ER stress and induced
autophagy in human hepatocellular carcinoma cells. J Biol Chem.
287:15661–15671. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu D, Yang Y, Liu Q and Wang J:
Inhibition of autophagy by 3-MA potentiates cisplatin-induced
apoptosis in esophageal squamous cell carcinoma cells. Med Oncol.
28:105–111. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen R, Dai RY, Duan CY, Liu YP, Chen SK,
Yan DM, Chen CN, Wei M and Li H: Unfolded protein response
suppresses cisplatin-induced apoptosis via autophagy regulation in
human hepatocellular carcinoma cells. Folia Biol (Praha). 57:87–95.
2011.PubMed/NCBI
|
|
14
|
Bao LJ, Jaramillo MC, Zhang Z, Zheng Y,
Yao M, Zhang DD and Yi X: Induction of autophagy contributes to
cisplatin resistance in human ovarian cancer cells. Mol Med Rep.
11:91–98. 2015.PubMed/NCBI
|
|
15
|
Lee AS: GRP78 induction in cancer:
Therapeutic and prognostic implications. Cancer Res. 67:3496–3499.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kang R, Zeh HJ, Lotze MT and Tang D: The
Beclin 1 network regulates autophagy and apoptosis. Cell Death
Differ. 18:571–580. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittqen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Method. 4:402–408. 2001.
View Article : Google Scholar
|
|
18
|
Kato H and Nishitoh H: Sress responses
from the endoplasmic reticulum in cancer. Front Oncol. 5:932015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shi S, Xu H, Li C, et al: Endoplasmic
reticulum stress and tumor. Medical Recapitulate. 15:525–527.
2009.
|
|
20
|
Yuan YF: Endoplasmic reticulum stress and
apoptosis of tumor cells. J Mol Diagn Ther. 2:128–134. 2010.
|
|
21
|
Liu Y and Ye Y: Roles of p97-associated
deubiquitinases in protein quality control at the endoplasmic
reticulum. Curr Protein Pept Sci. 13:436–446. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
White MC, Johnson GG, Zhang W, Hobrath JV,
Piazza GA and Grimaldi M: Sulindac sulfide inhibits
sarcoendoplasmic reticulum Ca2+ ATPase, induces endoplasmic
reticulum stress response and exerts toxicity in glioma cells:
Relevant similarities to and important differences from celecoxib.
J Neurosci Res. 91:393–406. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xu Y, Yu H, Qin H, Kang J, Yu C, Zhong J,
Su J, Li H and Sun L: Inhibition of autophagy enhances cisplatin
cytotoxicity through endoplasmic reticulum stress in human cervical
cancer cells. Cancer Lett. 314:232–243. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gills JJ, Lopiccolo J, Tsurutani J,
Shoemaker RH, Best CJ, Abu-Asab MS, Borojerdi J, Warfel NA, Gardner
ER, Danish M, et al: Nelfinavir, a lead HIV protease inhibitor, is
a broad-spectrum, anticancer agent that induces endoplasmic
reticulum stress, autophagy, and apoptosis in vitro and in vivo.
Clin Cancer Res. 13:5183–5194. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gills JJ, Lopiccolo J, Tsurutani J,
Shoemaker RH, Best CJ, Abu-Asab MS, Borojerdi J, Warfel NA, Gardner
ER, Danish M, et al: Nelfinavir, a lead HIV protease inhibitor, is
a broad-spectrum, anticancer agent that induces endoplasmic
reticulum stress, autophagy, and apoptosis in vitro and in vivo.
Clin Cancer Res. 13:5183–5194. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jiang W, Mikochik PJ, Ra JH, Lei H,
Flaherty KT, Winkler JD and Spitz FR: HIV protease inhibitor
nelfinavir inhibits growth of human melanoma cells by induction of
cell cycle arrest. Cancer Res. 67:1221–1227. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
McLean K, VanDeVen NA, Sorenson DR, Daudi
S and Liu JR: The HIV protease inhibitor saquinavir induces
endoplasmic reticulum stress, autophagy, and apoptosis in ovarian
cancer cells. Gynecol Oncol. 112:623–630. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Feldman DE, Chauhan V and Koong Ac: The
unfolded protein response: A novel component of the hypoxic stress
response in tumors. Mol Cancer Res. 3:597–605. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ni M and Lee AS: ER chaperones in
mammalian development and human diseases. FEBS Lett. 581:3641–3651.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lin Y, Wang Z, Liu L and Chen L: Akt is
the downstream target of GRP78 in mediating cisplatin resistance in
ER stress-tolerant human lung cancer cells. Lung Cancer.
71:291–297. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jiang CC, Mao ZG, Avery-Kiejda KA, Wade M,
Hersey P and Zhang XD: Glucose regulatedprotein78 antagonizes
cisplatin and adriamycin in human melanoma cells. Carcinogenesis.
30:197–204. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kato H, Nakajima S, Saito Y, Takahashi S,
Katoh R and Kitamura M: mTORC1 serves ER stress-triggered apoptosis
via selective activation of the IRE1-JNK pathway. Cell Death
Differ. 19:310–320. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Oh SH and Lim SC: Endoplasmic reticulum
stress- mediated autophagy/apoptosis induced by capsaicin
(8-methyl-N-vanillyl-6-nonenamide) and dihydrocapsaicin is
regulated by the extent of c-Jun NH2-terminal kinase/extracellular
signal-regulated kinase activation in WI38 lung epithelial
fibroblast cells. J Pharmacol Exp Ther. 329:112–122. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kadowaki H, Nishitoh H and Ichijo H:
Survival and apoptosis signals in ER stress: The role of protein
kinases. J Chen Neuroanat. 28:93–100. 2004. View Article : Google Scholar
|
|
35
|
Clarke R, Cook KL, Hu R, Facey CO,
Tavassoly I, Schwartz JL, Baumann WT, Tyson JJ, Xuan J, Wang Y, et
al: Endoplasmic reticulum stress, the unfolded protein response,
autophagy, and the integrated regulation of breast cancer cell
fate. Cancer Res. 72:1321–1331. 2012.PubMed/NCBI
|
|
36
|
He C and Klionsky DJ: Regulation
mechanisms and signaling pathways of autophagy. Ann Rev Genet.
43:67–93. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Takahashi Y, Coppola D, Matsushita N,
Cualing HD, Sun M, Sato Y, Liang C, Jung JU, Cheng JQ, Mulé JJ, et
al: Bif-1 interacts with Beclin1 through UVRAG and regulates
autophagy and tumorigenesis. Nat Cell Biol. 9:1142–1151. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Carew JS, Nawrocki ST and Cleveland JL:
Modulating autophagy for therapeutic benefit. Autophagy. 3:464–467.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Mathew R, Karantza-Wadsworth V and White
E: Role of autophagy in cancer. Nat Rev Cancer. 7:961–967. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sirichanchuen B, Pengsuparp T and
Chanvorachote P: Long-term cisplatin exposure impairs autophagy and
causes cisplatin resistance in human lung cancer cells. Mol Cell
Biochem. 364:11–18. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yu H, Su J, Xu Y, Kang J, Li H, Zhang L,
Yi H, Xiang X, Liu F and Sun L: p62/SQSTM1 involved in cisplatin
resistance in human ovarian cancer cells by clearing ubiquitinated
proteins. Eur J Cancer. 47:1585–1594. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Schönthal AH: Pharmacological targeting of
endoplasmic reticulum stress signaling in cancer. Biochem
Pharmacol. 85:653–666. 2013. View Article : Google Scholar : PubMed/NCBI
|