Introduction
Breast cancer is the most frequently occurring
cancer among women in 140 countries worldwide. This disease claimed
over half a million lives in 2012, and increased >20% in
incidence and 14% in mortality since 2008 (1). Postoperative adjuvant therapies
including radiotherapy are the main mode of treatment to improve
the overall prognosis. Adjuvant therapies prevent and control the
local recurrence of residual malignant tissue, however, the
underlying molecular mechanisms remain unknown. Improving knowledge
regarding molecular mechanisms involved in this process may be
useful for developing effective methods of therapy. In the past few
years, thousands of long non-coding RNAs (lncRNAs) were identified
and functional roles of lncRNAs in epigenetics have been discussed
(2).
The origin of lncRNAs varies from intergenic to
coding regions with sense or antisense orientations. Some of these
lncRNAs have been linked to oncogenic and tumor suppressor gene
regulation. LncRNAs, which play critical roles in cell regulation,
are deregulated in a number of cancers, demonstrating oncogenic
properties (2). These properties of
lncRNAs provide new tumor-related therapeutic targets for
successful intervention. HOTAIR is one of the oncogenic
lncRNAs.
A group of the genes with HOTAIR-induced polycomb
repressive complex 2 (PRC2) occupancy are involved in inhibiting
breast cancer progression. HOTAIR-induced PRC2 occupancy involves
transcription factors HOXD and PRG1 (coding for progesterone
receptors), cell adhesion molecules such as the protocadherin gene
family as well as EPHA1 (coding for an ephrin receptor) (3). Overexpression of HOTAIR has been linked
to breast cancer metastasis and poor survival rates. Previous
studies reported that overexpression of HOTAIR in breast
adenocarcinoma cells reduced the apoptotic rate, and increased cell
growth, migration, and invasion (4).
It has been reported that two types of ncRNAs, miRNA-10b (miR-10b)
and homeobox (HOX) RNA, can suppress the translation of the
HOXD10 gene. An mRNA encoding a transcriptional repressor
was reported to have inhibitory properties affecting genes
responsible for cell migration and invasion (5).
The aberrant proliferation of nucleus pulposus cells
through derepressing the RhoC-Akt pathway by targeting HOXD10 has
also been investigated (6). However,
whether a similar functional mechanism and molecular pathways of
HOTAIR are involved in the radiotherapy process of breast
adenocarcinoma cells remains to be determined. In the present
study, we investigated the expression of HOTAIR in breast
adenocarcinoma cells and identified that HOTAIR was markedly
overexpressed in a cohort of human breast cancer samples and in
advanced tumors. The results obtained from the functional analyses
of HOTAIR in radiotherapy revealed that the upregulation of HOTAIR
enhanced the resistance of human breast cancer cells to
radiotherapy by downregulating HOXD10 expression. Thus, HOTAIR can
be considered as a valid therapeutic target for the reversal of
radiotherapy resistance in breast cancer patients.
Materials and methods
Cell lines
The T47D, MCF-7, SKBR3, BT549, MDA-MB231 and MCF-10A
breast cancer cell lines were used in the present study. The T47D
and MCF-7 cell lines were grown in RPMI-1640 medium, while the
remaining cell lines were grown in DMEM supplemented with 10% fetal
bovine serum (FBS). The cell cultures were incubated at 37°C in a
humidified environment containing 5% CO2.
Reverse transcriptase-quantitative
PCR
Total RNA from fresh tissues was extracted by TRIzol
reagent according to the manufacturer's instructions with minor
modifications. The quality and quantity of RNA was determined using
a NanoDrop® ND-1000 spectrophotometer. Then, 1 µg total
RNA from each sample was reverse-transcribed using the RT reagent
kit according to the manufacturer's instructions. Real-time PCR was
performed to determine the relative expression levels of target
genes using the SYBR-Green RT-qPCR kit on the Step One Real-Time
PCR Detection system. The level of HOTAIR expression in each sample
was normalized to the respective β-actin expression level. The RNA
sequences targeting human HOTAIR were designed according to a
previous study (7) and produced by
Sangon Biotech Co., Ltd. (Shanghai, China). The sequences used to
construct the primers were: HOTAIR sense,
5′-ATAGGCAAATGTCAGAGGGTT-3′ and antisense,
5′-ATTCTTAAATTGGGCTGGGTC-3′; and β-actin sense,
5′-AAAGACCTGTACGCCAACAC-3′ and antisense,
5′-GTCATACTCCTGCTTGCTGAT-3′. The amplification profile was
pre-denatured at 95°C for 5 min, followed by 40 cycles of
denaturation at 95°C for 15 sec, annealing and extension at 60°C
for 30 sec, respectively. The accuracy of the PCR amplifications
was verified by melting curve analyses. The comparative threshold
cycle (Cq) method (ΔΔCq) was employed for the quantification of
HOTAIR gene expression. The relative expression amount of HOTAIR to
β-actin was calculated using the equation 2−ΔΔCq, where
ΔCq = Cq (HOTAIR) - Cq (β-actin). The HOTAIR expression levels in
tumors were compared to normal tissues. To minimize experimental
variability, each sample was analyzed in triplicates and the mean
expression levels were calculated.
Plasmids and transfection
Full length human HOTAIR DNA were cloned into a
pLVX-CMV-PGK-puro vector (BioWit Technologies Ltd., Shenzhen,
China). Recombinant plasmids were used for transfecting cells using
Lipofectamine 2000 (Invitrogen Life Technologies, Carlsbad, CA,
USA). MDA-MB231 cells were grown in culture media containing 10%
FBS. Lipofectamine 2000, opti-MEM (Gibco Life Technologies,
Carlsbad, CA, USA) and plasmid pLVX-CMV- HOTAIR-PGK-puro were mixed
and incubated for 20 min to be added to MDA-MB231 cells. On
reaching confluency, the MDA-MB231 cells were transfected at 25°C.
After 6 h of incubation, the cells were incubated in RPMI-1640
containing 10% FBS in 5% CO2 at 37°C. After
transfection, the cells were subjected to puromycin for selection.
After 10 days, the stable HOTAIR transfected MDA-MB231 cell line
(HOTAIR-MDA-MB231) was identified. Untransfected cells were used as
the blank control, and cells transfected with pLVX-CMV-PGK-puro
(empty vector) (vector-MDA-MB231) were used as the negative
control.
Clonogenic survival assay
The cells were seeded in 6-well plates for 24 h,
subjected to X-rays of 2, 4, 6, and 8 Gy, and then cultured at 37°C
for 12 days. After incubation, the cells were fixed with methanol
and stained with Giemsa. Any colonies containing ≥50 cells were
counted under the microscope (BX-51, Olympus, Tokyo, Japan).
Immunohistochemistry
The Ki67 expression level was measured using
immunohistochemistry, as previously described (8). Immunohistochemical studies were
performed on one representative block, using the avidin-biotin
complex horseradish peroxidase (HRP) method. Briefly, the sections
were fixed in formalin, embedded in paraffin, and treated with
heat-induced epitope retrieval. Ki67 staining was applied by mouse
anti-human monoclonal MIB-1 antibody (dilution: 1:100, cat no.:
ZM-0165, Beijing Zhongshan Jinqiao Biotechnology Co., Ltd.,
Beijing, China) according to the manufacturer's guidelines. Blank
control (MDA-MB231), negative control (Vector-MDA-MB231) and
positive tests (HOTAIR-MDA-MB231) were run simultaneously. Bound
secondary antibodies were visualized with diaminobenzidine (Beijing
Zhongshan Jinqiao Biotechnology Co., Ltd., Beijing, China) and
counterstained with Harris hematoxylin. Samples were examined by
two independent pathologists who had no prior knowledge of the
clinical data. The expression levels of Ki67 were graded based on
the percentage of tumor cells stained by the antibody.
Western blot analysis
Cells were treated with 10 µmol/l PI3K inhibitor
(LY294002) for 6 h (9,10). The cells were lysed in RIPA buffer
supplemented with protease and phosphatase inhibitors (Keygen,
Nanjing, China). Quantity of protein in the lysates was measured
using a BCA kit (Beyotime Institute of Biotechnology, Haimen,
China) and equal amounts of proteins were separated by SDS-PAGE and
transferred to PVDF membranes (Millipore, Alsace, France). The
membranes were blocked with 5% skim milk, incubated with primary
antibodies against HOXD10 (dilution: 1:5,000, cat no. AB76897;
Abcam, Cambridge, MA, USA), p-AKT (dilution: 1:300, cat no.
KG11054) and p-BAD (dilution: 1:300, cat no. KG11068) (both from
Nanjing Keygen) at 4°C overnight, and HRP-conjugated secondary
antibodies (Bioworld Technology, Inc., St. Louis Park, MN, USA) for
1 h at 25°C. Immunoblotted proteins were visualized by ECL reagents
and the signals were detected by ChemiDoc™ XRS imaging system
(Quantity One Quantitation software, Bio-Rad Laboratories, Inc.,
Hercules, CA, USA).
Statistical analysis
Comparisons of continuous data were conducted by the
independent test or paired t-test between the groups, whereas
categorical data were analyzed by the Chi-square test. Values were
shown as the mean ± SD. Data were analyzed by t-test or one-way
ANOVA analysis. The statistical analyses were performed using SPSS
for Windows version 16.0 (SPSS, Inc., Chicago, IL, USA) and
GraphPad Prism 5 (GraphPad Software Inc., La Jolla, CA, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
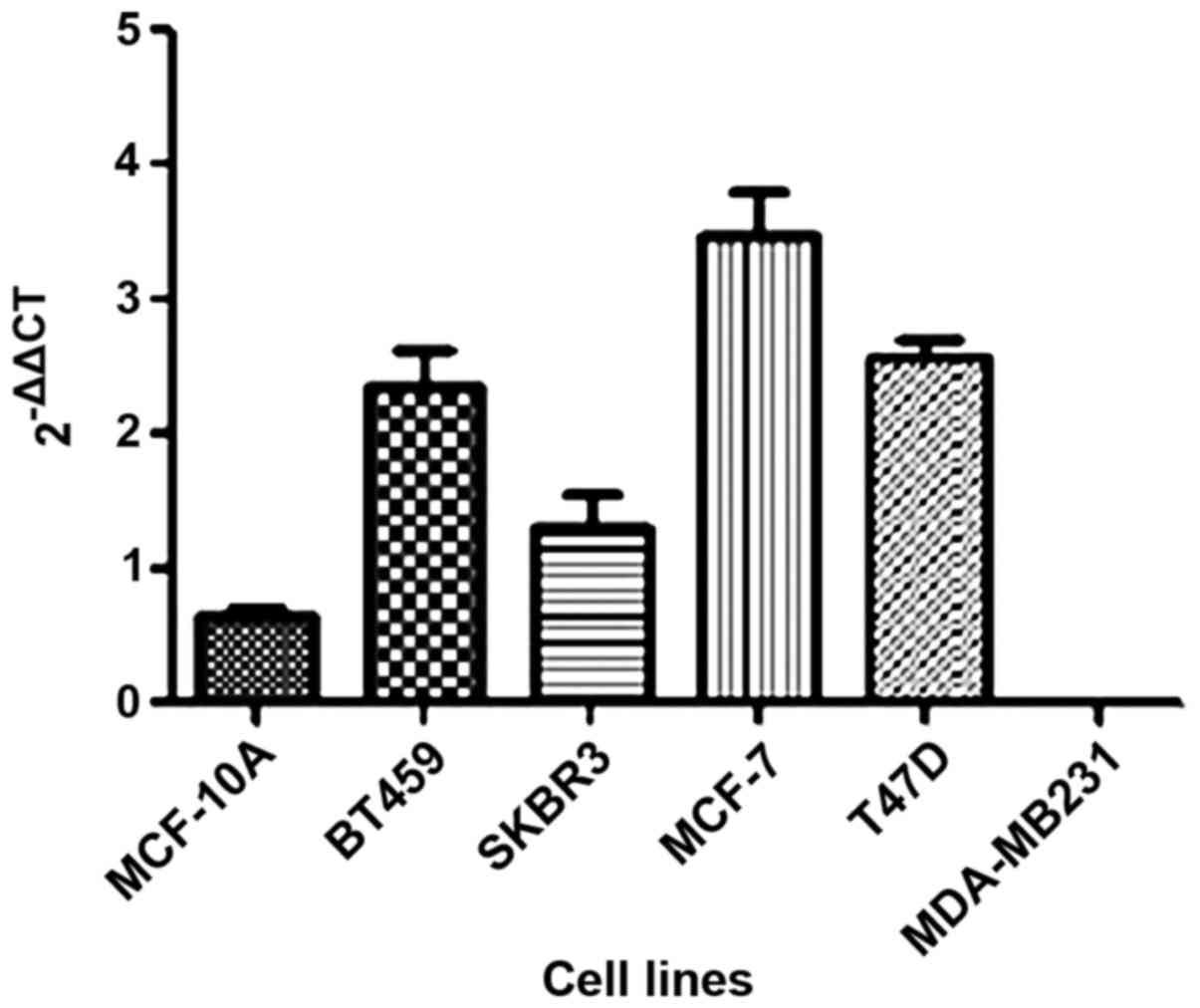
In the present study, we investigated the
HOTAIR gene expression in five breast cancer tumor cell
lines as well as one non-tumor (MCF-10A) cell line. We observed
that HOTAIR expression was lower only in the MDA-MB231 cell line
compared to the non-tumoral cell line (Fig. 1).
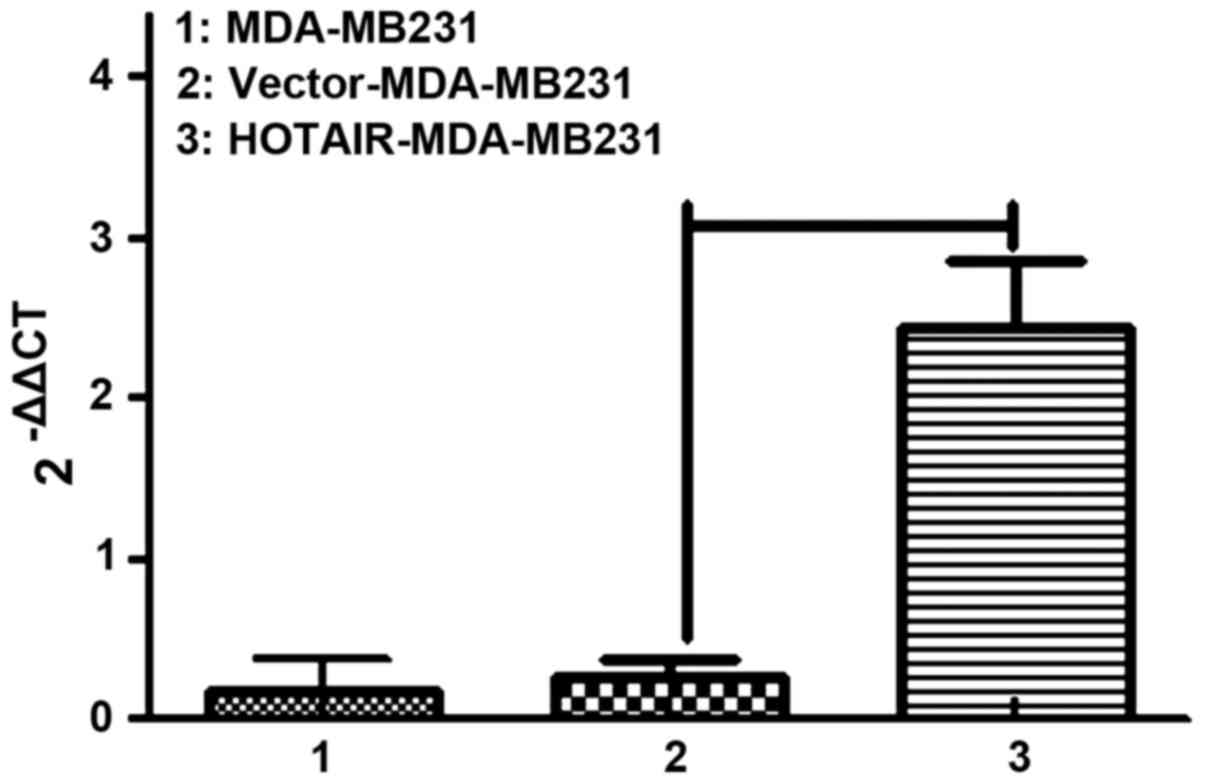
Thus, MDA-MB231 cells were selected to continue the
in vitro experiments. We labeled control and
HOTAIR-overexpressing cells with puro resistance. Lipofectamine
2000 transduction allowed the stable overexpression of HOTAIR to
almost 10-fold over the vector-transduced cells, which were
comparable to the levels seen in patients (Fig. 2).
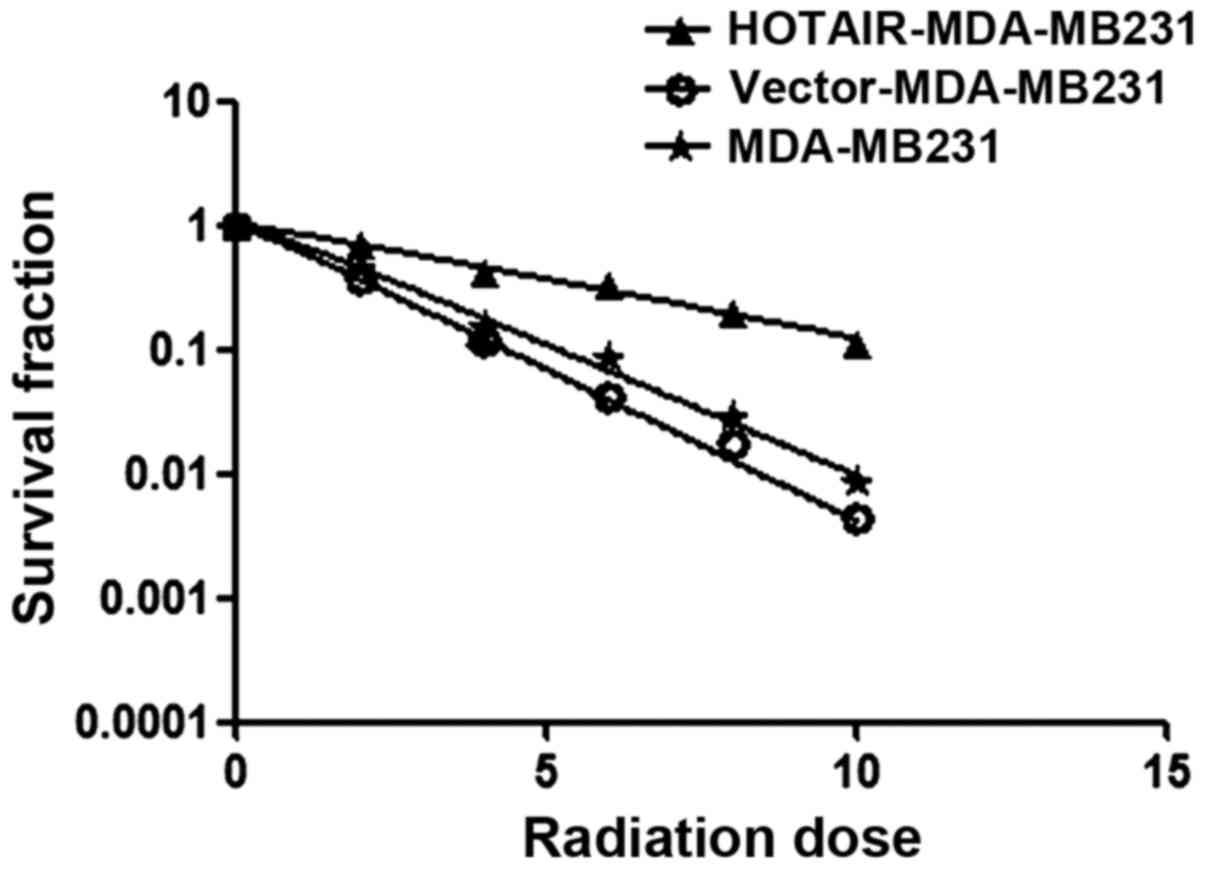
Investigations were performed to verify whether
HOTAIR induced radioresistance in MDA-MB231 cells. Using the
clonogenic survival assay test, overexpressed HOTAIR accelerated
the clonogenic survival of MDA-MB231 cells (Ser=0.58) was examined
(Fig. 3) (Table I).
 | Table I.Clonogenic survival assay test,
overexpressed HOTAIR accelerated clonogenic survival of MDA-MB231
cells (Ser=0.58). |
Table I.
Clonogenic survival assay test,
overexpressed HOTAIR accelerated clonogenic survival of MDA-MB231
cells (Ser=0.58).
| Cells | D0 | Dq | SF% | Ser D0 |
|---|
| MDA-MB231 | 2.05 | 1.42 | 0.46 |
|
| Vector-MDA-MB231 | 1.77 | 1.23 | 0.41 |
|
| HOTAIR-MDA-MB231 | 4.46 | 3.09 | 0.71 | 0.58 |
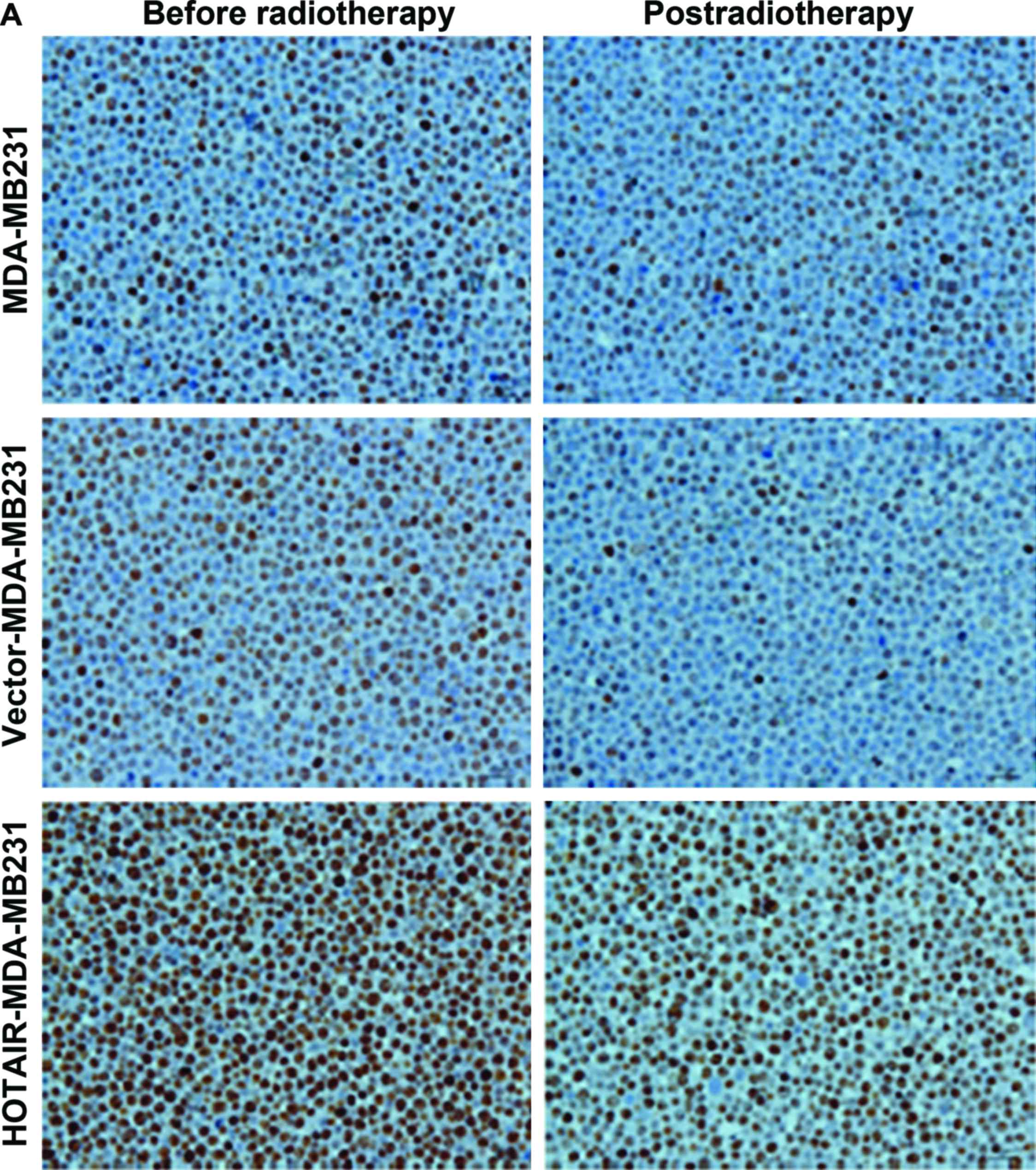
To understand the mechanism controlling HOTAIR-
induced radioresistance, the Ki67 expression level was measured.
This experiment was performed to determine whether HOTAIR-induced
radioresistance was a result of increased apoptosis or
proliferation. Null vector transfection (vector-MDA-MB231) served
as the control. The results showed that the proportion of apoptotic
and proliferative cells was altered (P<0.01) (Fig. 4).
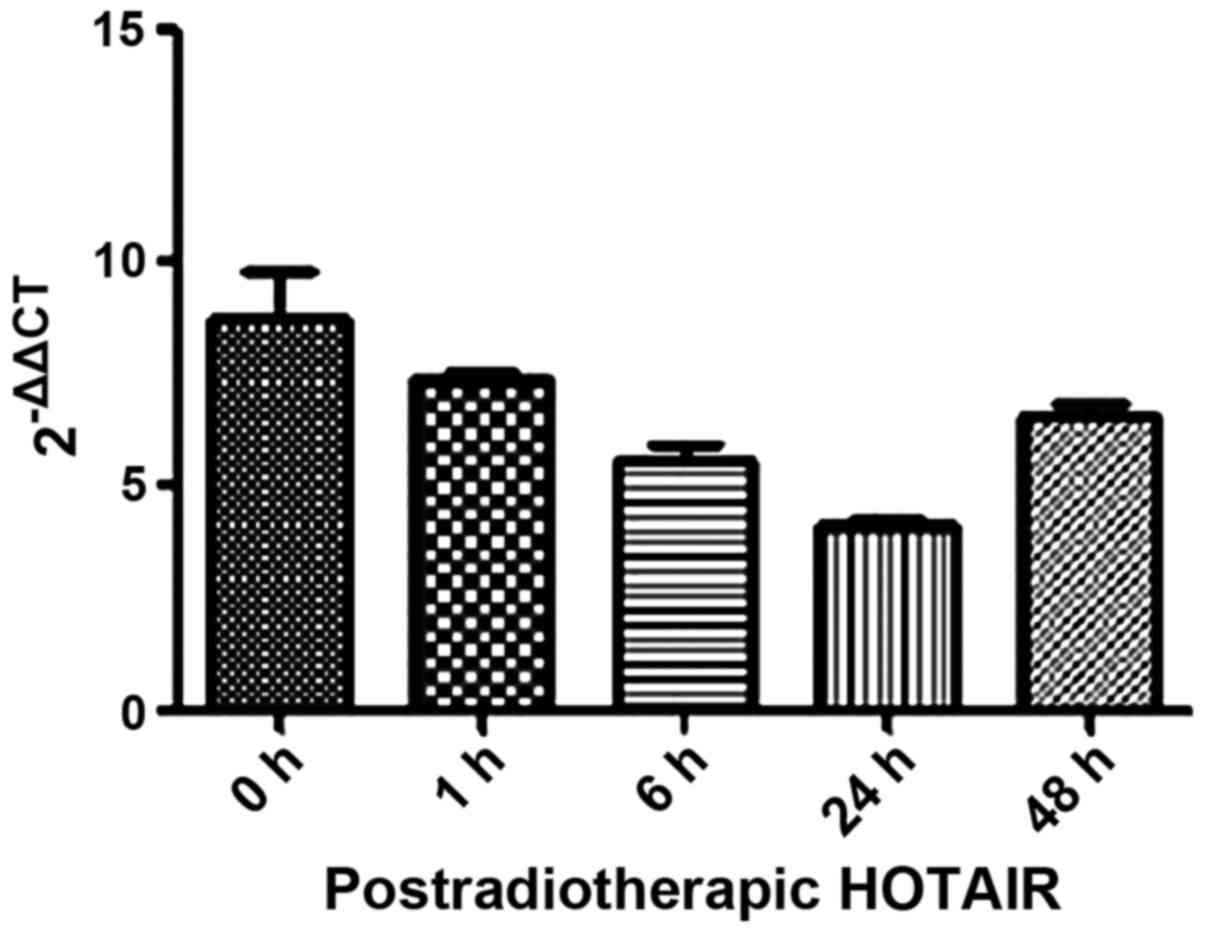
We also investigated the mechanism underlying the
anti-clonogenic effect identified in the overexpressed HOTAIR
MDA-MB231 cells. Firstly, we detected HOTAIR expression in
postradiotherapeutic breast cancer cells with a minimum level of
accumulation at 24 h (Fig. 5). Thus,
the 24-h time point was chosen for subsequent experiments.
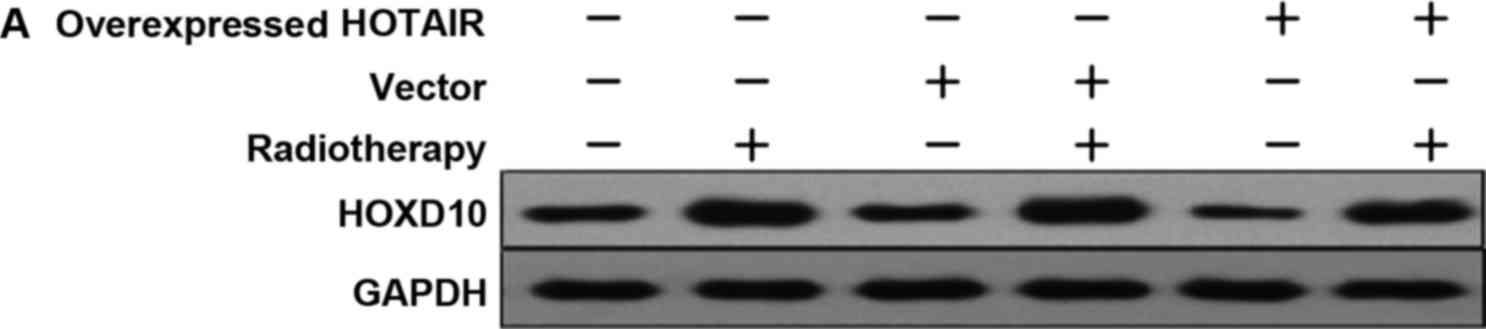
In order to investigate whether HOTAIR was involved
in HOXD10 expression and the PI3K/AKT-BAD pathway in MDA-MB231
cells, the expression levels of HOXD10, phosphorylated AKT (p-AKT)
and phosphorylated BAD (p-BAD) were measured. Subsequently, by
adding PI3K inhibitor (Ly294002), the relationship between p-AKT
and p-BAD proteins was investigated. The results showed that,
HOTAIR promoted the proliferation of breast cancer cells via HOXD10
and the PI3K/AKT-BAD pathway (Fig. 6A and
B).
The results suggested that radiation played an
effective role in the overexpression of HOTAIR breast cancer cells.
Therefore, an appropriate dose increase in radiation therapy may be
a useful strategy for those breast cancers with high expression
levels of HOTAIR.
Discussion
Radiotherapy is the leading method of therapy for
inoperable and locally advanced breast cancers. However, breast
cancer radioresistance occurs frequently in patients undergoing
radiotherapy. LncRNA HOTAIR recently emerged as a cancerogenic
promoter in various types of cancer including breast cancer
(10). Development and metastasis are
a common feature shared among some lncRNAs. It has been established
that lncRNAs were involved in numerous biological functions
including imprinting, epigenetic regulation, apoptosis, and cell
cycle (11). It appears that lncRNAs
are developing into promising biomarkers and novel strategies
against tumor (11). Recent findings
on lncRNAs revealed that HOTAIR was expressed from the HOXC locus,
whereas HOTAIR transcription was repressed in the more distal HOXD
locus (12). The interaction among
HOTAIR, PRC2 and the lysine-specific demethylase 1 negatively
influenced the expression of certain metastasis-suppressing genes
(3,12). Clinically, HOTAIR was significantly
overexpressed in a variety of tumors and was shown to induce the
proliferation and metastasis thereof (13–18). The
overexpression of HOTAIR is a useful predictor of survival and
progression in several cancer types, including colon cancer
(13), gastrointestinal stromal
tumors (14), pancreatic cancer
(15), oesophageal cancer (16), hepatocellular carcinoma (17) and nasopharyngeal carcinoma (18). HOTAIR is also involved in the
development of primary breast cancers and promotes invasion and
metastasis (3).
Our in vitro results demonstrated that the
overexpressed HOTAIR in MDA-MB231 cells had a significant effect in
cell proliferation. Notably, overexpressed HOTAIR in MDA-MB231
cells maintained higher proliferation rates after receiving
irradiation, suggesting that HOTAIR may play a role as a
radioresistance factor.
The exact mechanism of HOTAIR-induced
radioresistance remains unclear; however, details surrounding the
process are emerging gradually. HOTAIR overexpression induced an
aggressive phenotype in many cancer types and aberrant expression
of homeotic HOX transcription factors, especially HOXD10, which
regulated differentiation and tissue homeostasis (19).
Findings suggest that HOXD10 translation can be
repressed by two types of ncRNAs: HOTAIR and miR-10b. Liu et
al showed that miR-10b promoted cell invasion through the
RhoC-AKT signaling pathway by targeting HOXD10 in gastric cancer
(20). Previous findings showed that
HOXD10 downregulation resulting from miR-10b overexpression may
increase prometastatic gene products, such as MMP-14 and RHOC.
Additionally, HOXD10 downregulation also contributed to the
acquisition of metastatic phenotypes in epithelial ovarian cancer
cells (5). In the current study, we
investigated the effects of HOTAIR and/or irradiation on increasing
HOXD10 and PI3K/AKT-BAD activities. While we observed high levels
of HOXD10 expression during radiotherapy, HOXD10 was downregulated
in breast cancer cells in which HOTAIR was overexpressed suggesting
that, HOTAIR may serve as a limiting factor in HOXD10 expression.
Radiotherapy played a central role in the modulation of HOXD10
expression; nevertheless, HOTAIR antagonized and weakened the
effects of radiation. Furthermore, HOTAIR negatively affected the
BAD protein expression. After radiotherapy, the BAD level increased
significantly. BAD expression levels in the overexpressed HOTAIR
and radiotherapy group were lower than the null-vector and
radiotherapy groups. Our results revealed that after treatment with
Ly294002 (10 µmol/l) and radiotherapy, the p-AKT protein levels
changed and this change was time-dependent. The results suggest
that in breast cancer cells, HOTAIR had the ability to increase
radioresistance by interfering with activation of HOXD10 and the
PI3K/AKT-BAD signaling pathway.
We also showed that HOTAIR overexpressions may serve
as a predictor to stratify the risk of breast cancer
radioresistance. However, which oncogenic signal is responsible for
the increase observed in HOTAIR expression in breast tumor cells
remains to be determined. Additionally, the role of HOTAIR in
promoting proliferation through PI3K/AKT-Bad pathway by targeting
HOXD10 should be investigated. In conclusion, our findings reveal
that patients with HOTAIR-overexpression breast tumors have a low
sensitivity to radiotherapy and adjuvant or more aggressive
treatments should be administered to these patients.
References
|
1
|
Ferlay J, Soerjomataram I and Ervik M:
GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide. IARC
CancerBase No. 11 (Internet)International Agency for Research on
Cancer; Lyon, France: 2013, http://globocan.iarc.fr
|
|
2
|
Esteller M: Non-coding RNAs in human
disease. Nat Rev Genet. 12:861–874. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gupta RA, Shah N, Wang KC, Kim J, Horlings
HM, Wong DJ, Tsai MC, Hung T, Argani P, Rinn JL, et al: Long
non-coding RNA HOTAIR reprograms chromatin state to promote cancer
metastasis. Nature. 464:1071–1076. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Vardhini NV, Rao PJ, Murthy PB and
Sudhakar G: HOXD10 expression in human breast cancer. Tumour Biol.
35:10855–10860. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nakayama I, Shibazaki M, Yashima-Abo A,
Miura F, Sugiyama T, Masuda T and Maesawa C: Loss of HOXD10
expression induced by upregulation of miR-10b accelerates the
migration and invasion activities of ovarian cancer cells. Int J
Oncol. 43:63–71. 2013.PubMed/NCBI
|
|
6
|
ZLi X, Wu Z, Mei Q, Li X, Guo M, Fu X and
Han W: Long non-coding RNA HOTAIR, a driver of malignancy, predicts
negative prognosis and exhibits oncogenic activity in oesophageal
squamous cell carcinoma. Br J Cancer. 109:2266–2278. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Huuhtanen RL, Blomqvist CP, Wiklund TA,
Böhling TO, Virolainen MJ, Tukiainen EJ, Tribukait B and Andersson
LC: Comparison of the Ki-67 score and S-phase fraction as
prognostic variables in soft-tissue sarcoma. Br J Cancer.
79:945–951. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hu L, Zaloudek C, Mills GB, Gray J and
Jaffe RB: In vivo and in vitro ovarian carcinoma growth inhibition
by a phosphatidylinositol 3-kinase inhibitor (LY294002). Clin
Cancer Res. 6:880–886. 2000.PubMed/NCBI
|
|
9
|
Semba S, Itoh N, Ito M, Harada M and
Yamakawa M: The in vitro and in vivo effects of
2-(4-morpholinyl)-8-phenyl-chromone (LY294002), a specific
inhibitor of phosphatidylinositol 3′-kinase, in human colon cancer
cells. Clin Cancer Res. 8:1957–1963. 2002.PubMed/NCBI
|
|
10
|
Zhang J, Zhang P, Wang L, Piao HL and Ma
L: Long non-coding RNA HOTAIR in carcinogenesis and metastasis.
Acta Biochim Biophys Sin (Shanghai). 46:1–5. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tsai MC, Manor O, Wan Y, Mosammaparast N,
Wang JK, Lan F, Shi Y, Segal E and Chang HY: Long noncoding RNA as
modular scaffold of histone modification complexes. Science.
329:689–693. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kogo R, Shimamura T, Mimori K, Kawahara K,
Imoto S, Sudo T, Tanaka F, Shibata K, Suzuki A, Komune S, et al:
Long noncoding RNA HOTAIR regulates polycomb-dependent chromatin
modification and is associated with poor prognosis in colorectal
cancers. Cancer Res. 71:6320–6326. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xu ZY, Yu QM, Du YA, Yang LT, Dong RZ,
Huang L, Yu PF and Cheng XD: Knockdown of long non-coding RNA
HOTAIR suppresses tumor invasion and reverses
epithelial-mesenchymal transition in gastric cancer. Int J Biol
Sci. 9:587–597. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kim K, Jutooru I, Chadalapaka G, Johnson
G, Frank J, Burghardt R, Kim S and Safe S: HOTAIR is a negative
prognostic factor and exhibits pro-oncogenic activity in pancreatic
cancer. Oncogene. 32:1616–1625. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen FJ, Sun M, Li SQ, Wu QQ, Ji L, Liu
ZL, Zhou GZ, Cao G, Jin L, Xie HW, et al: Upregulation of the long
non-coding RNA HOTAIR promotes esophageal squamous cell carcinoma
metastasis and poor prognosis. Mol Carcinog. 52:908–915. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ishibashi M, Kogo R, Shibata K, Sawada G,
Takahashi Y, Kurashige J, Akiyoshi S, Sasaki S, Iwaya T, Sudo T, et
al: Clinical significance of the expression of long non-coding RNA
HOTAIR in primary hepatocellular carcinoma. Oncol Rep. 29:946–950.
2013.PubMed/NCBI
|
|
17
|
Nie Y, Liu X, Qu S, Song E, Zou H and Gong
C: Long non-coding RNA HOTAIR is an independent prognostic marker
for nasopharyngeal carcinoma progression and survival. Cancer Sci.
104:458–464. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tano K and Akimitsu N: Long non-coding
RNAs in cancer progression. Front Genet. 3:2192012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Heubach J, Monsior J, Deenen R, Niegisch
G, Szarvas T, Niedworok C, Schulz WA and Hoffmann MJ: The long
noncoding RNA HOTAIR has tissue and cell type-dependent effects on
HOX gene expression and phenotype of urothelial cancer cells. Mol
Cancer. 14:1082015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu Z, Zhu J, Cao H, Ren H and Fang X:
miR-10b promotes cell invasion through RhoC-AKT signaling pathway
by targeting HOXD10 in gastric cancer. Int J Oncol. 40:1553–1560.
2012.PubMed/NCBI
|