Introduction
Extranodal natural killer/T-cell lymphoma (ENKTL) is
considered to be an aggressive disease with particular morphologic,
immunohistochemical and clinical features (1). According to a review performed by the
Pathology of Lymphoma Collaborative Group (Beijing, China), ENKTL
is the second most common type of lymphoma following diffuse large
B-cell lymphoma (DLBCL) in China (2).
According to the anatomic site involved, ENKTL can be classified
into either upper aerodigestive tract (UAT) group or non-UAT group.
Subdivision of the 2 groups is necessary due to the difference in
clinical characteristics and prognosis (3–5). Due to
the poor prognosis of ENKTL, a great number of clinical and
pathological efforts have been made to identify prognostic markers
for ENKTL (6–8).
Tumor necrosis factor (TNF) receptor superfamily
member 8 (CD30) is a transmembrane glycoprotein belonging to the
TNF family (9). Among
lymphoproliferative disorders (LPDs), CD30 expression was
originally reported in classical Hodgkin's lymphoma and anaplastic
large cell lymphoma (10). CD30
expression has previously been reported in a set of peripheral
T-cell lymphomas (PTCL) including PTCL-not otherwise specified
(NOS) (11,12), angioimmunoblastic T-cell lymphoma
(13) and primary cutaneous LPDs
(14). There are few studies
investigating the expression of CD30 in ENKTL patients (15–23). In
addition, the association between CD30 and the clinicopathological
features and prognosis of ENKTL remains controversial.
In the present study, CD30 expression was determined
using immunohistochemistry in 122 patients with Chinese ENKTL, to
evaluate its association with clinicopathological features. The
prognostic significance of CD30 expression was also studied through
the analysis of selected patients with complete data.
Materials and methods
Patient selection
Biopsied tissues of patients with NK/T-cell
lymphomas between May 2011 and November 2013 at the First
Affiliated Hospital of Zhengzhou University (Zhengzhou, China) were
identified. All patients were diagnosed according to the World
Health Organization classification criteria (1): The morphological and immunophenotypic
characteristics of the tumor cells fulfilled the criteria of ENKTL,
and all the cases were EBV positive. The enrolled patients had
lymphomatous lesions on the upper aerodigestive tract and had not
received any previous treatment for lymphoma. Reactive hyperplasia
of lymph node tissue was collected as control. Written informed
consent was obtained from all patients prior to starting the study.
This project was approved by the First Affiliated Hospital of
Zhengzhou University Clinical Research Ethics Committee (Zhengzhou,
China).
Immunohistochemistry
Immunohistochemistry was performed using 10%
formalin-fixed paraffin-embedded tissue blocks that were divided
into 4 µm sections, followed by the modified avidin-biotin complex
method (Envision method) on an automated immunostainer (Ventana
Medical Systems, Inc., Tucson, AZ, USA) (24). The following antibodies were used:
Membrane spanning 4-domains A1 (CD20; dilution, ready-to-use;
catalogue no., 14357208; ZSGB-BIO, Beijing, China), membrane-bound
immunoglobulin-associated protein (CD79a; dilution, ready-to-use;
catalogue no., 15620806; Fuzhou Maixin Biotech Co., Ltd., Fuzhou,
China), cytoplasmic cluster of differentiation (CD) 3 (dilution,
ready-to-use; catalogue no., CRM01420309; Shanghai Jiehao Biotech
Co., Ltd., Shanghai, China), CD3 (dilution, ready-to-use; catalogue
no., 130801543E; Fuzhou Maixin Biotech Co., Ltd.), CD43 (dilution,
ready-to-use; catalogue no., 131006032b; Fuzhou Maixin Biotech Co.,
Ltd.), neural cell adhesion molecule (CD56; dilution, ready-to-use;
catalogue no., cm03510419; Shanghai Jiehao Biotech Co., Ltd.),
Granzyme-B (dilution, ready-to-use; catalogue no.,
16691906;ZSGB-BIO), T-cell-restricted intracellular antigen 1
(TIA-1; dilution, ready-to-use; catalogue no., 160426599E; Fuzhou
Maixin Biotech Co., Ltd.), CD30 (dilution, ready-to-use; catalogue
no., 15331003;ZSGB-BIO) and anaplastic lymphoma kinase (ALK;
dilution, ready-to-use; catalogue no., 151223281C; Fuzhou Maixin
Biotech Co., Ltd.). The percentage of CD30 expression was
determined by quantifying the number of positive cells with strong
membrane staining, excluding all necrotic area. Those with strong,
complete and uniform brown coloring at the cytoplasmic membrane
were considered as CD30-positive. The results were scored on a
5-tiered scale: 0, 0% positive cells or no staining; 1+, <25%
positive cells; 2+, 25–50% positive cells; 3+, >50–75% positive
cells; and 4+, >75% positive cells. A score of ≥1+ was taken to
represent CD30 positivity.
In situ hybridization for Epstein-Barr
virus (EBV)
The presence of EBV RNA was detected with an in
situ hybridization (ISH) technique, using the Epstein-Barr
Virus Early RNA kit (ZSGB-BIO) according to the manufacturer's
protocol. Briefly, 4–6 µm sections cut from paraffin tissues were
deparaffinized, rehydrated, predigested with gastric enzyme,
prehybridized, and then hybridized with a digoxigenin (DIG)-labeled
RNA probe (ZSGB-BIO). Subsequent to washing, the reaction was
performed using anti-DIG horseradish peroxidase conjugate antibody
(dilution, ready-to-use; catalogue no., 151110; ZSGB-BIO) followed
by development with diaminobenzidine (ZSGB-BIO, Beijing, China). A
known case of EBV-positive nasopharyngeal carcinoma was used as a
positive control.
Statistical analysis
All analyses were performed with SPSS 17.0 (SPSS,
Inc., Chicago, IL, USA). The association between CD30 expression
and clinicopathological parameters was assessed using a
χ2 test or Fisher's exact test. Survival rate analysis
was determined using the Kaplan-Meier method with the log-rank
test. Overall survival (OS) rate was defined as between the date of
diagnosis and the date of last follow-up or mortality.
Progression-free survival (PFS) rate was defined as between the
date of diagnosis and the time of progression or mortality.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Immunohistochemical analyses of CD30
expression
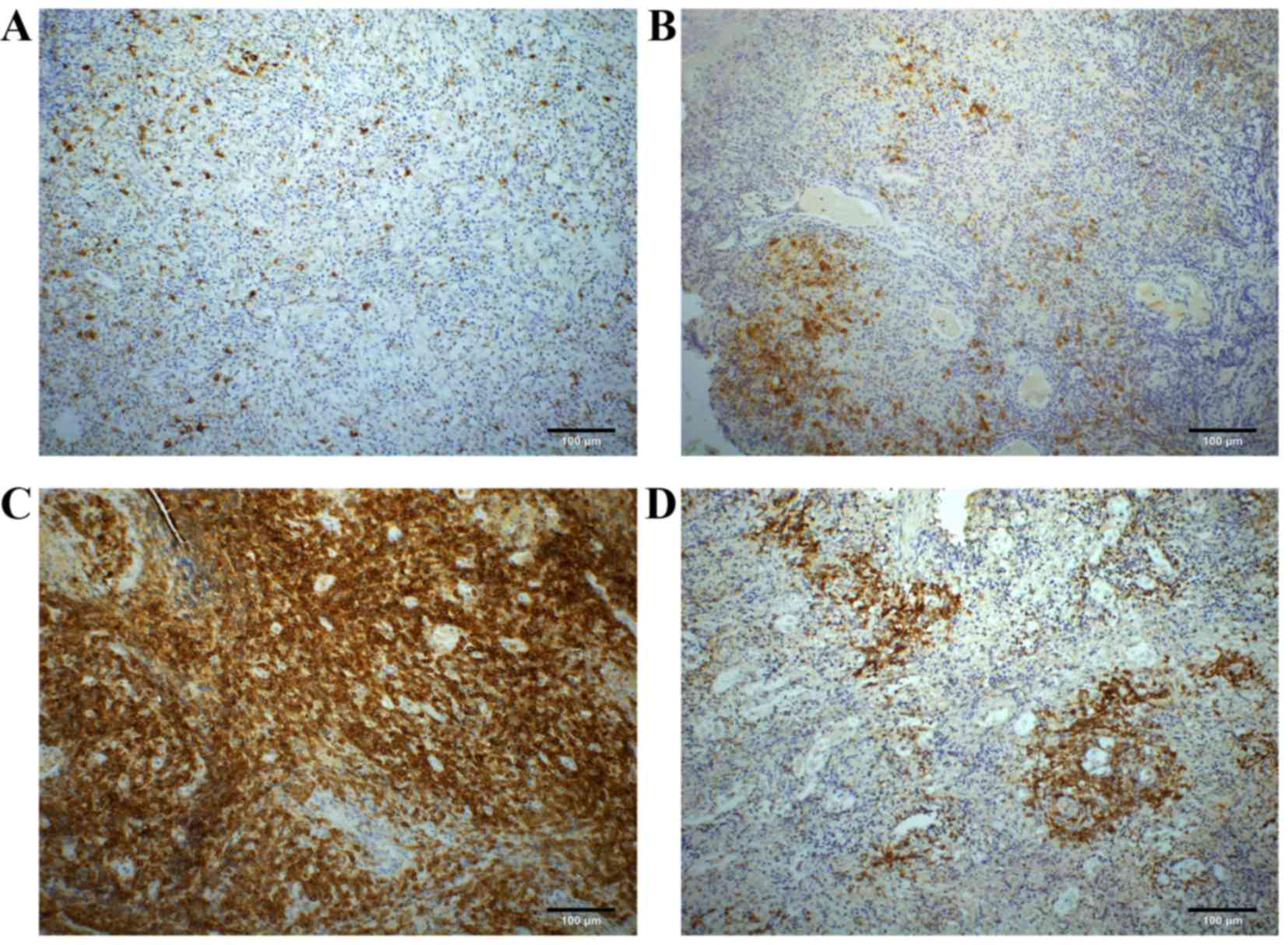
According to the scoring system aforementioned, the
numbers of CD30 expression scoring 0, 1+, 2+, 3+ and 4+ accounted
for 36 (29.5), 46 (37.7), 22 (18.0), 12 (9.8) and 6 (4.9%) of 122
patients with ENKTL, respectively. Overall, 14.7% (18 of 122) of
the considered patients showed a CD30 expression score of ≥3+.
Among the 86 patients with score 1+ to score 4+, the membranous
staining patterns of CD30 expression were sporadic (33.7%; 29 of
86), focal (43.0%; 37 of 86), diffuse (15.1%; 13 of 86) and
angiocentric (8.1%; 7 of 86) (Fig.
1A-D).
Association between CD30 expression
and clinical features
Complete clinical data was collected from 70
patients. All the patients received 1 of the following therapies:
Chemotherapy alone (50%; 35 of 70); radiotherapy alone (4.3%; 3 of
70); or concurrent chemo-radiotherapy (45.7%; 32 of 70). All the
patients were followed-up between 11 months and 50 months with a
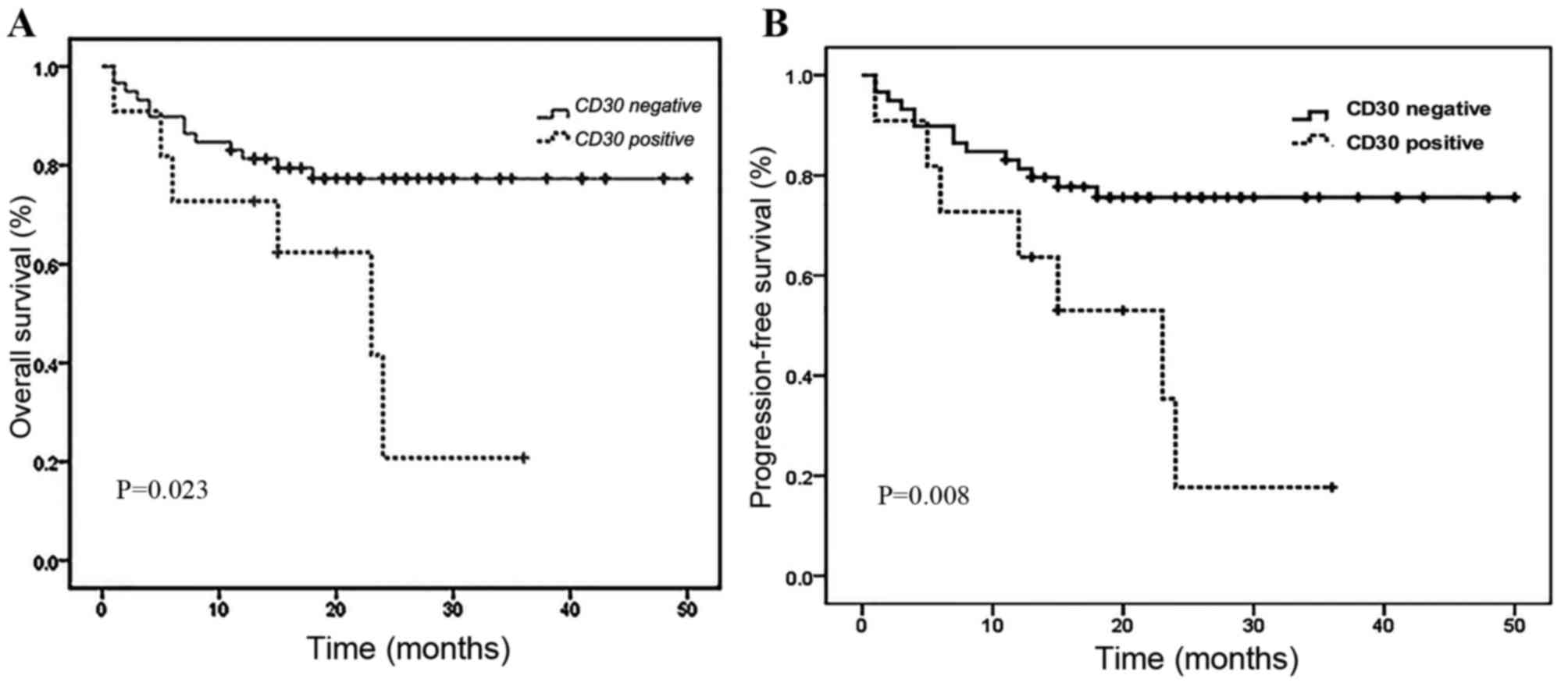
median follow-up length of 28 months. The Kaplan-Meier analysis of
OS for the whole group (n=70) was 72.9% at 5 years. Survival rate
analysis was performed between CD30+ and CD30-patients
with ENKTL based on the 5-tiered scale between 0 and 4+. When
considering a score of ≥3+ as CD30+, the
CD30+ group had a significantly shorter OS (P=0.0023)
and PFS (P=0.0008) compared with the CD30-group (Fig. 2). Thus, the criterion for CD30
positivity, ≥3+, was used for additional analysis.
Comparison of clinical and
histopathological features according to CD30 expression
The clinicopathological features of the 122 patients
with ENKTL stratified according to level of CD30 expression are
listed in Table I. Overall, there
were 84 men and 38 women (male/female, 2.2:1) with a median age of
45 years and a range of 15–80 years. Over half (53.3%) of the
patients presented with B symptoms, which consist of: Weight loss
of >10% within 6 months; night sweats; and fever of unknown
origin, and ~1/3 had advanced Ann Arbor staging of stage III or IV
(25). No clinical difference was
reported between patients in the CD30+ group compared
with the CD30-group when CD30 positivity was defined as ≥3+.
 | Table I.Clinicopathological features of ENKTL
stratified according to CD30 expression. |
Table I.
Clinicopathological features of ENKTL
stratified according to CD30 expression.
| Characteristic | Overall, n (%) | CD30+, n
(%) | CD30−, n
(%) | P-value |
|---|
| Gender |
|
|
|
|
| Male | 84 (68.9) | 12 (66.7) | 72 (69.2) | 0.828 |
|
Female | 38 (31.1) | 6 (33.3) | 32 (30.8) |
|
| Age, years |
|
|
|
|
| ≤60 | 97 (79.5) | 15 (83.3) | 82 (78.8) | 0.663 |
|
>60 | 25 (20.5) | 3 (16.7) | 22 (21.2) |
|
| B symptoms |
|
|
|
|
|
Absence | 57 (46.7) | 10 (55.6) | 47 (45.2) | 0.416 |
|
Presence | 65 (53.3) | 8 (44.4) | 57 (54.8) |
|
| Elevated serum
LDH |
|
|
|
|
| Yes | 49 (40.2) | 9 (50.0) | 40 (38.5) | 0.357 |
| No | 73 (59.8) | 9 (50.0) | 64 (61.5) |
|
| Bone marrow
involvement |
|
|
|
|
| Yes | 3
(2.5) | 1 (5.6) | 2 (1.9) | 0.358 |
| No | 119 (97.5) | 17 (94.4) | 102 (98.1) |
|
| Ann Arbor
staging |
|
|
|
|
| I,
II | 80 (65.6) | 14 (77.8) | 66 (63.5) | 0.238 |
| III,
IV | 42 (34.4) | 4 (22.2) | 38 (36.5) |
|
| Histological
types |
|
|
|
|
| SC | 27 (22.1) | 4 (22.2) | 23 (22.1) | 0.540 |
| MC | 65 (53.3) | 8 (44.4) | 57 (54.8) |
|
| LC | 10 (8.2) | 3 (16.7) | 7 (6.7) |
|
| PC | 20 (16.4) | 3 (16.7) | 17 (16.3) |
|
| Necrosis |
|
|
|
|
|
Yes | 105 (86.1) | 17 (94.4) | 88 (84.6) | 0.266 |
| No | 17 (13.9) | 1 (5.6) | 16 (15.4) |
|
| Vascular
destruction |
|
|
|
|
|
Yes | 45 (36.9) | 8 (44.4) | 37 (35.6) | 0.472 |
| No | 77 (63.1) | 10 (55.6) | 67 (64.4) |
|
Based on the prevailing population size of tumor
cells, ENKTL in the present study can be classified into 4
histological types: 27 patients with small cell type; 65 patients
with medium-sized cell (MC) type; 10 patients with large cell type;
and 20 patients with pleomorphic cell type. In total, 45 patients
exhibited an angiodestructive growth pattern and 105 patients
exhibited various degrees of coagulative necrosis. No statistically
significant association was observed between the expression of CD30
and histologic subtypes, the association between CD30 expression
and patients with vascular destruction and necrosis failed to reach
statistical significance as well.
In terms of immunohistochemical findings, the
neoplastic cells in all patients tested positive for cytoplasmic
CD3 and CD43, but negative for CD20. In total, 95.1% (116/122)
showed staining for CD3 and 91.8% (112/122) were positive for CD56.
The expression of the cytotoxic markers granzyme B and TIA-1 was
observed in 91.8% (112/122), and 92.6% (113/122) of all patients,
respectively. All 122 patients were EBV-positive according to ISH
detection.
Discussion
The rate of CD30 positivity in ENKTL that has been
reported in previous studies has been sporadic (range 20–70%)
(15–23). Different detection rates between the
immunohistochemistry techniques used perhaps attributed to
different numbers of patients and different cut-off values for CD30
being used by different researchers. Therefore, in order to figure
out how many tumor cells should be defined as CD30-positive, a
scoring system based on a 5-tiered scale was applied in the present
study. The CD30-positive rate was 14.7% at the cut-off point of
3+.
The association of CD30 expression in the prognosis
of ENKTL was analyzed first, as this has been controversial
(16,17,19,26). In
the present study (n=70), patients with CD30+ ENKTL
showed significantly inferior OS (P=0.023) and PFS (P=0.008)
compared with CD30-ENKTL. However, studies involving different
groups produced contradictory results. Kuo et al (16) (n=22) reported that there was no
significant statistical difference in survival rate according to
CD30 expression with respect to ENKTL. Mraz-Gernhard et al
(26) (n=30) reported that patients
with cutaneous CD30+ NK or NK/T cell lymphomas usually
demonstrated better outcomes compared withCD30 patients. By
contrast, Hong et al (19)
(n=22) found that CD30 expression was associated with a poor
prognosis. These studies mentioned that additional studies
involving larger numbers of patients were required due to limited
sample size. A study involving a greater number of patients
performed by Li et al (17)
(n=96) demonstrated that OS and PFS rates in the CD30+
group were significantly decreased compared with those in the
CD30-group, which is consistent with the findings of the present
study. Another focus of the present study was figuring out the
optimal cut-off for CD30+ that would be of statistical
significance, as previous studies used different criteria. In the
present study, survival rate analysis was performed between the
CD30+ and the CD30-group based on different cut-off
levels, when considering a score ≥3+ as CD30 positivity, a level of
significance of P<0.001 was observed. In the present study, CD30
expression was considered to be positive when >50% of the tumor
cells exhibited strong membrane staining. This criterion was used
in the analyses.
In the present study, the most common type of
morphological pattern was MC type, which accounted for 53.3% of the
122 patients with ENKTL. The majority of patients (86.1%) showed
coagulative necrosis. The association of the morphological
parameters in nasal NK/T cell lymphoma with CD30 expression has not
been clear thus far. Kuo et al (16) revealed that CD30 expression appeared
to correlate with vascular destruction and thrombosis, and 2 other
studies conducted by Kim and Heo (4)
and Gaal et al (15) reported
that CD30 positivity may be associated with large pleomorphic
(anaplastic) morphology. However, in these studies, no association
was found between CD30 expression and other morphological
parameters including histologic subtypes, vascular destruction and
necrosis. In the present study, 4 types of CD30+ pattern
were observed and almost half of the patients (43.2%) were CD30
focally positive. Notably, 7 patients with CD30 expression of
angiocentric positivity were observed, which was perhaps associated
with the angiodestructive growth pattern of the tumor cells. It
should be noted that 13 patients with ENKTL exhibited strong and
diffuse CD30 immunoreactivity, and this may be confused with
CD30+ anaplastic large cell lymphoma. Immunostains for
ALK and EBV in EBV-encoded RNA-ISH studies may be useful to
distinguish ENKTL and anaplastic large cell lymphoma in difficult
cases.
Certain studies have reported that CD30 expression
was an independent prognostic factor in several types of malignant
lymphoma. In a study on a large sample of patients with DLBCL
conducted by Hu et al (27),
CD30 expression defined a novel and unique subgroup of DLBCL with a
favorable clinical outcome and a distinct gene expression
signature. Another study investigating PTCL revealed, by gene
expression programming analyses, that patients with
CD30+ PTCL shared common molecular and phenotypic
features, and that there were significant differences between
CD30− and CD30+ PTCL-NOS (28). The 2 aforementioned observations
indicate that CD30 can be used as a valuable means to define 2
distinct biological subgroups, and it was demonstrated that CD30
expression was associated with improved outcomes. The mechanism
underlying the poor prognosis of CD30+ ENKTL has not
been well defined. EBV affects cell proliferation via latent
membrane protein (LMP) 1. CD30 and LMP1 belong to the TNF-receptor
family and their expression of protein has the potential to
activate nuclear transcription factor-κB (10). The authors hypothesize that LMP1 and
CD30 act cooperatively to affect the prognosis of ENKTL.
CD30+ ENKTL may exhibit a distinct gene expression
signature.
In conclusion, CD30 expression was associated with a
poor prognosis in ENKTL, but had no effect on clinical or
histopathological parameters when considering a score ≥3+ as CD30
positivity. The findings of the present study suggest that CD30
should be a good prognostic maker in ENKTL.
Acknowledgements
This study was funded by the National Natural
Science Foundation of China (grant nos. 81172118, 81402380 and
U1204814) and by a grant (no. 201303016) from the Programs for
Science and Technology Development of Henan, China.
References
|
1
|
Swerdlow SH, Campo E, Harris N, Jaffe ES,
Pileri SA, Stein H, Thiele J and Vardiman JW: World Health
Organization classification of tumors of hematopoietic and lymphoid
tissues. 4th. IARC; Lyon, France: 2008
|
|
2
|
Li XQ, Li GD, Gao ZF, Zhou XG and Zhu XZ:
The Chinese Lymphoma Study Group: Distribution pattern of lymphoma
subtypes in China: A nationwide multicenter study of 10,002 cases.
J Diagn Concepts Pract. 11:111–115. 2012.
|
|
3
|
Au WY, Weisenburger DD, Intragumtornchai
T, Nakamura S, Kim WS, Sng I, Vose J, Armitage JO and Liang R:
Clinical differences between nasal and extranasal natural
killer⁄T-cell lymphoma: A study of 136 cases from the International
Peripheral T-Cell Lymphoma Project. Blood. 113:3931–3937. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kim TM and Heo DS: Extranodal NK/T-cell
lymphoma, nasal type: New staging system and treatment strategies.
Cancer Sci. 100:2242–2248. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Oshimi K, Kawa K, Nakamura S, Suzuki R,
Suzumiya J, Yamaguchi M, Kameoka J, Tagawa S, Imamura N, Ohshima K,
et al: NK-cell neoplasms in Japan. Hematology. 10:237–245. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ye Z, Cao Q, Niu G, Liang Y, Liu Y, Jiang
L, Yu X and Han A: p63 and p53 expression in extranodal NK/T cell
lymphoma, nasal type. J Clin Pathol. 66:676–680. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang H, Wang L, Liu WJ, Xia ZJ, Huang HQ,
Jiang WQ, Li ZM and Lu Y: High post-treatment serum levels of
soluble programmed cell death ligand 1 predict early relapse and
poor prognosis in extranodal NK/T cell lymphoma patients.
Oncotarget. 7:3035–3345. 2016.
|
|
8
|
Wang H, Li P, Zhang X, Xia Z, Lu Y and
Huang H: Histological vascular invasion is a novel prognostic
indicator in extranodal natural killer/T-cell lymphoma, nasal type.
Oncol Lett. 12:825–836. 2016.PubMed/NCBI
|
|
9
|
Chiarle R, Podda A, Prolla G, Gong J,
Thorbecke GJ and Inghirami G: CD30 in normal and neoplastic cells.
Clin Immunol. 90:157–164. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Deutsch YE, Tadmor T, Podack ER and
Rosenblatt JD: CD30: An important new target in hematologic
malignancies. Leuk Lymphoma. 52:1641–1654. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sabattini E, Pizzi M, Tabanelli V, Baldin
P, Sacchetti CS, Agostinelli C, Zinzani PL and Pileri SA: CD30
expression in peripheral T-cell lymphomas. Haematologica.
98:e81–e82. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bossard C, Dobay MP, Parrens M, Lamant L,
Missiaglia E, Haioun C, Martin A, Fabiani B, Delarue R, Tournilhac
O, et al: Immunohistochemistry as a valuable tool to assess CD30
expression in peripheral T-cell lymphomas: High correlation with
mRNA levels. Blood. 124:2983–2986. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nicolae A, Pittaluga S, Venkataraman G,
Vijnovich-Baron A, Xi L, Raffeld M and Jaffe ES: Peripheral T-cell
lymphomas of follicular T-helper cell derivation with
Hodgkin/Reed-Sternberg cells of B-cell lineage: Both EBV-positive
and EBV-negative variants exist. Am J Surg Pathol. 37:816–826.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Duvic M: CD30+ neoplasms of the skin. Curr
Hematol Malig Rep. 6:245–250. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gaal K, Sun NC, Hernandez AM and Arber DA:
Sinonasal NK/T-cell lymphomas in the United States. Am J Surg
Pathol. 24:1511–1517. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kuo TT, Shih LY and Tsang NM: Nasal NK/T
cell lymphoma in Taiwan: A clinicopathologic study of 22 cases,
with analysis of histologic subtypes, Epstein-Barr virus LMP-1 gene
association, and treatment modalities. Int J Surg Pathol.
12:375–387. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li P, Jiang L, Zhang X, Liu J and Wang H:
CD30 expression is a novel prognostic indicator in extranodal
natural killer/T-cell lymphoma, nasal type. BMC Cancer. 14:8902014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kim WY, Nam SJ, Kim S, Kim TM, Heo DS, Kim
CW and Jeon YK: Prognostic implications of CD30 expression in
extranodal natural killer/T-cell lymphoma according to treatment
modalities. Leuk Lymphoma. 56:1778–1786. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hong J, Park S, Baek HL, Jung JH, Kang IG,
Sym SJ, Park J, Ahn JY, Cho EK, Kim ST, et al: Tumor cell nuclear
diameter and CD30 expression as potential prognostic parameter in
patients with extranodal NK/T-cell lymphoma, nasal type. Int J Clin
Exp Pathol. 5:939–947. 2012.PubMed/NCBI
|
|
20
|
Li T, Zhang B, Ye Y and Yin H:
Immunohistochemical and genetic analysis of Chinese nasal natural
killer/T-cell lymphomas. Hum Pathol. 37:54–60. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Schwartz EJ, Molina-Kirsch H, Zhao S,
Marinelli RJ, Warnke RA and Natkunam Y: Immunohistochemical
characterization of nasal-type extranodal NK/T-cell lymphoma using
a tissue microarray: An analysis of 84 cases. Am J Clin Pathol.
130:343–351. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ko YH, Ree HJ, Kim WS, Choi WH, Moon WS
and Kim SW: Clinicopathologic and genotypic study of extranodal
nasal-type natural killer/T-cell lymphoma and natural killer
precursor lymphoma among Koreans. Cancer. 89:2106–2116. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lu D, Lin CN, Chuang SS, Hwang WS and
Huang WT: T-cell and NK/T-cell lymphomas in southern Taiwan: A
study of 72 cases in a single institute. Leuk Lymphoma. 45:923–928.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ramos-Vara JA and Miller MA: Comparison of
two polymer-based immunohistochemical detection systems: ENVISION+
and ImmPRESS. J Microsc. 224:135–139. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Oshimi K: Progress in understanding and
managing natural killer-cell malignancies. Br J Haematol.
139:532–544. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mraz-Gernhard S, Natkunam Y, Hoppe RT,
LeBoit P, Kohler S and Kim YH: Natural killer/natural killer-like
T-cell lymphoma, CD56, presenting in the skin: An increasingly
recognized entity with an aggressive course. J Clin Oncol.
19:2179–2188. 2001.PubMed/NCBI
|
|
27
|
Hu S, Xu-Monette ZY, Balasubramanyam A,
Manyam GC, Visco C, Tzankov A, Liu WM, Miranda RN, Zhang L,
Montes-Moreno S, et al: CD30 expression defines a novel subgroup of
diffuse large B-cell lymphoma with favorable prognosis and distinct
gene expression signature: A report from the International DLBCL
Rituximab-CHOP Consortium Program Study. Blood. 121:2715–2724.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bisig B, de Reyniès A, Bonnet C, Sujobert
P, Rickman DS, Marafioti T, Delsol G, Lamant L, Gaulard P and de
Leval L: CD30-positive peripheral T-cell lymphomas share molecular
and phenotypic features. Haematologica. 98:1250–1258. 2013.
View Article : Google Scholar : PubMed/NCBI
|