Introduction
The sinonasal cavities are anatomical regions
affected by a number of tumours that are clinically, genetically
and etiologically different from classical carcinomas of the head
and neck (1). Sinonasal carcinoma
(SNc) is a rare disease that accounts for <3% of all head and
neck tumours, with a 5-year overall survival (OS) rate of 30%
across all stages. SNc is etiologically-associated with
professional exposure to leather and wood dust particles, and is
therefore defined as an occupational disease (2–4). Tumours
of the maxillary sinuses are more prevalent than those of the nasal
cavities and ethmoid sinuses (5).
Squamous cell carcinoma and adenocarcinoma are the most frequent
histological types, accounting for 80% of all SNcs, while
neuroendocrine, adenoid cystic and undifferentiated entities are
much less frequent (6).
Patients with SNcs are often asymptomatic in early
stages and are therefore commonly diagnosed at an advanced stage
(T3-4), presenting with a large primary tumour that invades the
surrounding bone structures and is associated with a high frequency
of poor outcome and local failure (7). Due to their rarity, there is a lack of
randomized clinical trials assessing the standard treatment options
for SNcs, with no clear guidelines concerning their treatment.
Generally, surgery, whenever possible, represents the cornerstone
of therapy in early (T1-2) and advanced stage (T3-4) patients, and
should always be followed by adjuvant radiation therapy, except in
cases of T1 low-risk disease (absence of involved surgical
margins). Chemotherapy should be administered concomitantly with
radiation therapy in cases of high-risk disease (T3-4 and/or N+
and/or involved surgical margins) (1,8–11). The outcome of patients with SNc also
depends on histological type (12),
and prognosis is poorer in patients with squamous cell carcinoma
compared with adenocarcinoma (25–50 vs. 40–60%, respectively)
(13,14). Undifferentiated sinonasal carcinoma
(SNUc) often presents as a rapidly enlarging and decaying mass,
which is associated with the poorest prognosis among all SNcs
(15).
Adjuvant radiotherapy may offer an increased chance
for local control, particularly in cases of high-risk disease;
however, its utility must be balanced against its frequent optic
nerve toxicity (16).
Radiotherapy-induced blindness may occur in up to 40% of treated
patients (17). Exclusive
radiotherapy may be administered in cases of unresectable disease
and should be coupled with chemotherapy, even if this does not
represent the standard of care (18).
Advanced disease is often treated with exclusive chemotherapy even
if SNCs are poorly chemosensitive. The most effective drugs are the
platinum-derived compounds, either associated with or not with
5-fluorouracil, and in certain cases they are administered with
taxanes (19–22). At present, there are only a few
clinical trials assessing the efficacy of targeted therapy in this
category of tumours (23).
The present retrospective study mainly aimed to
describe the survival of patients affected by this rare disease
treated at University of Naples ‘Federico II’ (Naples, Italy). The
current study also discusses the best therapy choice for locally
advanced disease.
Patients and methods
A total of 30 patients with SNc treated between
January 1999 and January 2013 at the Department of Radiation
Therapy, University of Naples ‘Federico II’ were included in the
present retrospective study. Pretreatment evaluation consisted of a
complete patient history, physical examination and fine-needle
aspiration with cytological examination to provide a diagnosis. A
core-biopsy of the lesion was performed in all patients, which
aimed to confirm initial diagnoses and histologically characterize
lesions. Staging was completed with head and neck magnetic
resonance imaging and thorax computed tomography (CT). All patients
attended a multidisciplinary head and neck conference at the
Department of Radiation Therapy, University of Naples ‘Federico
II’. Based on the National Comprehensive Cancer Network guidelines
(24) and, primarily, the shared
opinion of the multidisciplinary team, patients were selected for
treatment with surgery followed by adjuvant radio- or
chemoradiotherapy (group A), or definitive radiotherapy with or
without concomitant chemotherapy (group B). Surgery aimed to
achieve complete resection of the tumour with negative margins. The
type and extension of the surgery was dictated by the onset site
and the extent of the disease, in addition to functional
considerations. The surgical techniques employed included total or
subtotal maxillectomy and/or ethmoidectomy, and craniofacial
resection. Lymph node resection was performed only in the presence
of their clinical involvement. Criteria for unresectability
included wide intradural or intracranial spread, encasement of the
carotid artery and invasion of the cavernous sinus. Patients who
were unsuitable for resection underwent definitive radiotherapy
with or without concomitant chemotherapy. All patients were treated
with three-dimensional-conformal radiotherapy. Treatment planning
was based on CT examination performed with patients in a supine
position using head-neck-shoulder thermoplastic devices. The target
and organs at risk (OARs) were defined on a CT planning scan.
Clinical target volume (CTV) included the macroscopic extent of
disease/resection cavity/postoperative residual mass plus all
paranasal sinuses that had been invaded or were at high risk of
invasion. In case of orbital invasion, CTV included the medial
region of the orbit. Elective nodal irradiation was not performed,
but only the clinically positive lymph nodes were enclosed in the
CTV. Planning target volume was defined as CTV plus a 5-mm
isotropic margin. OARs included the retina, optic nerve and optic
chiasm. A median total dose of 60 Gy (range, 50–64 Gy) in 30
fractions daily of 2 Gy was planned. The maximum dose of 54 Gy was
used as dose constraint for OARs for planning elaboration. The
fields were arranged and weighted to achieve the maximum possible
uniform distribution in the target volume (95% of prescription dose
delivered to at least 95% of the PTV) without exceeding the dose
constraints for the OARs. Concomitant cisplatin was administered at
a dose of 100 mg/m2 on days 1, 22 and 43 for 3 cycles
during radiotherapy. This schedule was used for adjuvant and
exclusive radiotherapy settings. Demographic, disease and treatment
characteristics of all patients are presented in Table I.
 | Table I.Demographic, clinical and treatment
characteristics of patients. |
Table I.
Demographic, clinical and treatment
characteristics of patients.
| Characteristic | n (%) |
|---|
| Gender |
|
|
Female | 12 (40.0) |
| Male | 18 (60.0) |
| T onset site |
|
| Maxillary
sinus | 17 (56.7) |
| Nasal
cavity | 7 (23.3) |
| Ethmoidal
sinus | 4 (13.3) |
|
Sphenoidal sinus | 2 (6.7) |
| Histological
type |
|
| Squamous
cell carcinoma | 16 (53.3) |
|
Adenocarcinoma | 5 (16.7) |
| Adenoid
cystic carcinoma | 5 (16.7) |
| Sinonasal
undifferentiated carcinoma | 3 (10.0) |
|
Intestinal-like | 1 (3.3) |
| Stage |
|
| III | 8 (26.7) |
| IV | 22 (73.3) |
| T |
|
| T3 | 8 (26.7) |
| T4 | 22 (73.3) |
| N |
|
| N- | 27 (90.0) |
| N+ | 3 (10.0) |
| Grading |
|
| G1 | 11 (36.7) |
| G2 | 10 (33.3) |
| G3 | 9 (30.0) |
| Surgery |
|
| No | 12 (40.0) |
|
Yes |
|
|
R0 | 11 (36.7) |
|
R1 | 7 (23.3) |
| Chemotherapy |
|
| No | 8 (26.7) |
|
Yes |
|
|
Neoadjuvant | 1 (3.3) |
|
Concentrated | 17 (56.7) |
|
Neoadjuvant +
concentrated | 4 (13.3) |
Acute and chronic treatment-related toxicities were
registered according to the Radiation Therapy Oncology
Group/European Organization for Research and Treatment of Cancer
toxicity scale (25).
Statistical analysis
The actuarial OS, cause-specific survival (CSS;
percent of patients who succumbed to tumour progression) and
progression-free survival (PFS) rates were estimated using the
Kaplan-Meier method. Univariate analysis was performed using the
log-rank test, aiming to investigate the effect of clinical and
treatment-related variables on the 2-year CSS and PFS rates. All
statistical tests were two-sided and P<0.05 was considered to
indicate a statistically significant difference. All statistical
analyses were performed using SPSS 18.0 (SPSS, Inc., Chicago, IL,
USA).
Results
A total of 19/30 patients were treated with upfront
surgery followed by adjuvant radio- or chemoradiotherapy (group A),
while the remaining 11 received exclusive radiotherapy with or
without concomitant chemotherapy (group B). Concomitant
chemotherapy was performed in 6/19 patients in group A, and in 6/11
patients in group B. A total of 8/30 patients were diagnosed as
stage III [according to Tumour-Node-Metastasis staging (26)], while the remaining 22 were diagnosed
as having stage IV disease. Histological types widely varied, with
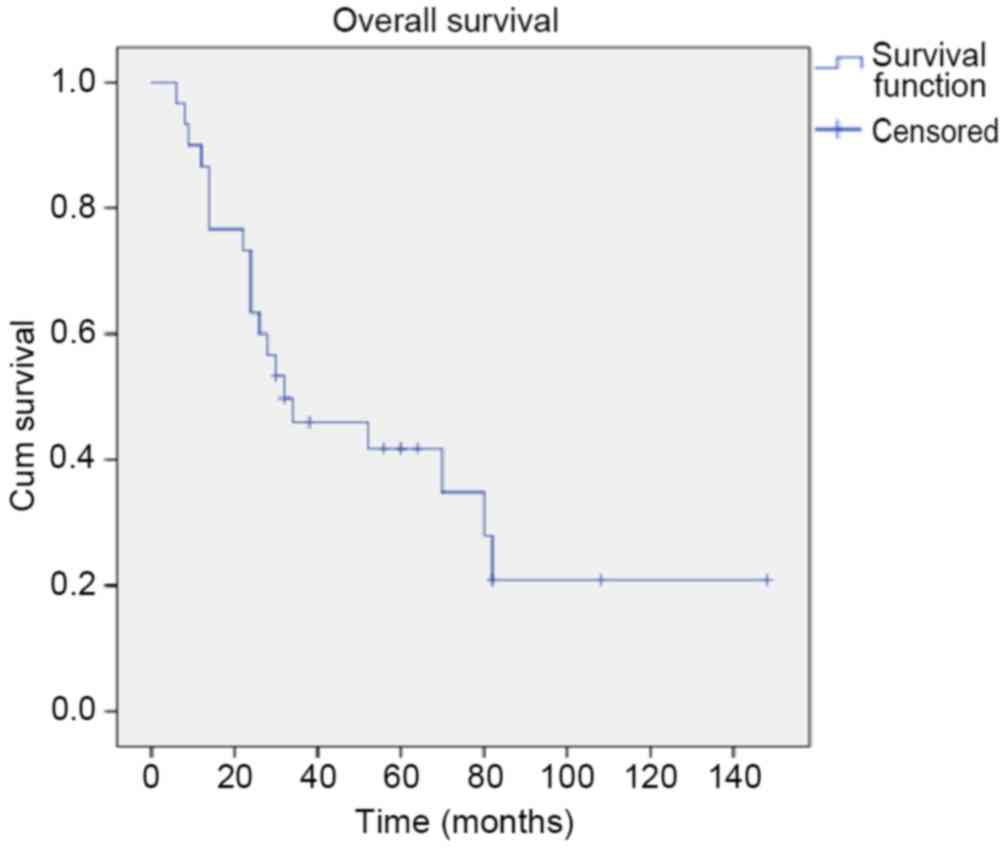
the majority determined as squamous cell carcinoma (Table I). At a median follow-up of 31 months
(range, 6–148 months), 33.3% of patients were alive and 90% of
these did not experience relapse. Overall, the relapse rate was
76.7% (23/30), and the rate of local and distant recurrence was
82.6% (19/23) and 8.7% (2/23), respectively. The estimated median
CSS and PFS times were 32 and 12 months, respectively. Univariate
analysis determined that only disease stage significantly affected
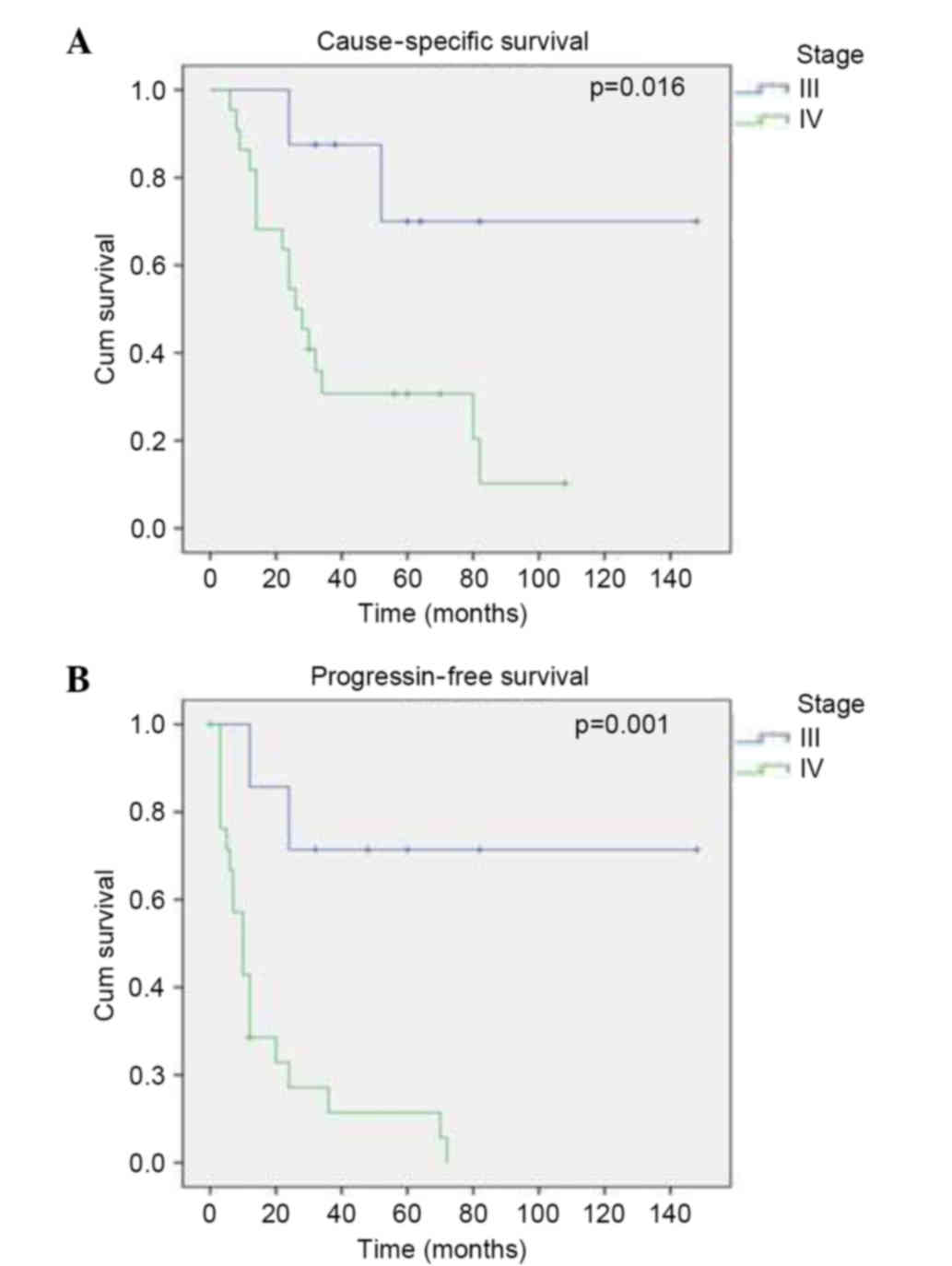
the CSS (P=0.002) and PFS (P=0.0001) rates. The 2-year CSS rate was
87.5% for stage III and 54.5% for stage IV (P=0.016), and the
2-year PFS rate was 71.4% for stage III and 17.1% for stage IV
(P=0.001) (Fig. 1). The effect of
stage on CSS and PFS rates was essentially linked to the primary
tumour size (T), and no contribution was observed for nodal status
(N); this was most likely due to the low number of lymph
node-positive cases (3/30 patients). Notably, no significant
differences in the 2-year CSS and PFS rates were observed between
the surgery (group A) and no surgery (group B) subgroups. However,
this result may be due to non-radical surgery, which was performed
in 7/11 resected patients. Non-radical surgery (R1 resection) is
not as effective as radical surgery, being associated with lower
survival in clinical trials. The constant presence of disease in
the tumour bed is frequently associated with early local recurrence
(27).
Similarly, no significant differences in CSS and PFS
rates were observed between the chemotherapy and no chemotherapy
subgroups in groups A and B. The results of the survival analysis
are presented in Fig. 2. The
remaining variables, including histological type, grade of
differentiation and site of origin, did not significantly affect
the CSS and PFS rates.
With regard to acute toxicity, 66.7% of patients
(20/30) experienced side effects during radiation therapy. The most
frequent side effects were oral mucositis and xerostomia, while
other side effects included dysphagia and skin toxicity. These
effects occurred at a median radiation dose of 30 Gy, with a peak
incidence at a median dose of 45 Gy. With regard to acute toxicity
grading, 43.3% (13/30) of patients developed grade 1 or grade 2
toxicity, while 23.3% of patients (7/30) developed grade 3
toxicity. Chronic side effects were observed in 13.3% of patients
(4/30), represented by the presence of xerostomia in all 4 cases.
No events of chronic toxicity to the optic pathways were noted. No
patterns of acute and chronic toxicity in relation to various
treatment-related variables (surgery, chemotherapy or both) were
observed.
Discussion
SNc is a rare disease that accounts for 3% of all
head and neck carcinomas (28). As
SNcs exhibit particular behaviours, such as chemo- and
radioresistance, diagnosis at an advanced stage and lack of
association with common risk factors including alcohol and tobacco,
they should be considered as separate entities and therefore should
not be included in the miscellany of head and neck carcinomas
(29). Due to the rarity of these
lesions, there is lack of consensus regarding their management, and
the majority of data are derived from retrospective analyses.
Treatment options vary according to disease extension, and surgery
is the preferred therapy in the majority of SNc cases. Recurrence
rates widely differ among patients and depend on several factors,
including histological type, stage, lymph node metastasis and
multimodal treatment strategy (12,16,30).
Historically, the median OS rate for locally
advanced SNc ranges from 25–50% in clinical trials, depending on
the aforementioned factors (31). In
the present study, a CSS rate of 33.3% was reported, which is
consistent with data in the literature.
Advanced stage at diagnosis is acknowledged to have
a strong impact on prognosis and, in particular, on the probability
of recurrence (32). In clinical
trials, it has been observed that locally advanced diseases, namely
those with intracranial extension and/or orbital apex involvement,
are characterized by a poor prognosis and shorter disease-free
survival, OS and PFS (12,23). This was confirmed by the present
study, which demonstrated that patients with stage IV disease had a
poorer outcome, in terms of CSS rate (87.5 vs. 57.5%; P=0.016) and
PFS rate (71.4 vs. 17.1%; P=0.001) compared with patients with
stage III disease.
Patients require accurate selection of treatment in
order to maximise response to therapy and survival. For example,
there is a wealth of data suggesting that the expression of certain
biomarkers, including epidermal growth factor receptor, P16 and
survivin, is associated with a more positive response to
chemoradiotherapy (33,34). The possibility to pursue a multimodal
strategy, namely surgery followed by adjuvant radio or
chemoradiotherapy, has been associated with positive outcomes in
clinical trials (35,36). The addition of surgical resection did
not impact the 2-year CSS rate in the present study, and patients
that were treated with upfront surgery followed by adjuvant radio-
or chemoradiotherapy did not demonstrate a better survival rate
compared with patients treated with exclusive radiotherapy with or
without chemotherapy. This may be explained by the fact that
surgery was non-radical in the majority of patients, with 7/11
patients undergoing an R1 resection.
SNUc, in addition to intestinal-like and squamous
cell histologies, have been reported to correlate with poor
prognosis in clinical trials (37,38).
Conversely, in the present analysis, histological type did not
affect patient prognosis; however, this may be due to the small
sample size and the low number of SNUc cases included (3/30).
Finally, the presence of lymph node metastasis,
concurrent chemotherapy administration, site of origin and tumour
grade did not impact patient prognosis in the current study;
however, this may also be due to the small sample size in the
framework of a wide heterogeneity.
In the present analysis, acute toxicity was mild and
was characterized by mucositis, xerostomia, dysphagia and in-field
skin erythema. Grade 3 toxicity was observed in 23.3% of the
patients, while only grade 1 and 2 toxicity were reported in the
remaining 76.7%. This toxicity spectrum is considered to be
uniquely linked to radiation therapy and, notably, the addition of
surgery did not exacerbate it.
Additionally, late toxicity was mild and presented
as xerostomia in 13.3% of patients, while no blindness or optic
pathway disruption was reported. This is most likely due the fact
that the dose constraints for the OARs considered were not violated
in any cases.
In conclusion, the current study was a small
retrospective analysis, but did observe a fairly positive survival
rate in a group of poor prognosis patients with locally advanced
SNc, at the cost of acute and chronic moderate toxicity. Disease
stage was the only factor identified to impact prognosis. It is
therefore considered that radical surgery should be avoided, given
the high rate of recurrence observed in patients, even if they are
subsequently treated with adjuvant radio- or chemoradiotherapy.
References
|
1
|
Mahalingappa YB and Khalil HS: Sinonasal
malignancy: Presentation and outcomes. J Laryngol Otol.
128:654–657. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dulguerov P, Jacobsen MS, Allal AS,
Lehmann W and Calcaterra T: Nasal and paranasal sinus carcinoma:
Are we making progress? A series of 220 patients and a systematic
review. Cancer. 92:3012–3029. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Edge S, Bird D and Compton C: AJCC Cancer
Staging Manual. 7th. New York: Springer; 2010
|
|
4
|
Van Eyken E: Cancer incidence in Flanders,
1997–1999VLK. Flemish Cancer Network; Brussels: 2002
|
|
5
|
Périé S, Meyers M, Mazzaschi O, De Crouy
Chanel O, Baujat B and St Guily J Lacau: Epidemiology and anatomy
of head and neck cancers. Bull Cancer. 101:404–410. 2014.(In
French). PubMed/NCBI
|
|
6
|
Katz TS, Mendenhall WM, Morris CG, Amdur
RJ, Hinerman RW and Villaret DB: Malignant tumors of the nasal
cavity and paranasal sinuses. Head Neck. 24:821–829. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Khademi B, Moradi A, Hoseini S and
Mohammadianpanah M: Malignant neoplasms of the sinonasal tract:
Report of 71 patients and literature review and analysis. Oral
Maxillofac Surg. 13:191–199. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dirix P, Nuyts S, Vanstraelen B, Nulens A,
Hermans R, Jorissen M, Poorten V Vander and Van den Bogaert W:
Post-operative intensity-modulated radiotherapy for malignancies of
the nasal cavity and paranasal sinuses. Radiother Oncol.
85:385–391. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hanna E, DeMonte F, Ibrahim S, Roberts D,
Levine N and Kupferman M: Endoscopic resection of sinonasal cancers
with and without craniotomy: Oncologic results. Arch Otolaryngol
Head Neck Surg. 135:1219–1224. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Blanco AI, Chao KS, Ozyigit G, Adli M,
Thorstad WL, Simpson JR, Spector GJ, Haughey B and Perez CA:
Carcinoma of paranasal sinuses: Long-term outcomes with
radiotherapy. Int J Radiat Oncol Biol Phys. 59:51–58. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hoffman TK: Systemic therapy strategies
for head and neck carcinomas: Current status. GMS Curr Top
Otorhinolaryngol Head Neck Surg. 11:Doc032012.PubMed/NCBI
|
|
12
|
Kazi M, Awan S, Junaid M, Qadeer S and
Hassan NH: Management of sinonasal tumors: Prognostic factors and
outcomes: A 10 year experience at a tertiary care hospital. Indian
J Otolaryngol Head Neck Surg. 65:(Suppl 1). S155–S159. 2013.
View Article : Google Scholar
|
|
13
|
Harvey RJ and Dalgorf DM: Chapter 10:
Sinonasal malignancies. Am J Rhinol Allergy. 27:(Suppl 1). S35–S38.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Smith SP, Russell JL, Chen NW, Kuo YF and
Resto VA: Sinonasal carcinoma: Racial and ethnic disparities in
survival-a review of 4,714 patients. Otolaryngol Head Neck Surg.
153:551–560. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Enepekides DJ: Sinonasal undifferentiated
carcinoma: An update. Curr Opin Otolaryngol Head Neck Surg.
13:222–225. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dirix P, Vanstraelen B, Jorissen M,
Poorten V Vander and Nuyts S: Intensity-modulated radiotherapy for
sinonasal cancer: Improved outcome compared to conventional
radiotherapy. Int J Radiat Oncol Biol Phys. 78:998–1004. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shukovsky LJ and Fletcher GH: Retinal and
optic nerve complications in a high dose irradiation technique of
ethmoid sinus and nasal cavity. Radiology. 104:629–634. 1972.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ellingwood K and Million R: Cancer of the
nasal cavity and ethmoid/sphenoid sinuses. Cancer. 43:1517–1526.
1979. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Katz TS, Mendenhall WM, Morris CG, Amdur
RJ, Hinerman RW and Villaret DB: Malignant tumors of the nasal
cavity and paranasal sinuses. Head Neck. 24:821–829. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jacobs C, Lyman G, Velez-García E, Sridhar
KS, Knight W, Hochster H, Goodnough LT, Mortimer JE, Einhorn LH,
Schacter L, et al: A phase III randomized study comparing cisplatin
and fluorouracil as single agents and in combination for advanced
squamous cell carcinoma of the head and neck. J Clin Oncol.
10:257–263. 1992.PubMed/NCBI
|
|
21
|
Okano S, Tahara M, Zenda S, Fuse N,
Yoshino T, Doi T, Kawashima M, Ogino T, Hayashi R and Ohtsu A:
Induction chemotherapy with docetaxel, cisplatin and S-1 followed
by proton beam therapy concurrent with cisplatin in patients with
T4b nasal and sinonasal malignancies. Jpn J Clin Oncol. 42:691–696.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Caponigro F, Longo F, Perri F and Ionna F:
Docetaxel in the management of head and neck cancer. Anticancer
Drugs. 20:639–645. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Burtness B, Goldwasser MA, Flood W, Mattar
B and Forastiere AA: Eastern Cooperative Oncology Group: Phase III
randomized trial of cisplatin plus placebo compared with cisplatin
plus cetuximab in metastatic/recurrent head and neck cancer: An
eastern cooperative oncology group study. J Clin Oncol.
23:8646–8654. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
National Comprehensive Cancer Network
(NCCN), . NCCN Clinical Practice Guidelines in Oncology. https://www.nccn.org/professionals/physician_gls/f_guidelines.asp#siteAccessed.
January 21–2016.
|
|
25
|
Cox JD, Stetz J and Pajak TF: Toxicity
criteria of the Radiation Therapy Oncology Group (RTOG) and the
European Organization for Research and Treatment of Cancer (EORTC).
Int J Radiat Oncol Biol Phys. 31:1341–1346. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
National Comprehensive Cancer Network
(NCCN), . Head and Neck Cancers. Version 1.2015. https://www.nccn.org/professionals/physician_gls/pdf/head-and-neck.pdfAccessed.
January 21–2016.
|
|
27
|
Sakata K, Maeda A, Rikimaru H, Ono T, Koga
N, Takeshige N, Tokutomi T, Umeno H, Kiyokawa K and Morioka M:
Advantage of extended craniofacial resection for advanced malignant
tumors of the nasal cavity and paranasal sinuses: Long-term outcome
and surgical management. World Neurosurg. 89:240–254. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ansa B, Goodman M, Ward K, Kono SA,
Owonikoko TK, Higgins K, Beitler JJ, Grist W, Wadsworth T, El-Deiry
M, et al: Paranasal sinus squamous cell carcinoma incidence and
survival based on Surveillance, Epidemiology, and End Results data,
1973 to 2009. Cancer. 119:2602–2610. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lopez F, Suárez V, Vivanco B, Suárez C and
Llorente JL: Current management of sinonasal undifferentiated
carcinoma. Rhinology. 53:212–220. 2015.PubMed/NCBI
|
|
30
|
Hoppe BS, Nelson CJ, Gomez DR, Stegman LD,
Wu AJ, Wolden SL, Pfister DG, Zelefsky MJ, Shah JP, Kraus DH and
Lee NY: Unresectable carcinoma of the paranasal sinuses: Outcomes
and toxicities. Int J Radiat Oncol Biol Phys. 72:763–769. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Budihna M and Smid L: Carcinoma of the
paranasal sinuses: Results of treatment and some prognostic
factors. Strahlenther Onkol. 168:322–327. 1992.PubMed/NCBI
|
|
32
|
Bossi P, Saba NF, Vermorken JB, Strojan P,
Pala L, de Bree R, Rodrigo JP, Lopez F, Hanna EY, Haigentz M, et
al: The role of systemic therapy in the management of sinonasal
cancer: A critical review. Cancer Treat Rev. 41:836–843. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Stasikowska-Kanicka O,
Wagrowska-Danilewicz M and Danilewicz M: Immunohistochemical study
on survivin in sinonasal tumors and its relationship with the
immunoexpression of Ki67 and Bcl-2. Folia Histochem Cytobiol.
51:225–231. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Takahashi Y, Bell D, Agarwal G, Roberts D,
Xie TX, El-Naggar A, Myers JN and Hanna EY: Comprehensive
assessment of prognostic markers for sinonasal squamous cell
carcinoma. Head Neck. 36:1094–1102. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Michel J, Fakhry N, Mancini J, Braustein
D, Moreddu E, Giovanni A and Dessi P: Sinonasal squamous cell
carcinomas: Clinical outcomes and predictive factors. Int J Oral
Maxillofac Surg. 43:1–6. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Devaraja K, Sikka K, Kumar R and Thakar A:
Sinonasal malignancies: Long term follow up after surgical
management-an analysis of outcomes. Indian J Otolaryngol Head Neck
Surg. 67:28–33. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Levine PA, Frierson HF Jr, Stewart FM,
Mills SE, Fechner RE and Cantrell RW: Sinonasal undifferentiated
carcinoma: A distinctive and highly aggressive neoplasm.
Laryngoscope. 97:905–908. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Deutsch BD, Levine PA, Stewart FM,
Frierson HF Jr and Cantrell RW: Sinonasal undifferentiated
carcinoma: A ray of hope. Otolaryngol Head Neck Surg. 108:697–700.
1993. View Article : Google Scholar : PubMed/NCBI
|