Introduction
Ovarian cancer is the leading cause of gynecological
malignancy-associated mortality. Patients diagnosed during the
early stages of the disease (stage I–II) have a more favorable
prognosis compared with patients who are diagnosed during later
stages (stage III–IV) (1,2). Advanced-stage disease, dissemination,
metastasis and resistance to chemotherapeutic drugs all contribute
to the high mortality rate (~60%) associated with ovarian cancer
(1,2).
Novel therapies with higher success rates are urgently required, in
addition to the identification of potential therapeutic targets and
prognostic markers.
Matriptase is a type II transmembrane serine
protease that facilitates cellular invasiveness, and is also
considered to activate oncogenic pathways (3). The enzyme was first identified in human
breast carcinoma (3). Matriptase is
expressed in epithelial cells (4) and
has been detected in a large proportion of human epithelium-derived
tumor tissues, including ovarian carcinoma (5), prostate cancer (6), breast cancer (3), colorectal carcinoma (7), stomach cancer (8), renal cancer (9), cervical cell carcinoma (10) and endometrial cancer (11). The protein has also been reported to
promote malignant progression in a number of animal models
(12). Matriptase is considered to
have pleiotropic functions, including modulation of tumor
cell-substratum adhesion, degradation of the extracellular matrix,
tissue remodeling, tumor growth and metastasis (3). In the current study, the expression of
matriptase was examined using immunohistochemical techniques, and
the association between matriptase expression and
clinicopathological characteristics and prognosis in patients with
ovarian serous adenocarcinoma was investigated.
Materials and methods
Patients
Ovarian serous adenocarcinoma specimens were
obtained from 80 patients during surgery at the Department of
Obstetrics and Gynecology of The First Affiliated Hospital of
Zhengzhou University (Zhengzhou, China) between February 2006 and
March 2011. Optimal surgery was attempted in all patients.
Laparoscopic comprehensive staging surgery was performed in early
stage patients, while reduction surgery was performed in later
stage patients, according to the National Comprehensive Cancer
Network guidelines (13). Patients
were not subjected to radiotherapy or chemotherapy prior to
surgery. Stage Ia/Ib and grade 1 patients received no further
treatment, whilst stage Ic, II, III or IV and grade 2/3 patients
were treated with platinum-based chemotherapy (80 kg/m2
intravenous nedaplatin, 6–8 cyles). The age of the patients ranged
from 28 to 83 years (mean, 55.78 years). All specimens were used in
the present study after obtaining written informed consent from the
patients, and ethical approval was provided by the Ethics Review
Board of Life Sciences, Zhengzhou University. According to the 2010
International Federation of Gynecology and Obstetrics (FIGO)
grading system (14), tumor
histological grade was classified as poorly-differentiated (grade
3; 11/80 patients), moderately-differentiated (grade 2; 20/80
patients) or well-differentiated (grade 1; 49/80 patients).
Surgical staging was reviewed based on the FIGO staging system as
follows: 9 patients were allocated to stage I, 20 patients to stage
II, 33 patients to stage III and 18 patients to stage IV. In the
follow-up care clinic, patients were evaluated every month in the
first 6 months, every 2 months in the subsequent 6 months, and
every 3 months in the second year. Subsequently, follow-up care was
conducted annually.
In total, 12 normal ovarian tissue specimens were
obtained from patients undergoing hysterectomies for non-ovarian
disease: 7 patients underwent oophorectomy for uterine myoma and 5
patients underwent surgery for adenomyosis. The age of the patients
ranged from 53 to 83 years (mean, 69.58 years). Written informed
consent was obtained from these patients.
Immunohistochemistry
Paraffin-embedded tissues were obtained from the
archive of the Department of Pathology, of The First Affiliated
Hospital of Zhengzhou University, and were cut to a thickness of 4
µm. The tissue sections were baked at 60°C, dewaxed in xylene and
rehydrated in a graded alcohol series following 3 washes (each for
3 min) in phosphate-buffered saline (PBS). Antigen retrieval was
performed by heating each section to 100°C for 20 min in 0.01 mol/l
sodium citrate buffer (pH 6.0). Sections were subsequently immersed
in 3% hydrogen peroxide for 10 min to suppress endogenous
peroxidase activity. Following 3 additional washes with PBS,
sections were immersed in goat serum (Gibco®; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) for 10 min and incubated
with a rabbit polyclonal anti-matriptase/suppression of
tumorigenicity 14 (ST14) antibody (catalog no. ab106842; 1:100;
Abcam, Cambridge, UK) diluted in PBS at 4°C overnight. The sections
were washed again 3 times (each for 3 min) in PBS, and incubated
with horseradish peroxidase-labeled mouse anti-rabbit polyclonal
immunoglobulin antibody (catalog no. E0433; dilution, 1:250; Dako,
Glostrup, Denmark) for 20 min at room temperature. The sections
underwent a further 3 washes (each for 3 min) in PBS and were
developed in 3,3′-diaminobenzidine (Biosynthesis Biotechnology Co.,
Ltd., Beijing, China). Following washing with distilled water, the
sections were placed into hematoxylin (ComWin Biotech Co., Ltd.,
Beijing, China) for 5 min. Lastly, the sections were dehydrated in
a graded alcohol series, cleared in xylene and resealed in natural
resin (ComWin Biotech Co., Ltd.). PBS was used as a negative
control instead of ST14 antibody. Olympus IMT-2 Microscope (Olympus
Corporation, Tokyo, Japan) was used to view the slides.
Statistical analysis
SPSS version 17.0 (SPSS, Inc., Chicago, IL, USA) was
used to perform statistical analysis. The χ2 or Fisher's
exact test was used to analyze the association between
clinicopathological parameters and matriptase expression.
Kaplan-Meier analysis was used to construct survival curves, and
the impact of matriptase expression on survival was assessed using
the log-rank test and Cox proportional hazards regression model.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Immunohistochemical analysis of
matriptase
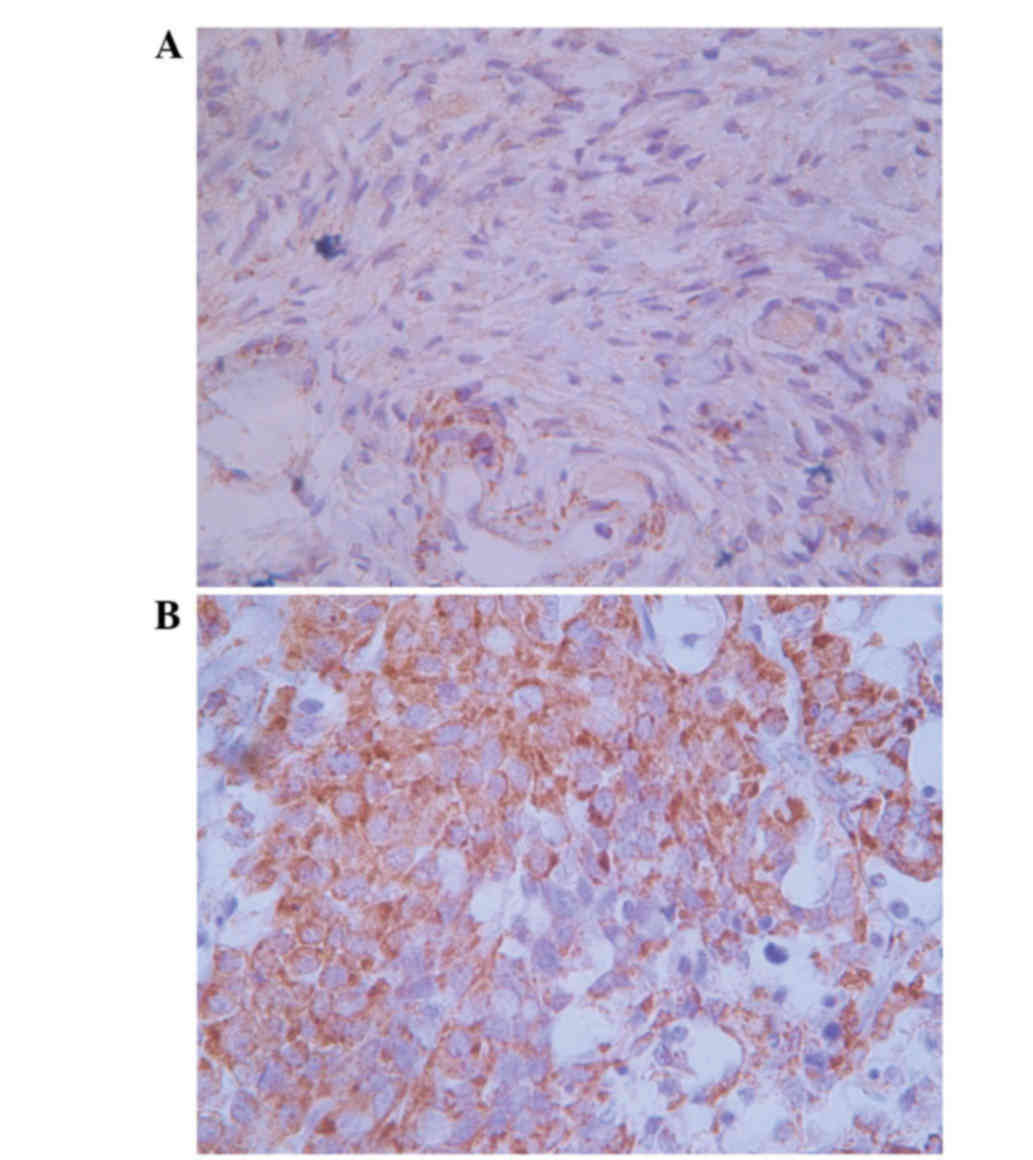
Positive matriptase staining was observed in the
cytoplasm and cell surface of the ovarian carcinoma cells, while
the normal ovarian cells exhibited negative staining (Fig. 1). Matriptase staining was observed in
45 (56.3%) out of 80 ovarian serous adenocarcinoma tissues, while
all the normal ovarian tissues demonstrated negative staining
(P=0.0003). Positive matriptase expression was significantly
associated with clinical stage (P=0.0077) and lymph node metastasis
(P=0.0111), and matriptase was detected more frequently in early
stage cases than in advanced stage cases [stage I–II, 22/29
(75.9%); stage III–IV, 23/51 (45.1%); P=0.0077]. No significant
association was observed between positive matriptase expression and
patient age, histological grade, residual tumor post-surgery or
ascites (Table I).
 | Table I.Association between matriptase and
clinicopathological parameters in ovarian serous
adenocarcinoma. |
Table I.
Association between matriptase and
clinicopathological parameters in ovarian serous
adenocarcinoma.
| Variable | Total patients,
n | Positive matriptase
expression, n (%) | P-value |
|---|
| Age, years |
|
| 0.2240 |
|
<50 | 38 | 22 (57.8) |
|
| ≥50 | 42 | 23 (54.8) |
|
| Clinical stage |
|
| 0.0077a |
| I/II | 29 | 22 (75.9) |
|
|
III/IV | 51 | 23 (45.1) |
|
| Histological
grade |
|
| 0.2595 |
| 1 | 49 | 30 (61.2) |
|
| 2/3 | 31 | 15 (48.4) |
|
| Lymph node
metastasis |
|
| 0.0111a |
| N0 | 38 | 27 (71.1) |
|
| N1 | 42 | 18 (42.9) |
|
| Residual tumor
post-surgery |
|
| 0.7636 |
| ≤1
cm | 54 | 31 (57.4) |
|
| >1
cm | 26 | 14 (53.8) |
|
| Ascites |
|
| 0.7968 |
| Yes | 33 | 18 (54.5) |
|
| No | 47 | 27 (57.4) |
|
Survival analysis
For the univariate analysis, log-rank testing
identified that advanced clinical stage (P<0.0001), high
histological grade (P<0.0001), lymph node metastasis
(P<0.0001), large residual tumor post-surgery (P<0.0001) and
negative matriptase expression (P=0.0008) were significantly
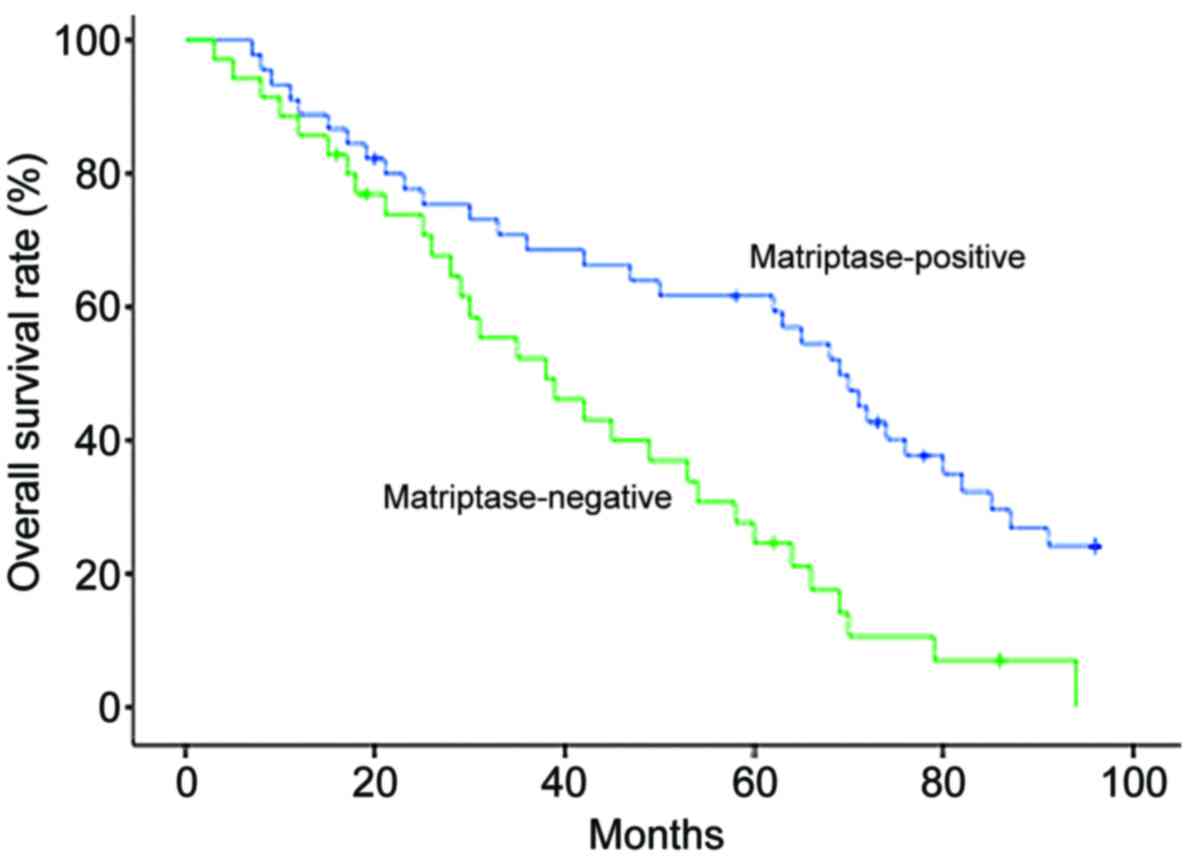
correlated with poor overall survival times (Table II). Patients with ovarian serous
adenocarcinoma were categorized along a Kaplan-Meier survival curve
according to negative vs. positive matriptase expression and a
statistically significant association was observed between negative
matriptase expression and poor overall survival (Fig. 2; P=0.0008). For the multivariate
analysis, clinical stage and lymph node metastasis were identified
as significant and independent variables affecting survival
(P<0.0001 and P=0.0005, respectively). None of the other
variables were significantly associated with overall survival time
in multivariate models (Table
III).
 | Table II.Univariate analysis of overall
survival in patients with ovarian serous adenocarcinoma. |
Table II.
Univariate analysis of overall
survival in patients with ovarian serous adenocarcinoma.
| Factor | P-value |
|---|
| Age (<50 vs. ≥50
years) | 0.5572 |
| Matriptase expression
(positive vs. negative) | 0.0008a |
| Clinical stage (I/II
vs. III/IV) |
<0.0001a |
| Histological grade (1
vs. 2/3) |
<0.0001a |
| Lymph node metastasis
(N0 vs. N1) |
<0.0001a |
| Residual tumor
post-surgery (≤1 vs. >1 cm) |
<0.0001a |
| Ascites (yes vs.
no) |
0.5354 |
 | Table III.Multivariate analysis of overall
survival in patients with ovarian serous adenocarcinoma. |
Table III.
Multivariate analysis of overall
survival in patients with ovarian serous adenocarcinoma.
| Factor | RR | 95% CI | P-value |
|---|
| Age (<50 vs. ≥50
years) | 1.149 | 0.647–2.040 | 0.6359 |
| Matriptase expression
(positive vs. negative) | 0.754 | 0.390–1.460 | 0.6994 |
| Clinical stage (I/II
vs. III/IV) | 16.962 | 7.383–38.966 |
<0.0001a |
| Histological grade (1
vs. 2/3) | 1.126 | 0.756–1.676 | 0.5607 |
| Lymph node metastasis
(N0 vs. N1) | 22.337 | 4.632–107.724 | 0.0001a |
| Residual tumor
post-surgery (≤1 vs. >1 cm) | 1.109 | 0.581–2.118 | 0.7530 |
| Ascites (yes vs.
no) | 1.125 | 0.651–1.944 | 0.6735 |
Discussion
In the present study, a significantly higher rate of
positive matriptase expression was detected by immunohistochemistry
in ovarian serous adenocarcinoma tissues compared with normal
ovarian tissue, thus indicating that matriptase may serve as a
biomarker for ovarian cancer.
It is widely known that tumor cells utilize cell
surface and extracellular proteolytic enzymes to degrade basement
membrane proteins and achieve invasion (15,16).
Proteolytic enzymes participate in various cellular activities,
including degradation of the extracellular matrix, migration, blood
coagulation, adhesion, cell growth, apoptosis and differentiation
(17). Pro-urokinase plasminogen
activator and pro-hepatocyte growth factor are substrates for
matriptase (18,19), and all serve an important role in
neoplastic progression, ranging from degradation of the
extracellular matrix and basement membrane proteins to ovarian
carcinoma cell proliferation (20).
Among the clinicopathological data examined in the
present study, the expression of matriptase was significantly more
frequent in patients with early stage ovarian cancer (stage I–II)
compared with patients with advanced stages (stage III–IV), and in
patients without lymph node metastasis compared with those with
lymph node metastasis. These results indicate that matriptase
expression primarily affects initial tumor development, and that
the level of its expression is downregulated during cancer
progression. Furthermore, it suggests that matriptase may be
involved in the development of primary ovarian neoplasia and that,
with cancer progression and invasion, its function becomes
inhibitory. The exact role of matriptase in the progression and
invasion of ovarian cancer remains unclear. A previous study
reported that an imbalance in the ratio of matriptase to its
inhibitor, hepatocyte growth factor activator inhibitor-1, may be
important in the development of tumors (20). According to Kaplan-Meier plots from
the present study, patients with positive matriptase expression
have a longer survival time compared with patients with negative
matriptase expression, however multivariate analyses have not
demonstrated similar significant results. Matriptase may not be an
independent prognostic factor for ovarian carcinoma; however, the
protease is significantly associated with early stages of the
disease and greater patient survival time.
In conclusion, the results of the present study
indicate that a high level of matriptase expression is frequent in
early stage ovarian cancer relative to that in normal ovarian
tissues. This suggests that matriptase may participate in the
formation of ovarian tumors, and may therefore serve as a marker
for early diagnosis of this disease. Further study is required to
confirm that this gene has clinical implications as an
individualized treatment strategy for ovarian cancer.
Glossary
Abbreviations
Abbreviations:
|
FIGO
|
International Federation of Gynecology
and Obstetrics
|
|
PBS
|
phosphate-buffered saline
|
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Uhland K: Matriptase and its putative role
in cancer. Cell Mol Life Sci. 63:2968–2978. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nakamura K, Hongo A, Kodama J, Abarzua F,
Nasu Y, Kumon H and Hiramatsu Y: Expression of matriptase and
clinical outcome of human endometrial cancer. Anticancer Res.
29:1685–1690. 2009.PubMed/NCBI
|
|
5
|
Tanimoto H, Underwood LJ, Wang Y,
Shigemasa K, Parmley TH and O'Brien TJ: Ovarian tumor cells express
a transmembrane serine protease: A potential candidate for early
diagnosis and therapeutic intervention. Tumour Biol. 22:104–114.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Saleem M, Adhami VM, Zhong W, Longley BJ,
Lin CY, Dickson RB, Reagan-Shaw S, Jarrard DF and Mukhtar H: A
novel biomarker for staging human prostate adenocarcinoma:
Overexpression of matriptase with concomitant loss of its
inhibitor, hepatocyte growth factor activator inhibitor-1. Cancer
Epidemiol Biomarkers Prev. 15:217–227. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Vogel LK, Saebø M, Skjelbred CF, Abell K,
Pedersen ED, Vogel U and Kure EH: The ratio of Matriptase/HAI-1
mRNA is higher in colorectal cancer adenomas and carcinomas than
corresponding tissue from control individuals. BMC Cancer.
6:1762006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zeng L, Cao J and Zhang X: Expression of
serine protease SNC19/matriptase and its inhibitor hepatocyte
growth factor activator inhibitor type 1 in normal and malignant
tissues of gastrointestinal tract. World J Gastroenterol.
11:6202–6207. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jin JS, Chen A, Hsieh DS, Yao CW, Cheng MF
and Lin YF: Expression of serine protease matriptase in renal cell
carcinoma: Correlation of tissue microarray immunohistochemical
expression analysis results with clinicopathological parameters.
Int J Surg Pathol. 14:65–72. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Santin AD, Cane' S, Bellone S, Bignotti E,
Palmieri M, De Las Casas LE, Anfossi S, Roman JJ, O'Brien T and
Pecorelli S: The novel serine protease tumor-associated
differentially expressed gene-15 (matriptase/MT-SP1) is highly
overexpressed in cervical carcinoma. Cancer. 98:1898–1904. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nakamura K, Hongo A, Kodama J, Abarzua F,
Nasu Y, Kumon H and Hiramatsu Y: Expression of matriptase and
clinical outcome of human endometrial cancer. Anticancer Res.
29:1685–1690. 2009.PubMed/NCBI
|
|
12
|
List K, Bugge TH and Szabo R: Matriptase:
Potent proteolysis on the cell surface. Mol Med. 12:1–7. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bristow RE, Chang J, Ziogas A, Campos B,
Chavez LR and Anton-Culver H: Impact of National Cancer Institute
Comprehensive Cancer Centers on ovarian cancer treatment and
survival. J Am Coll Surg. 220:940–950. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zeppernick F and Meinhold-Heerlein L: The
new FIGO staging system for ovarian, fallopian tube, and primary
peritoneal cancer. Arch Gynecol Obstet. 290:839–842. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chang C and Werb Z: The many faces of
metalloproteases: Cell growth, invasion, angiogenesis and
metastasis. Trends Cell Biol. 11:S37–S43. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Del Rosso M, Fibbi G, Pucci M, D'Alessio
S, Del Rosso A, Magnelli L and Chiarugi V: Multiple pathways of
cell invasion are regulated by multiple families of serine
proteases. Clin Exp Metastasis. 19:193–207. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lisk K, Bugge TH and Szabo R: Matriptase:
Potent proteolysis on the cell surface. Mol Med. 12:1–7. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Takeuchi T, Harris JL, Huang W, Yan KW,
Coughlin SR and Craik CS: Cellular localization of membrane-type
serine protease1 and identification of protease-activated
receptor-2 and single-chain urokinase-type plasminogen activator as
substrates. J Biol Chem. 275:26333–26342. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lee SL, Dickson RB and Lin CY: Activation
of hepatocyte growth factor and urokinase/plasminogen activator by
matriptase, an epithelial membrane serine protease. J Biol Chem.
275:36720–36725. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Oberst MD, Johnson MD, Dickson RB, Lin CY,
Singh B, Stewart M, Williams A, al-Nafussi A, Smyth JF, Gabra H and
Sellar GC: Expression of the serine protease matriptase and its
inhibitor HAI-1 in epithelial ovarian cancer: Correlation with
clinical outcome and tumor clinicopathological parameters. Clin
Cancer Res. 8:1101–1107. 2002.PubMed/NCBI
|