Introduction
Pancreatic cancer often has a poor prognosis due to
the difficulty associated with early detection. The majority of
cases of pancreatic cancer are identified at an advanced stage,
resulting in estimated 5-year survival rates of ~5% worldwide
(1). In an effort to enhance early
detection methods, the mechanisms driving pancreatic cancer
carcinogenesis are currently being investigated worldwide; however,
they require further elucidation. Pancreatic intraductal papillary
mucinous neoplasms (IPMNs) are a type of pancreatic epithelial
tumor. The incidence of IPMNs has increased due to developments in
diagnostic imaging modalities, including computer tomography and
magnetic resonance imaging. IPMNs can be classified into four
types, intestinal, gastric foveolar, oncocytic and
pancreatobiliary, based on their histology and expression of mucin
proteins. IPMN progresses from benign pancreatic intraductal
papillary mucinous adenoma (IPMA) to malignant pancreatic
intraductal papillary mucinous carcinoma (IPMC) (2,3), however,
the details of this process of carcinogenesis remain to be fully
elucidated. In the present study, the factors affecting the
carcinogenesis of IPMN were investigated.
p27 is a well-known tumor suppressor. It was first
identified as an inhibitor of cyclin-dependent kinase (CDK)
complexes in transforming growth factor β-arrested cells and was
classified as a member of the Cip/Kip family of CDK inhibitors
(CKIs) (4). CKIs associate with a
broad spectrum of cyclin-CDK complexes to negatively regulate
progression through the G1 phase of the cell cycle (5). Previously, the expression of p27 was
reported to suppress carcinogenesis in types of cystic cancer,
including ovarian tumors and pancreatic mucinous cystic tumors
(6). However, the role of p27 in the
progression of IPMN to IPMC remains to be elucidated.
Stathmin 1 (STMN1) is an important cytoplasmic
phosphoprotein, which regulates microtubule dynamics by preventing
the polymerization of tubulin and through promoting microtubule
destabilization. STMN1 is expressed at high levels in various types
of human malignancy and is referred to as oncoprotein 18; the
expression of STMN1 correlates with tumor progression and poor
prognosis in several types of cancer, including breast cancer
(7), prostate cancer (8), gastric cancer (9), hepatocellular carcinoma (10), oral squamous cell carcinoma (11), colorectal cancer (12), malignant mesothelioma (13) and urothelial carcinoma (14). These previous studies indicate that
STMN1 is a fundamental cancer-associated gene, and a
potential target for diagnosis and treatment. In our previous
study, STMN1 contributed to poor prognosis and cancer progression
in patients with extrahepatic cholangiocarcinoma (15). Additionally, there is evidence to
suggest that STMN1 regulates the function of p27, a cytoplasmic
protein shown to regulate cell migration. For example, Baldassarre
et al (16) reported that
STMN1 binds to p27 in the cytoplasm of cancer cells and enhances
their proliferation by suppressing p27 function. In our previous
study, a similar association between STMN1 and p27 was found in the
cytoplasm of extrahepatic cholangiocarcinoma cells, which resulted
in suppressed proliferation by inhibiting the intranuclear
transport of p27 (15). The present
study aimed to provide a follow up by investigating the correlation
between STMN1 and p27 in the carcinogenesis of IPMN.
In the present study, the expression of STMN1 and
cell cycle regulators, p27 and S-phase kinase-associated protein 2
(SKP2) were evaluated in tissue samples of IPMN using
immunohistochemical analyses. The aim of the present study was to
clarify the function of STMN1 in the carcinogenesis of IPMN and to
investigate the mechanisms by which STMN1 drives this process of
carcinogenesis.
Materials and methods
Patients and samples
The immunohistochemical analyses were performed on
tissue samples obtained from 27 patients with IPMNs, who had
undergone potentially curative surgery between 1995 and 2011 at
Gunma University Hospital (Maebashi, Japan). All patients signed
written informed consent forms as required by the institutional
guidelines of Gunma University Graduate School of Medicine
(Maebashi, Japan).
Immunohistochemical staining
Each 4-µm-thick tissue section was mounted on a
silane-coated glass slide, deparaffinized, and soaked for 30 min at
room temperature in 0.3% H2O2/methanol to
block endogenous peroxidases. The sections were then heated in
boiling water and Immunosaver (Nishin EM, Tokyo, Japan) at 98°C for
45 min. Nonspecific binding sites were then blocked by incubating
the slides with Protein Block Serum-Free (Dako North America, Inc.,
Carpinteria, CA, USA) for 30 min at room temperature. Subsequently,
the slides were incubated for 24 h at 4°C with the following
primary antibodies (dilution, 1:100): Anti-STMN1 antibody (catalog
no., sc-48362; Santa Cruz Biotechnology, Inc., Santa Cruz, CA,
USA); anti-p27 antibody (catalog no., sc-56454; Santa Cruz
Biotechnology, Inc.); anti-SKP2 antibody (catalog no., D3G5; Cell
Signaling Technology, Inc., Danvers, MA, USA) and anti-Ki-67
antibody (catalog no., ab833; Abcam, Cambridge, UK). The primary
antibody was visualized using the Histofine Simple Stain PO (Mouse)
kit (Nichirei, Tokyo, Japan) with a 5-min incubation time. The
3,3′-diaminobenzidine tetrahydrochloride chromogen was applied as a
0.02% solution containing 0.005% H2O2 in 50
mM ammonium acetate-citrate acid buffer (pH 6.0). The sections were
lightly counterstained with Mayer's hematoxylin and mounted.
Negative controls were established by omitting the primary
antibody. As it was previously confirmed that esophageal carcinomas
expressed STMN1, p27 and SKP2 (17),
esophageal carcinoma tissues that were preserved in our department
were used as a positive control.
The protocol for the subsequent analysis of the
immunohistochemical staining has been described in previous reports
(15,18). The intensity of the STMN1, p27 and
SKP2 staining was scored as no expression (0), weak positive
expression (1+), positive expression (2+) or strong positive
expression (3+). In addition, the number of tumor cells within each
tissue sample were counted, and the percentage of positively
stained cells was determined. All the samples were then scored as
0, 1, 3, 4, 6 or 9, according to the criteria presented in Table I. Tissue samples with scores of 0–4
were considered negative for expression, whereas tissue samples
with scores of 6–9 were considered positive for expression. Each
case was evaluated by two observers. The expression of Ki-67 was
determined as the percentage of cells with a high level of nuclear
expression in ~1,000 cells per sample, according to a previous
report (19).
 | Table I.Scoring criteria of
immunohistochemical evaluation. |
Table I.
Scoring criteria of
immunohistochemical evaluation.
|
| Positively stained
cells (%) |
|---|
|
|
|
|---|
| Stain intensity | 0 | 1–10 | 10–50 | ≥50 |
|---|
| 0 | Score 0 | Score 0 | Score 0 | Score 0 |
| 1+ | Score 0 | Score 1 | Score 2 | Score 3 |
| 2+ | Score 0 | Score 2 | Score 4 | Score 6 |
| 3+ | Score 0 | Score 3 | Score 6 | Score 9 |
Statistical analysis
The data for the continuous variables are expressed
as the mean ± standard error of the mean. The significance of
differences between the values was determined using Student's
t-test and analysis of variance. Statistical analysis of the
immunohistochemical staining was performed using the χ2
test. P<0.05 was considered to indicate a statistically
significant difference. All statistical analyses were performed
using JMP software, version 5.01 (SAS Institute, Inc., Cary, NC,
USA).
Results
Immunohistochemical staining of STMN1,
SKP2 and p27 in IPMN tissue samples
The expression of STMN1 was evaluated using
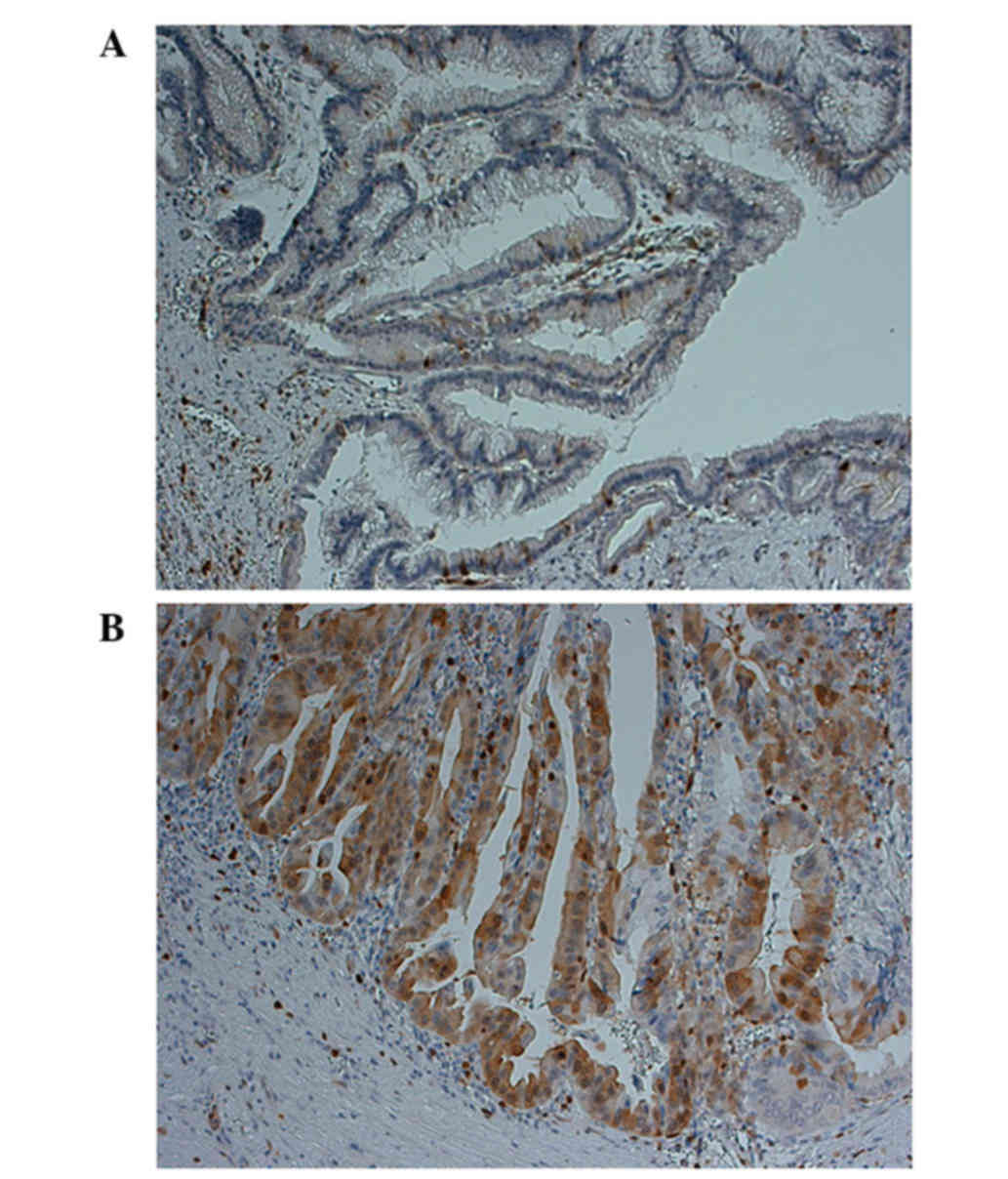
immunohistochemistry in 27 IPMN samples. Overall, 17 IPMN samples
(41.2%) were negative for the expression of STMN1 (Fig. 1A), whereas 10 samples (58.8%)
exhibited positive cytoplasmic staining for STMN1 (Fig. 1B). Although no correlations were found
between the expression of STMN1 and age, gender, cystic diameter or
presence of an intracystic nodule, the samples exhibiting
significantly high expression levels of STMN1 tended to be from
patients diagnosed with IPMC (Table
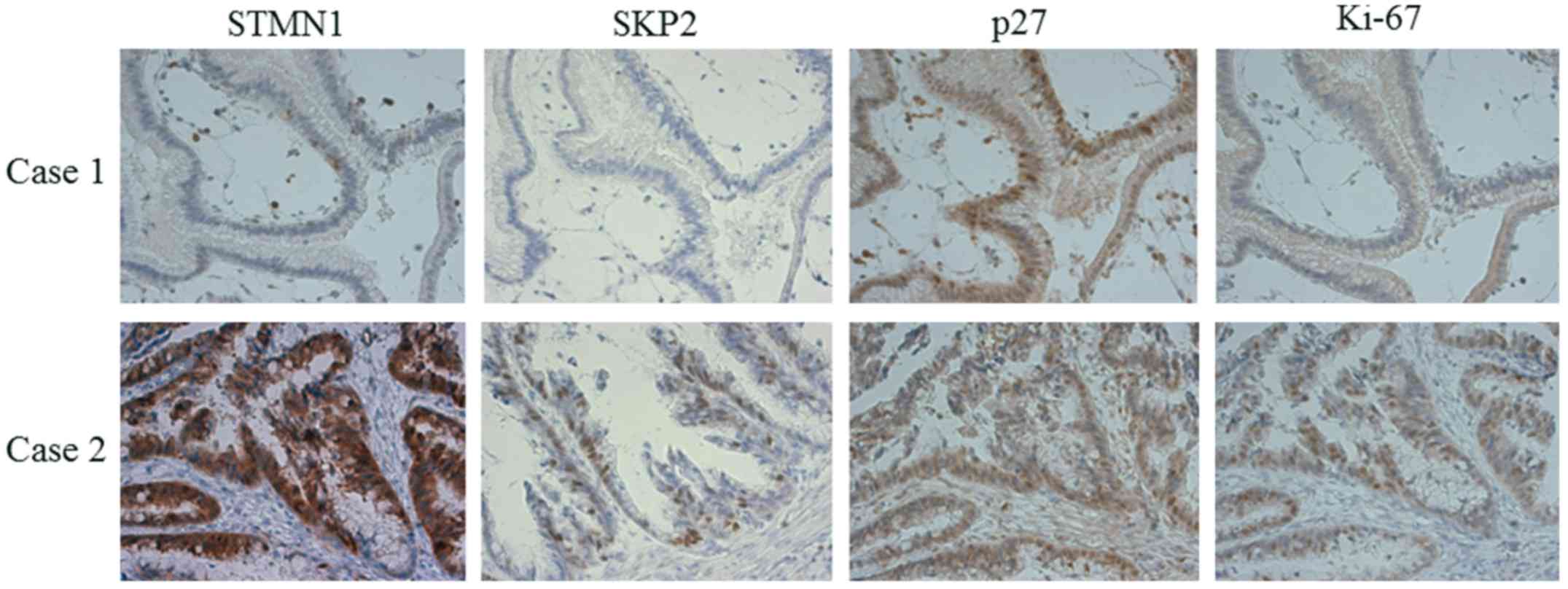
II). In the samples, high expression levels of STMN1 were also
associated with high expression levels of SKP2, low nuclear
expression levels of p27 and a high Ki-67 index (Fig. 2). No correlations were found between
the expression of STMN1 and the histological subclassification of
IPMN (Table I). Of note, the samples
with low expression levels of STMN1 and SKP2 tended to be IPMA,
whereas those with high expression levels of STMN1 and SKP2 tended
to be IPMC (Table III).
 | Table II.Correlation between expression levels
of STMN1 and clinicopathological factors using
immunohistochemistry. |
Table II.
Correlation between expression levels
of STMN1 and clinicopathological factors using
immunohistochemistry.
| Factor | STMN1 low (n=17) | STMN1 high
(n=10) | P-value |
|---|
| Age (years) |
|
| 0.4007 |
|
<60 | 6 | 2 |
|
| ≥60 | 11 | 8 |
|
| Gender |
|
| 0.2847 |
| Male | 5 | 5 |
|
|
Female | 12 | 5 |
|
| Cystic diameter
(mm) |
|
| 0.9521 |
|
<30 | 10 | 6 |
|
| ≥30 | 7 | 4 |
|
| Intracystic
nodule |
|
| 0.1588 |
| − | 13 | 5 |
|
| + | 4 | 5 |
|
| Diagnosis |
|
| 0.0004a |
| IPMA | 15 | 2 |
|
| IPMC | 2 | 8 |
|
| Histological
subclassification |
|
| 0.262 |
|
Gastric | 5 | 2 |
|
|
Intestinal | 14 | 5 |
|
|
Pancreatobiliary | 1 | 2 |
|
|
Oncocytic | 0 | 1 |
|
| SKP2 expression |
|
| 0.0286a |
| Low | 15 | 5 |
|
| High | 2 | 5 |
|
| Nuclear p27
expression |
|
|
|
| Low | 7 | 8 | 0.0499a |
| High | 10 | 2 |
|
| Ki-67 labeling index
(permille), mean±SD | 9.71±10.4 | 78.8±58.8 | 0.0002a |
 | Table III.Correlation between STMN1 and
SKP2. |
Table III.
Correlation between STMN1 and
SKP2.
|
| Expression of
STMN1/SKP2 (n) |
|
|---|
|
|
|
|
|---|
| Diagnosis | Low/low | High/low or
low/high | High/high | P-value |
|---|
| IPMA | 13 | 2 | 1 | <0.05 |
| IPMC | 1 | 5 | 5 |
|
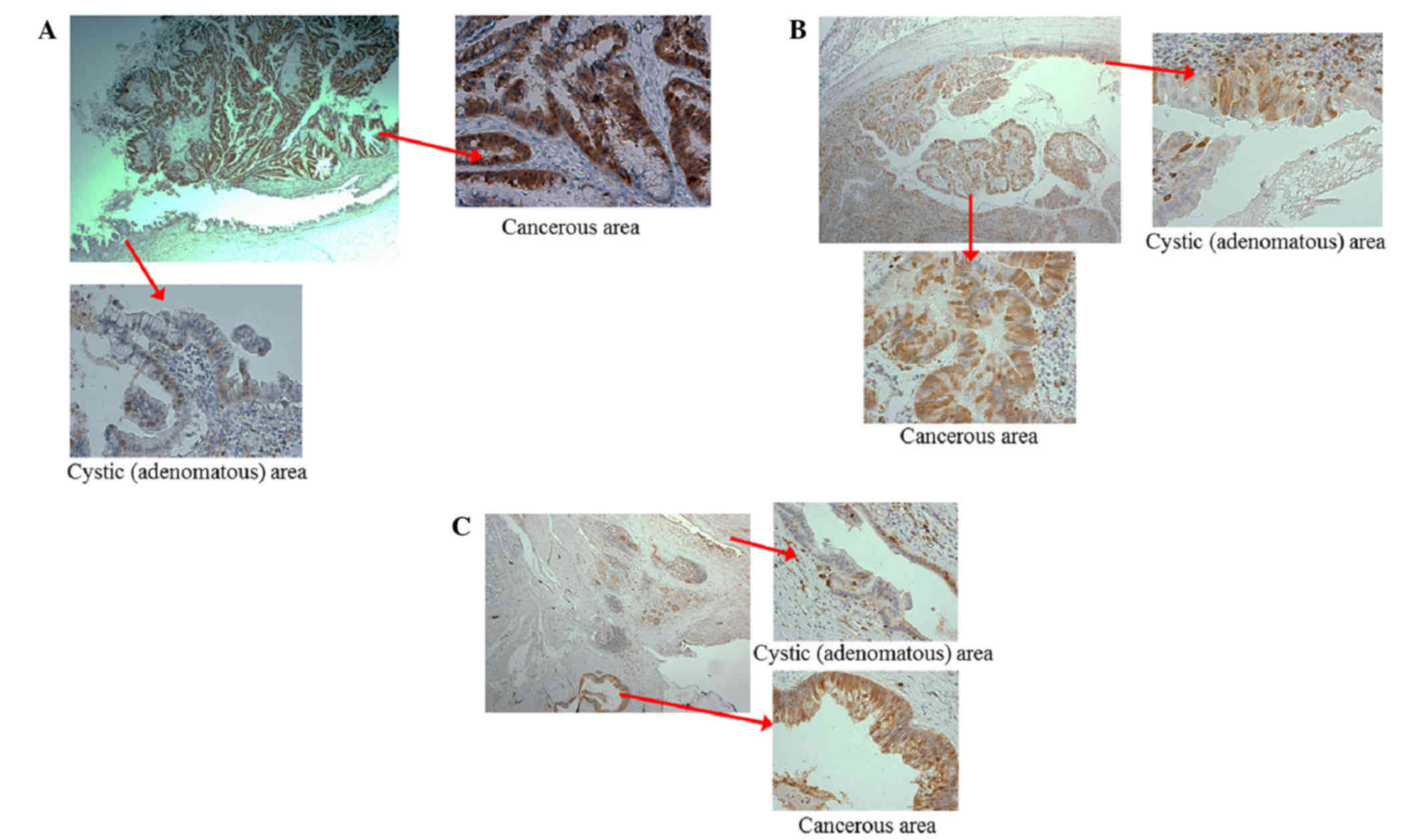
Upregulation of STMN1 in cancerous
areas of IPMN tissue samples
As the results of the immunohistochemical staining
detected an association between high expression levels of STMN1 and
IPMC, the present study evaluated the localization of STMN1 in the
IPMC cases. The images in Fig. 3A-C
show the adenomatous and cancerous areas in each of three IPMC
cases. In each case, the expression of STMN1 was weak in the
adenomatous regions and high in the cancerous regions (Fig. 3A-C).
Discussion
In the present study, it was demonstrated that high
expression levels of STMN1 were associated with tumor proliferation
and malignant transformation in IPMN tissue samples. The cases of
IPMC tended to have high expression levels of STMN1. The increased
expression of STMN1 correlated with high expression levels of SKP2,
low nuclear expression levels of p27 and a high Ki-67 index. In
addition, STMN1 was expressed at high levels in the cancerous
regions, compared with the adenomatous regions of the IPMC
samples.
Previous reports have described an association
between the expression of STMN1 and the proliferation and malignant
transformation of cancer cells. Karst et al (20) reported that STMN1 is expressed at
higher levels in tubal intraepithelial carcinoma and invasive
carcinoma, compared with normal epithelium in pelvic serous
carcinoma, and that this expression is associated with tumor
initiation. Singer et al (10)
reported that the expression of STMN1 is increased in hepatic
carcinoma, compared with normal tissue, and that the expression of
STMN1 is associated with protumorigenic events in human
hepatocarcinoganesis. Additionally, Ghosh et al (8) reported the increased expression of STMN
1 in human prostate cancer, and concluded that it contributes to
tumorigenesis. The results of the present study were in agreement
with these previous reports. In analyzing the immunohistochemical
staining of IPMN tissues, the cases of IPMC consistently
demonstrated high expression levels of STMN1. Specifically, the
expression of STMN1 was weak in adenomatous regions, but high in
cancerous regions. In addition, thee tissues with high expression
levels of STMN1 exhibited low nuclear expression levels of p27 and
higher Ki-67 indices. These results suggested that STMN1
contributed to the proliferation and malignant transformation of
IPMNs.
Previous studies have established an association
between STMN1 and p27. Baldassarre et al (16) reported that STMN1 binds to p27 in the
cytoplasm, and that sarcoma cells with high expression levels of
STMN1 and low expression levels of p27 demonstrate increased
proliferation and invasion (16). In
our previous report, a similar association was found between STMN1
and p27 in extrahepatic cholangiocarcinoma, in which samples with
high expression levels of STMN1 tended to have low expression
levels of p27. Subsequently, STMN1 was shown to interact with p27
directly in the cytoplasm of cholangiocarcinoma cells in
vitro. The knockdown of STMN1 by small interfering RNA
triggered an increase in the expression of p27 and suppressed the
proliferation of cholangiocarcinoma cells. It was demonstrated that
STMN1 interacts with p27 in the cytoplasm, inhibiting the function
of nuclear p27, thereby leading to the progression of cancer
(15). In the present study, a
similar inverse association was found between the expression of
STMN1 and the expression of nuclear p27 in the cases of IPMN, and
it was further demonstrated that these cases were associated with a
high Ki-67 index, as shown in Table
I. These data suggested that STMN1 inhibited the function of
p27 in the nucleus, upregulating proliferation and consequently
contributing to the malignant transformation of IPMNs.
SKP2 promotes the degradation of p27 via the
ubiquitin-proteasome system (21). As
indicated in Tables I and II, high expression levels of STMN1 were
significantly correlated with high expression levels of SKP2.
Additionally, low expression levels of STMN1 and SKP2 tended to be
observed in IPMA, whereas high expression levels of STMN1 and SKP2
tended to be observed in the cases of IPMC. These results suggested
that STMN1 and SKP2 functioned cooperatively in the malignant
transformation of IPMN.
At present, it is difficult to diagnose cases of
cancerous IPMN, and there is no sensitive marker to diagnose IPMC.
As pancreatectomy is a high-risk surgical procedure, diagnostic
markers are critically required to distinguish malignant
transformation in IPMN. Bhagirath et al (22) reported that serum and urine
concentrations of STMN1 were elevated in patients with bladder
cancer, compared with healthy subjects, suggesting that this may be
a viable marker for malignancy. In the present study, it was
demonstrated that STMN1 contributed to the proliferation and
malignant transformation of IPMNs. Although further investigations
are required, STMN1 has the potential to be a diagnostic marker for
cancer progression in IPMNs.
In conclusion, the present study demonstrated that
the expression of STMN1 was correlated with tumor proliferation and
malignant transformation in patients with IPMN. Therefore,
evaluating the expression of STMN1 in patients with IPMN may be a
useful predictor of malignant transformation. Additionally, it was
demonstrated that the suppression of p27 by STMN1/SKP2 was critical
for IPMN carcinogenesis. Although further investigations are
required, STMN1, SKP2 and p27 may be promising therapeutic targets
for IPMNs. As the present study indicated that STMN1 contributed to
proliferative ability and malignant transformation in IPMN,
although further investigations are required, STMN1 may offer
potential as a marker for cancer progression in IPMNs.
Acknowledgements
The authors would like to thank Ms. Yukie Saito, Ms.
Tomoko Yano, Ms. Tomoko Ubukata, Ms. Yuka Matsui, Ms. Ayaka Ishida
and Ms. Ayaka Ishikubo (Department of General Surgical Science,
Gunma University Graduate School of Medicine) for their
assistance.
References
|
1
|
Poruk KE and Weiss MJ: The current state
of surgery for pancreatic cancer. Minerva Gastroenterol Dietol.
61:101–115. 2015.PubMed/NCBI
|
|
2
|
Okabayashi T, Shima Y, Kosaki T, Sumiyoshi
T, Kozuki A, Iiyama T, Takezaki Y, Kobayashi M, Nishimori I, Ogawa
Y and Hanazaki K: Invasive carcinoma derived from branch duct-type
IPMN may be a more aggressive neoplasm than that derived from main
duct-type IPMN. Oncol Lett. 5:1819–1825. 2013.PubMed/NCBI
|
|
3
|
Nakata K, Nagai E, Ohuchida K, Aishima S,
Hayashi A, Miyasaka Y, Yu J, Mizumoto K, Tanaka M and Tsuneyoshi M:
REG4 is associated with carcinogenesis in the ‘intestinal’ pathway
of intraductal papillary mucinous neoplasms. Mod Pathol.
22:460–468. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nakayama K, Ishida N, Shirane M, Inomata
A, Inoue T, Shishido N, Horii I, Loh DY and Nakayama K: Mice
lacking p27(Kip1) display increased body size, multiple organ
hyperplasia, retinal dysplasia, and pituitary tumors. Cell.
85:707–720. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Denicourt C and Dowdy SF: Cip/Kip
proteins: More than just CDKs inhibitors. Genes Dev. 18:851–855.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Suzuki Y, Sugiyama M, Abe N, Fujioka Y and
Atomi Y: Immunohistochemical similarities between pancreatic
mucinous cystic tumor and ovarian mucinous cystic tumor. Pancreas.
36:e40–e46. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Alli E, Yang JM and Hait WN: Silencing of
stathmin induces tumor-suppressor function in breast cancer cell
lines harboring mutant p53. Oncogene. 26:1003–1012. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ghosh R, Gu G, Tillman E, Yuan J, Wang Y,
Fazli L, Rennie PS and Kasper S: Increased expression and
differential phosphorylation of stathmin may promote prostate
cancer progression. Prostate. 67:1038–1052. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jeon TY, Han ME, Lee YW, Lee YS, Kim GH,
Song GA, Hur GY, Kim JY, Kim HJ, Yoon S, et al: Overexpression of
stathmin1 in the diffuse type of gastric cancer and its roles in
proliferation and migration of gastric cancer cells. Br J Cancer.
102:710–718. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Singer S, Ehemann V, Brauckhoff A, Keith
M, Vreden S, Schirmacher P and Breuhahn K: Protumorigenic
overexpression of stathmin/Op18 by gain-of-function mutation in p53
in human hepatocarcinogenesis. Hepatology. 46:759–768. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kouzu Y, Uzawa K, Koike H, Saito K,
Nakashima D, Higo M, Endo Y, Kasamatsu A, Shiiba M, Bukawa H, et
al: Overexpression of stathmin in oral squamous-cell carcinoma:
Correlation with tumour progression and poor prognosis. Br J
Cancer. 94:717–723. 2006.PubMed/NCBI
|
|
12
|
Zheng P, Liu YX, Chen L, Liu XH, Xiao ZQ,
Zhao L, Li GQ, Zhou J, Ding YQ and Li JM: Stathmin, a new target of
PRL-3 identified by proteomic methods, plays a key role in
progression and metastasis of colorectal cancer. J Proteome Res.
9:4897–4905. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kim JY, Harvard C, You L, Xu Z,
Kuchenbecker K, Baehner R and Jablons D: Stathmin is overexpressed
in malignant mesothelioma. Anticancer Res. 27:39–44.
2007.PubMed/NCBI
|
|
14
|
Lin WC, Chen SC, Hu FC, Chueh SC, Pu YS,
Yu HJ and Huang KH: Expression of stathmin in localized upper
urinary tract urothelial carcinoma: Correlations with prognosis.
Urology. 74:1264–1269. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Watanabe A, Suzuki H, Yokobori T,
Tsukagoshi M, Altan B, Kubo N, Suzuki S, Araki K, Wada S,
Kashiwabara K, et al: Stathmin1 regulates p27 expression,
proliferation and drug resistance, resulting in poor clinical
prognosis in cholangiocarcinoma. Cancer Sci. 105:690–696. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Baldassarre G, Belletti B, Nicoloso MS,
Schiappacassi M, Vecchione A, Spessotto P, Morrione A, Canzonieri V
and Colombatti A: p27(Kip1)-stathmin interaction influences sarcoma
cell migration and invasion. Cancer Cell. 7:51–63. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Akhtar J, Wang Z, Yu C, Zhang ZP and Bi
MM: STMN-1 gene: A predictor of survival in stage iia esophageal
squamous cell carcinoma after Ivor-Lewis esophagectomy? Ann Surg
Oncol. 21:315–321. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Altan B, Yokobori T, Mochiki E, Ohno T,
Ogata K, Ogawa A, Yanai M, Kobayashi T, Luvsandagva B, Asao T and
Kuwano H: Nuclear karyopherin-α2 expression in primary lesions and
metastatic lymph nodes was associated with poor prognosis and
progression in gastric cancer. Carcinogenesis. 34:2314–2321. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Suzuki S, Miyazaki T, Tanaka N, Sakai M,
Sano A, Inose T, Sohda M, Nakajima M, Kato H and Kuwano H:
Prognostic significance of CD151 expression in esophageal squamous
cell carcinoma with aggressive cell proliferation and invasiveness.
Ann Surg Oncol. 18:888–893. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Karst AM, Levanon K, Duraisamy S, Liu JF,
Hirsch MS, Hecht JL and Drapkin R: Stathmin 1, a marker of PI3K
pathway activation and regulator of microtubule dynamics, is
expressed in early pelvic serous carcinomas. Gynecol Oncol.
123:5–12. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hao Z and Huang S: E3 ubiquitin ligase
Skp2 as an attractive target in cancer therapy. Front Biosci
(Landmark Ed). 20:474–490. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bhagirath D, Abrol N, Khan R, Sharma M,
Seth A and Sharma A: Expression of CD147, BIGH3 and Stathmin and
their potential role as diagnostic marker in patients with
urothelial carcinoma of the bladder. Clin Chim Acta. 413:1641–1646.
2012. View Article : Google Scholar : PubMed/NCBI
|