Introduction
Oncocytic tumors, including oncocytomas and
oncocytic carcinomas, are comprised of oncocytic cells. The term
‘oncocyte’ was initially used to refer to a cellular change in the
salivary glands by Hamperl in 1931 (1). Oncocytes are cells with an eosinophilic
granular and reticular cytoplasm and, in the majority of cases,
immunohistological analysis by light microscopy reveals that
>60% of the cytoplasm is occupied by mitochondria (2). Oncocytic tumors are occasionally
observed in the salivary gland, thyroid gland, kidney, parathyroid
gland and pituitary gland (3–8).
Oncocytomas and oncocytic carcinomas, which are
commonly used terms for benign and malignant tumors, respectively
(3), are comprised of oncocytic
cells. Oncocytic carcinoma of the breasts is extremely rare
(9). It was first reported by
Gădăleanu and Craciun in 1987 (10),
and 48 cases have since been reported, 15 of which were diagnosed
retrospectively (9).
Oncocytes and apocrine cells exhibit similar
appearances following hematoxylin and eosin staining. Apocrine
metaplasia is frequently observed in breast lesions. When
eosinophilic tumor cells are observed, a diagnosis of oncocytic
carcinoma should be considered, and immunohistochemical or electron
microscopic analysis is advisable (2,11,12). A previous study reported that the
clinical features of oncocytic carcinoma are similar to those of
other types of invasive carcinomas (9).
The present study reports a case of synchronous
bilateral oncocytic carcinoma of the breast, which is extremely
rare but is considered important to the pathogenesis of oncocytic
carcinoma.
Case report
A 78-year-old Japanese woman exhibited bilateral
breast tumors on chest computed tomography (CT) during surveillance
following an operation for colon cancer. The patient's medical
history included non-insulin-dependent diabetes, acromegaly,
hypertension and colon cancer. Six months previously, the patient
had been diagnosed with colon cancer, and an ileocecal resection
was performed. Adjuvant chemotherapy with 300 mg/day oral
tegafur/uracil plus 75 mg/day calcium folinate (Taiho
Pharmaceutical, Co., Ltd., Tokyo, Japan) was administered for 4
weeks (including a 1 week rest) and continued for a total of four
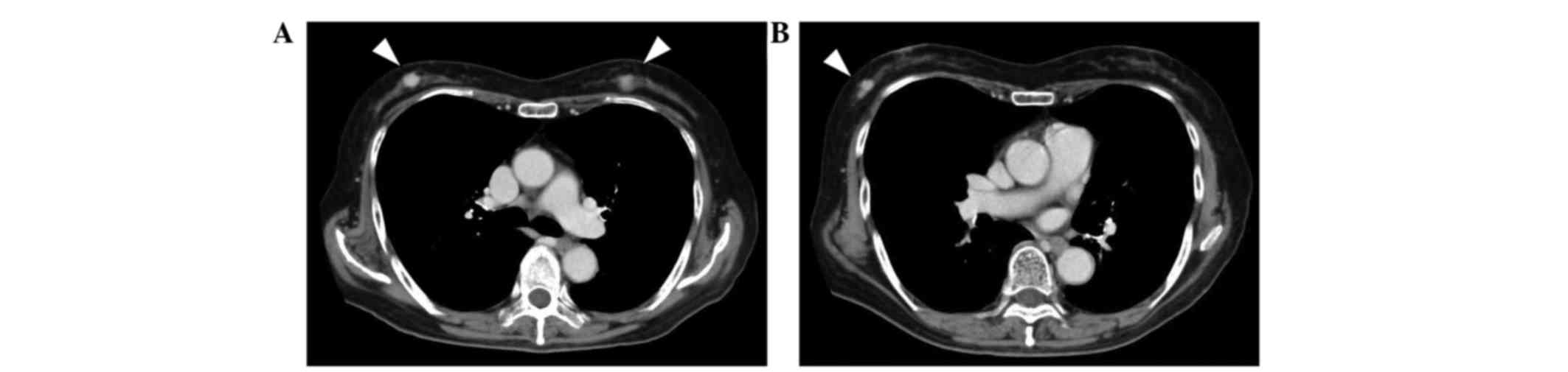
cycles. Contrast-enhanced chest CT revealed three hyperdense masses
in the breasts: Two in the right breast and one in the left
(Fig. 1). Core needle biopsies were
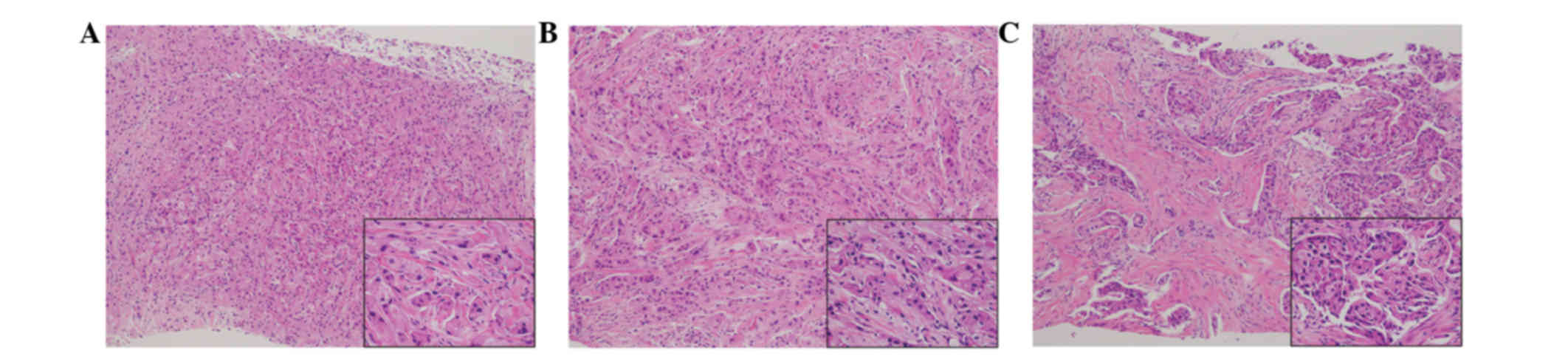
performed on the three tumors. Tumor cells with eosinophilic
cytoplasm were identified in all three lesions (Fig. 2). The tumors were diagnosed as
invasive ductal carcinoma, with potential apocrine carcinoma. As no
metastasis was detected radiologically, the patient was treated
with a bilateral modified radical mastectomy and sentinel lymph
node biopsy in a single session. Intraoperative pathological
diagnosis showed no metastasis in the sentinel nodes. The patient
did not undergo further treatment and was followed-up every 3
months by blood and imaging examinations. She remained free of
relapse and metastasis for 7 months after the surgery.
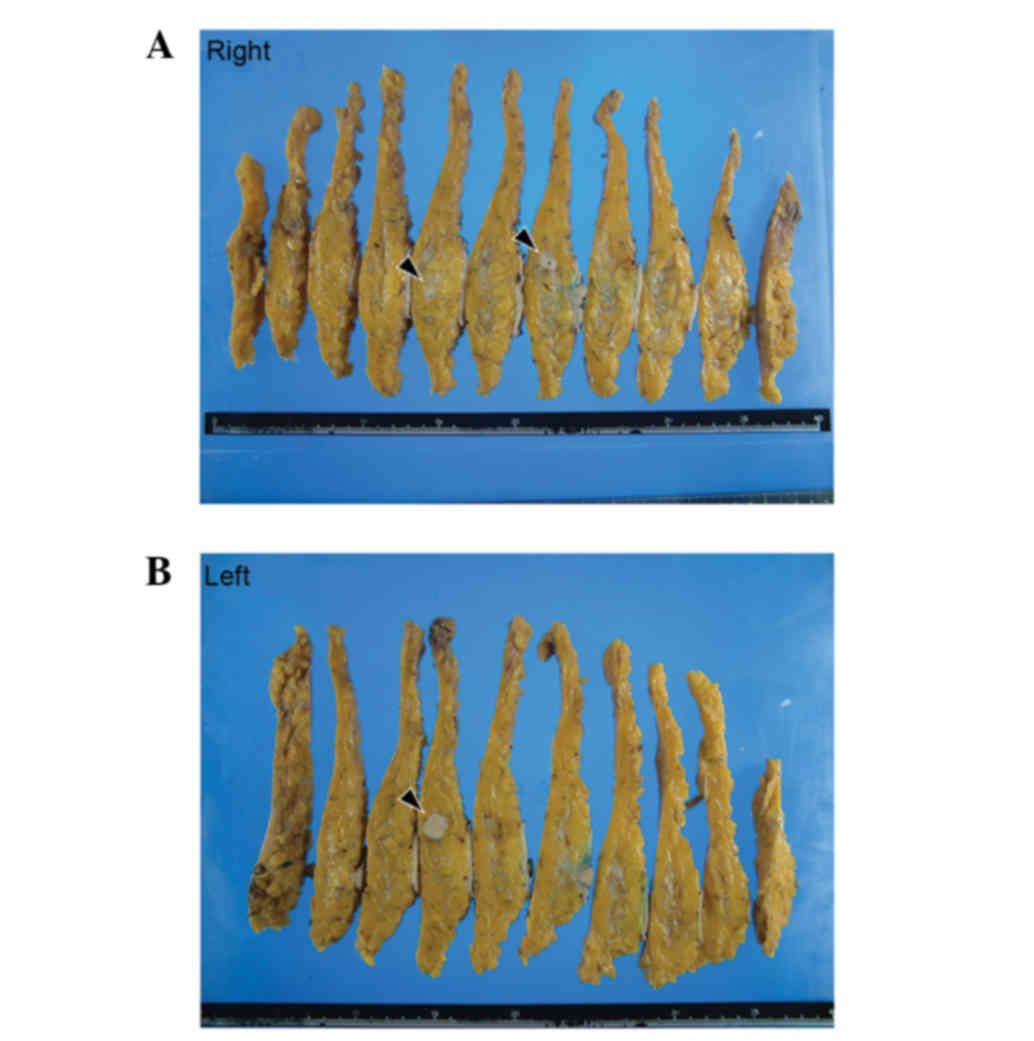
Macroscopic examination of the resected breasts
revealed three white solid masses: One in the right upper outer
quadrant (13×12×10 mm), one in the right outer quadrant (10×7×10
mm) and one in the left upper inner quadrant (17×15×12 mm)
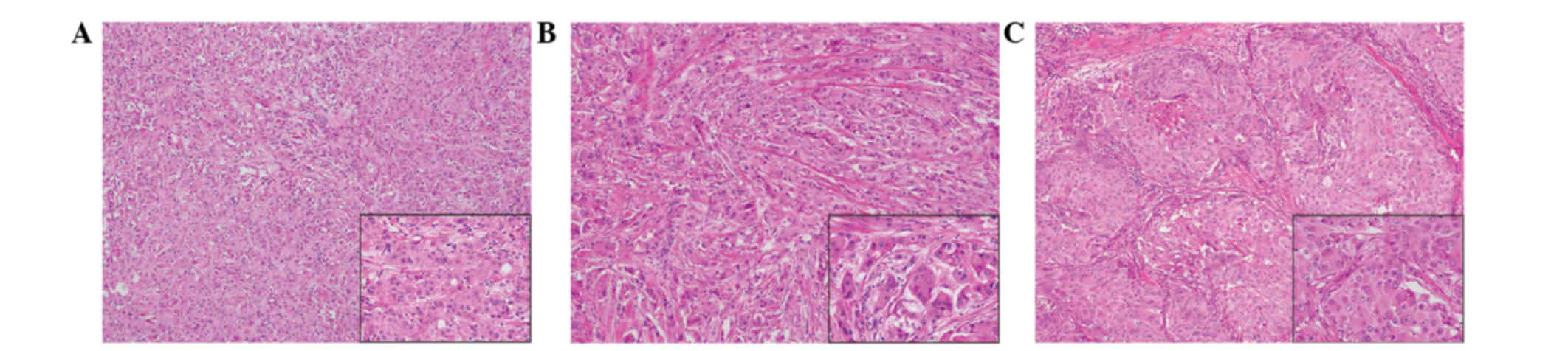
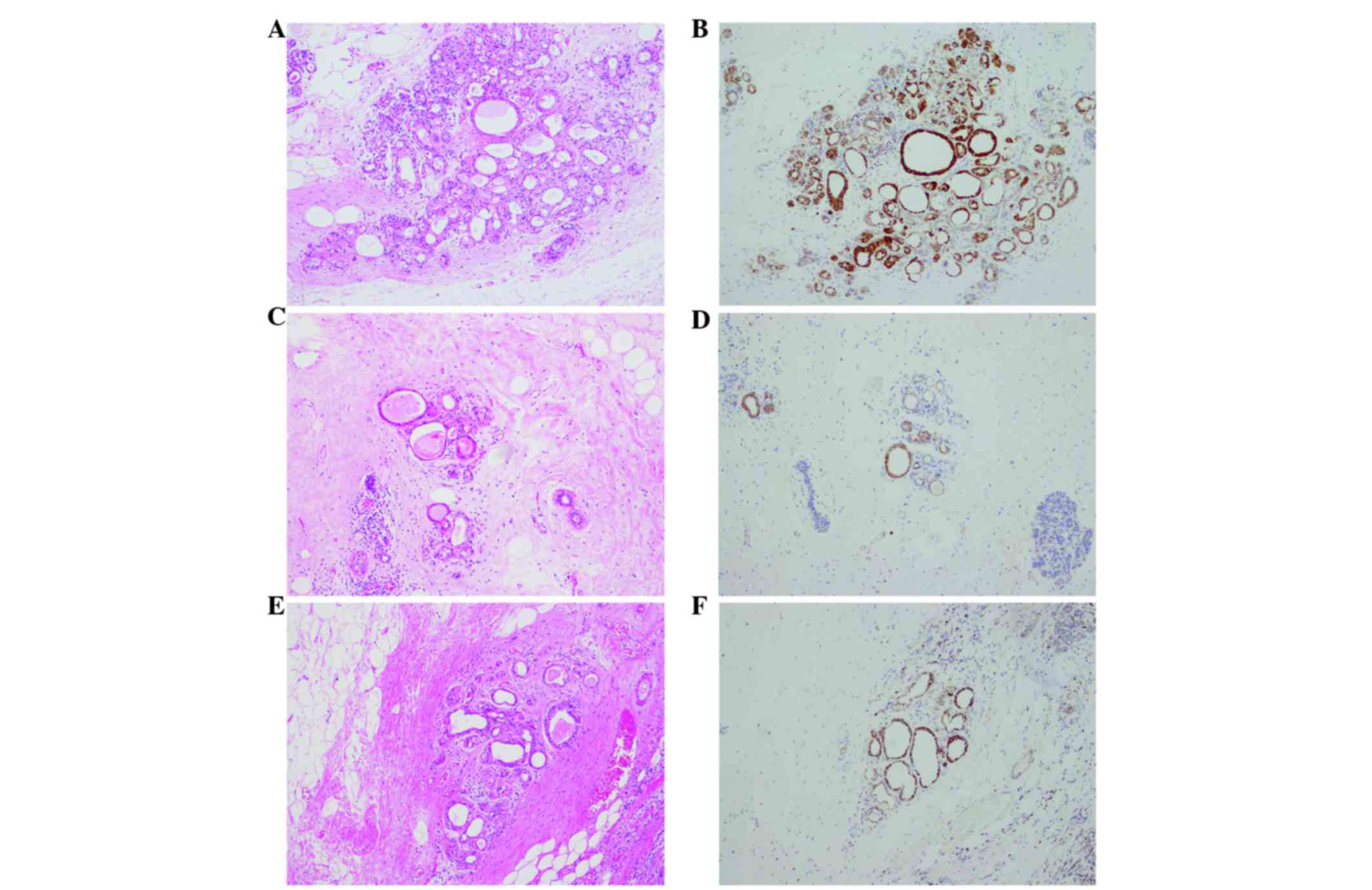
(Fig. 3). Histologically, all of the
tumors were comprised of solid cell sheets and nests of tumor
cells. The tumor cells possessed abundant eosinophilic granular
cytoplasm (Fig. 4). Invasion into the
fat was observed in the right breast.
Immunohistochemical analysis was performed using an
autostainer (Ventana Medical Systems, Inc. Tucson, AZ, USA). Breast
tissue sections (4-µm) were incubated with mouse anti-cytokeratin-7
(1:200; cat. no. M7018; Dako, Glostrup, Denmark), mouse
anti-epithelial membrane antigen (1:100; cat. no. 247M-96; Cell
Marque Corporation, Rocklin, CA, USA), mouse anti-E-cadherin
(1:100; cat. no. M3612; Dako), mouse anti-gross cystic disease
fluid protein-15 (1:50; cat. no. SIG-3611-1000; Covance, Inc.,
Princeton, NJ, USA), mouse anti-mitochondria (1:500; cat. no.
B-MU213UC; BioGenex, San Ramon, CA, USA), rabbit anti-estrogen
receptor (prediluted; cat. no. 518-107925; Roche Diagnostics,
Basel, Switzerland), rabbit anti-progesterone receptor (prediluted;
cat. no. 790-2223; Roche Diagnostics) and anti-human epidermal
growth factor receptor 2 (prediluted; cat. no. 518107918; Roche
Diagnostics) antibodies. The primary antibodies were visualized
using horseradish peroxidase-conjugated secondary antibodies and
the ultraView Universal DAB Detection kit (cat. no. 760-500;
Ventana Medical Systems, Inc.), after which the sections were
counterstained for 1 min with Carrazzi's hematoxylin solution.
Immunohistochemically, the tumor cells were positive for
cytokeratin-7, epithelial membrane antigen and E-cadherin (data not
shown). Additionally, gross cystic disease fluid protein-15 showed
a diffuse and weakly positive reaction (data not shown). Notably,
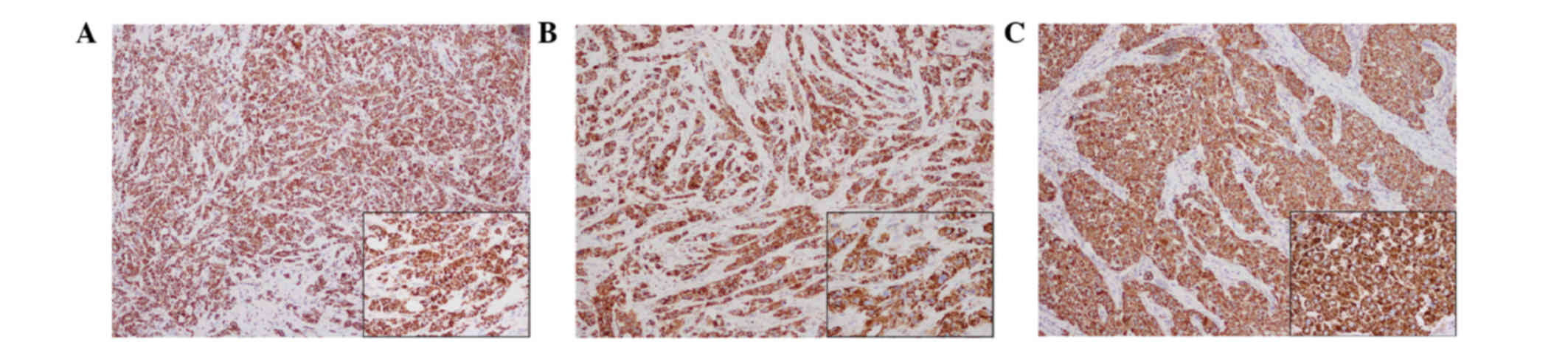
all three tumors were strongly positive for mitochondria in the
majority of the tumor cells (Fig. 5).
The peritumoral lesion contained ductal epithelia with eosinophilic
cytoplasm, which also demonstrated strong reactivity for
anti-mitochondrial antibody (Fig. 6).
The tumor cells were negative for estrogen and progesterone
receptors. Human epidermal growth factor receptor 2 was weakly
positive along the membrane and was scored 1+ using the HercepTest
(Dako), according to the manufacturer's protocol (data not shown)
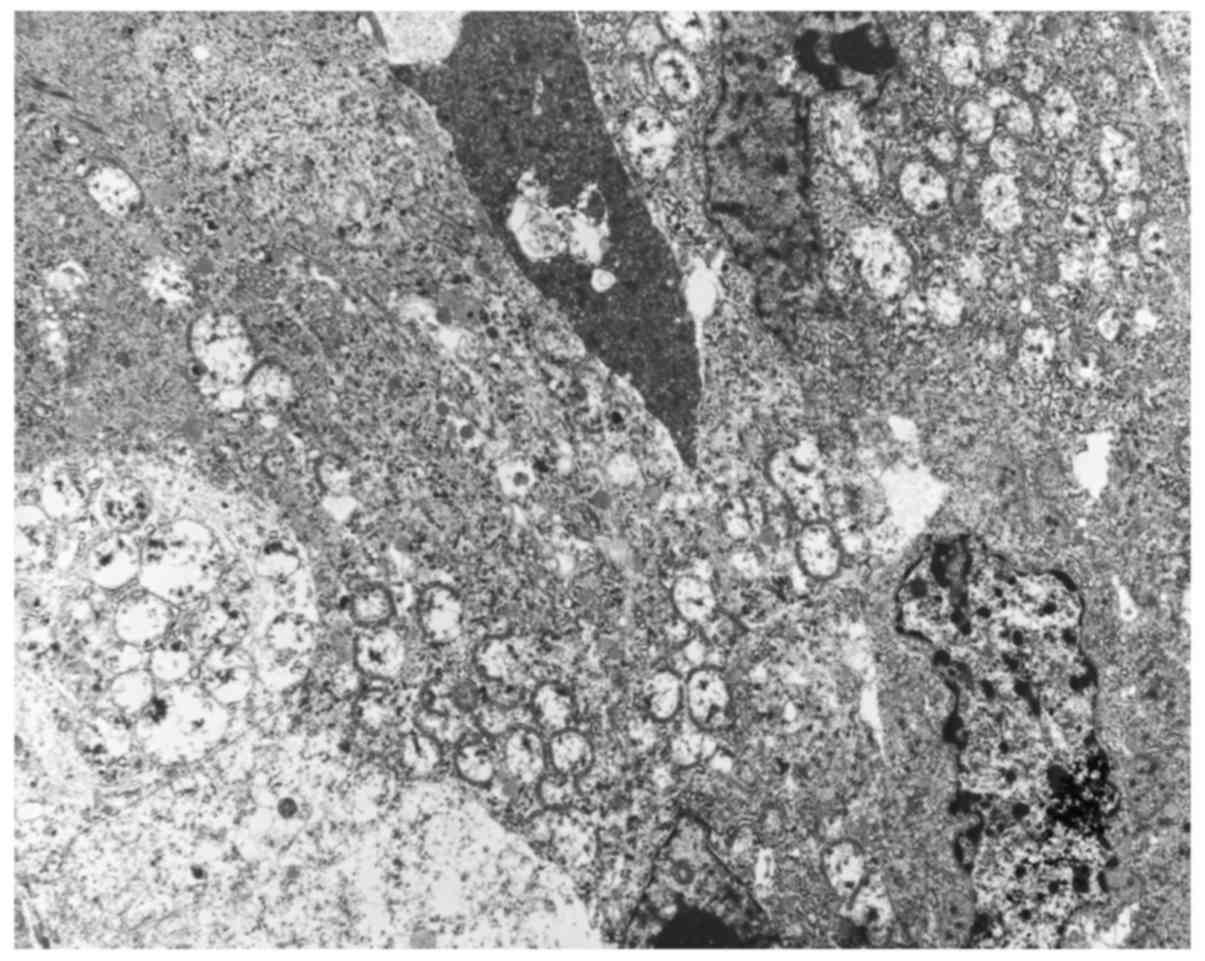
(13). Electron microscopy performed
on formalin-fixed tumor tissue revealed numerous mitochondria
(Fig. 7). Based on these findings,
the tumors were diagnosed as synchronous bilateral oncocytic
carcinoma of the breast.
Discussion
Oncocytic carcinoma of the breast is defined as a
tumor in which >70% of tumor cells demonstrate oncocytic
characteristics, and is classified as ‘uncommon’ according to the
World Health Organization classification (14). To the best of our knowledge, only six
cases of oncocytic carcinoma of the breast had been previously
reported before Ragazzi et al reviewed 32 cases in 2011
(9–11,15,16). The
tumor is characterized by oncocytic tumor cells containing numerous
mitochondria (14).
Candidates for the differential diagnosis of
oncocytic carcinoma include ductal carcinoma with partial apocrine
differentiation, acinic cell carcinoma, apocrine carcinoma,
granular cell tumors and metastatic carcinoma arising from
eosinophilic granular cytoplasm (17,18). On
routine hematoxylin and eosin staining of specimens, it is
difficult to recognize eosinophilic cells as oncocytes (19). Immunohistochemical or electron
microscopic analyses are required to distinguish these neoplastic
cells (3,9). Apocrine metaplasia is frequently
observed in breast lesions (20). The
cells of apocrine metaplasia typically exhibit abundant
eosinophilic cytoplasm containing brightly eosinophilic granules,
and secretory ‘snouts’ are typically observed by light microscopy
(20). Ultrastructurally, the
cytoplasm of granular cell tumors is packed with numerous
lysosomes, and apocrine cells contain abundant granules surrounding
the nuclei (12,19). Oncocytic carcinoma is characterized by
strong immunopositivity for mitochondria, and numerous mitochondria
fill the cytoplasm when examined by electron microscopy (2). The present study reported a case
involving a patient with synchronous and bilateral oncocytic
carcinoma. In the present case, immunohistochemistry for
mitochondria revealed markedly positive signals in the cytoplasm,
and electron microscopy demonstrated large numbers of mitochondria.
These findings supported the diagnosis of oncocytic carcinoma of
the breast.
Oncocytic cells are occasionally observed in
glandular epithelia that have high metabolic activity, including
that in the salivary glands, thyroid gland, kidney, parathyroid
gland and pituitary gland (3–8). However, the mechanism of oncocytic
change remains to be fully elucidated; it has been reported that
oncocytic change may be a type of senescent change. Previous
studies have noted that increased oncocytic changes in the salivary
glands and liver are associated with senescence and the
administration of certain drugs (21–23).
Frequent mitochondrial DNA abnormalities have been observed in
oncocytic lesions in the thyroid gland, kidney, salivary glands,
adrenal cortex and parathyroid gland (6,24). A small
number of cases in the thyroid have exhibited mutations in nuclear
DNA genes encoding oxidative phosphorylation proteins (24). Geyer et al (25) reported chromosomal changes in
oncocytic carcinoma of the breast. Oncocytic carcinoma of the
breast frequently exhibits gains of 11q13.1–13.2 and 19p13, similar
to oncocytic tumors of the kidney and thyroid. In the present case,
non-neoplastic duct epithelia adjacent to the tumor demonstrated
oncocytic changes. Although gene mutations were not analyzed in the
present case, the oncocytic carcinoma of the breast may have been
derived from ducts with oncocytic changes.
A review of the literature reveals that the clinical
features of oncocytic carcinomas are similar to those of invasive
ductal carcinoma, not otherwise specified (9). Thus, the therapeutic strategies are
identical. However, to the best of our knowledge, no previous
reports have described the use and effects of radiation therapy for
the treatment of oncocytic carcinoma of the breast, due to the
rarity of this tumor. Resistance to radiotherapy has been reported
in oncocytic tumors at other sites, including the rectum and
meninges (26,27). Thus, certain modifications may be
required to utilize radiotherapy in the treatment of oncocytic
carcinoma of the breast.
Bilateral breast cancer is uncommon and represents
2–6% of all breast carcinomas (28).
Synchronous carcinoma is defined as a second cancer diagnosed
within 3 months of the diagnosis of a first cancer (28). Although both synchronous breast cancer
and oncocytic carcinoma of the breast are uncommon, synchronous
bilateral oncocytic carcinoma of the breast is extremely rare
(9,29,30).
Thorough follow-up is important, and additional clinicopathological
studies are required to analyze effective treatments for oncocytic
carcinoma.
In conclusion, the present study demonstrated that
it is difficult to diagnose oncocytic carcinoma of the breast
merely by light microscopy. To distinguish breast neoplasms
composed of tumor cells with abundant eosinophilic cytoplasms from
oncocytic carcinoma, immunohistochemical or electron microscopy
analyses should be performed.
References
|
1
|
Hamperl H: Beiträge zur normalen und
pathologischen Histologie menschlieher Speicheldriisen. Z Mikrosk
Anat Forsch. 27:1–55. 1931.(In German).
|
|
2
|
Ghadially FN: Diagnostic electron
microscopy of tumours. 2nd. Butterworth & Company; London:
1985, View Article : Google Scholar
|
|
3
|
Tallini G: Oncocytic tumours. Virchows
Arch. 433:5–12. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kim YL, Jang YW, Kim JT, Sung SA, Lee TS,
Lee WM and Kim HJ: A rare case of primary hyperparathyroidism
associated with primary aldosteronism, Hürthle cell thyroid cancer
and meningioma. J Korean Med Sci. 27:560–564. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hoang MP, Ayala AG and Albores-Saavedra J:
Oncocytic adrenocortical carcinoma: A morphologic,
immunohistochemical and ultrastructural study of four cases. Mod
Pathol. 15:973–978. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Máximo V, Rios E and Sobrinho-Simões M:
Oncocytic lesions of the thyroid, kidney, salivary glands, adrenal
cortex, and parathyroid glands. Int J Surg Pathol. 22:33–36. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yamakita N, Ikeda T, Murai T, Kimura M,
Komaki T, Miura K, Iwamura M, Hirata T and Umezaki:
Panhypopituitarism due to Rathke's cleft cyst associated with
pituitary oncocytoma. Intern Med. 36:107–112. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chakrabarti I, Basu A and Ghosh N:
Oncocytic lesion of parotid gland: A dilemma for cytopathologists.
J Cytol. 29:80–82. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ragazzi M, de Biase D, Betts CM, Farnedi
A, Ramadan SS, Tallini G, Reis-Filho JS and Eusebi V: Oncocytic
carcinoma of the breast: Frequency, morphology and follow-up. Hum
Pathol. 42:166–175. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gădăleanu V and Craciun C: Malignant
oncocytoma of the breast. Zentralbl Allg Pathol. 133:279–283.
1987.PubMed/NCBI
|
|
11
|
Damiani S, Eusebi V, Losi L, D'Adda T and
Rosai J: Oncocytic carcinoma (malignant oncocytoma) of the breast.
Am J Surg Pathol. 22:221–230. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hashimoto K, Gross BG and Lever WF:
Electron microscopic study of apocrine secretion. J Invest
Dermatol. 46:378–390. 1966. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wolff AC, Hammond ME, Schwartz JN, Hagerty
KL, Allred DC, Cote RJ, Dowsett M, Fitzgibbons PL, Hanna WM, Langer
A, et al: American Society of Clinical Oncology; College of
American Pathologists: American Society of Clinical
Oncology/College of American Pathologists guideline recommendations
for human epidermal growth factor receptor 2 testing in breast
cancer. J Clin Oncol. 25:118–145. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lakhani SR, Ellis IO, Schnitt SJ, Tan PH
and van de Vijver MJ: WHO Classification of Tumours of the Breast.
4th. IARC Press; Lyon: 2012
|
|
15
|
Hamperl H: Oncocytes and hyaline
inclusions in the human breast. Virchows Arch B Cell Pathol.
10:88–92. 1972.(In German). PubMed/NCBI
|
|
16
|
Costa MJ and Silverberg SG: Oncocytic
carcinoma of the male breast. Arch Pathol Lab Med. 113:1396–1399.
1989.PubMed/NCBI
|
|
17
|
Yamazaki M, Nagata Y, Monji S, Shigematsu
Y, Baba T, Shimokawa H, Uramoto H, Yamada S, Hanagiri T and Tanaka
F: Apocrine carcinoma of the breast. J UOEH. 33:293–301.
2011.PubMed/NCBI
|
|
18
|
Coyne JD and Dervan PA: Primary acinic
cell carcinoma of the breast. J Clin Pathol. 55:545–547. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chang A and Harawi SJ: Oncocytes,
oncocytosis, and oncocytic tumors. Pathol Annu. 27:263–304.
1992.PubMed/NCBI
|
|
20
|
Durham JR and Fechner RE: The histologic
spectrum of apocrine lesions of the breast. Am J Clin Pathol.
113(5): Suppl 1. S3–S18. 2000.PubMed/NCBI
|
|
21
|
Mete O and Asa SL: Oncocytes, oxyphils,
Hürthle, and Askanazy cells: Morphological and molecular features
of oncocytic thyroid nodules. Endocr Pathol. 21:16–24. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Baris O, Savagner F, Nasser V, Loriod B,
Granjeaud S, Guyetant S, Franc B, Rodien P, Rohmer V, Bertucci F,
et al: Transcriptional profiling reveals coordinated up-regulation
of oxidative metabolism genes in thyroid oncocytic tumors. J Clin
Endocrinol Metab. 89:994–1005. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Martinez-Madrigal F and Micheau C:
Histology of the major salivary glands. Am J Surg Pathol.
13:879–899. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Máximo V, Botelho T, Capela J, Soares P,
Lima J, Taveira A, Amaro T, Barbosa AP, Preto A, Harach HR, et al:
Somatic and germline mutation in GRIM-19, a dual function gene
involved in mitochondrial metabolism and cell death, is linked to
mitochondrion-rich (Hurthle cell) tumours of the thyroid. Br J
Cancer. 92:1892–1898. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Geyer FC, de Biase D, Lambros MBK, Ragazzi
M, Lopez-Garcia MA, Natrajan R, Mackay A, Kurelac I, Gasparre G,
Ashworth A, et al: Genomic profiling of mitochondrion-rich breast
carcinoma: Chromosomal changes may be relevant for mitochondria
accumulation and tumour biology. Breast Cancer Res Treat.
132:15–28. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Marucci G, Betts CM, Frank G and Foschini
MP: Oncocytic meningioma: Report of a case with progression after
radiosurgery. Int J Surg Pathol. 15:77–81. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ambrosini-Spaltro A, Salvi F, Betts CM,
Frezza GP, Piemontese A, Del Prete P, Baldoni C, Foschini MP and
Viale G: Oncocytic modifications in rectal adenocarcinomas after
radio and chemotherapy. Virchows Arch. 448:442–448. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hartman M, Czene K, Reilly M, Adolfsson J,
Bergh J, Adami HO, Dickman PW and Hall P: Incidence and prognosis
of synchronous and metachronous bilateral breast cancer. J Clin
Oncol. 25:4210–4216. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chandrika Permi HS, Prasad HL Kishan,
Mohan R, Shetty KJ and Patil C: Synchronous bilateral medullary
carcinoma of breast: Is it metastasis or second primary? J Cancer
Res Ther. 8:129–131. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Adami HO, Hansen J, Jung B, Lindgren A and
Rimsten A: Bilateral carcinoma of the breast. Epidemiology and
histopathology. Acta Radiol Oncol. 20:305–309. 1981. View Article : Google Scholar : PubMed/NCBI
|