Introduction
Cervical cancer is the twelfth most common
malignancy in women in the USA, with 12,340 cases annually. The
cancer is the second leading cause of cancer-associated mortality
in women aged 20–39 years, and 4,030 patients succumb to the
disease every year (1). Cervical
cancer is also the third most common cancer worldwide, with an
annual incidence of 530,000 cases and 250,000 mortalities expected
in 2011 (2,3). Endometrial carcinoma is the most common
malignancy of the female genital tract in the United States, with
an estimated incidence of 49,560 cases and 8,190 mortalities
annually (1). Ovarian cancer is the
fifth most common cancer in women, with an estimated 22,240 new
cases annually, resulting in 14,030 mortalities, as it often
presents with widespread metastasis (1). Ovarian cancer often spreads
loco-regionally in the abdomen and distant metastasis are
infrequently observed (4). Amongst
these abdominal diseases, brain metastases from malignancies of the
female genital tract have rarely been reported, with an incidence
of only 0.4–1.2% in metastatic cervical cancer patients (5–7), 0.3–0.9%
in the majority of metastatic endometrial cancer cases (4,8) and <2%
in metastatic ovarian cancer cases (9,10). Despite
decades of studies on the management of brain metastases from lung,
renal and gastrointestinal cancer, melanoma and other cancers,
there is hardly any available literature on brain metastases from
gynecological malignancies. Furthermore, other than results from
case series, there are few guidelines on how to manage these
patients.
There have been significant advances in the systemic
management of metastatic cervical, endometrial and ovarian cancer
(11–13). This has been purported to increase
recognition of unusual metastatic sites such as the brain (14–17). These
advances have also led to prolonged survival in such patients,
meaning that quality of life, including neurocognitive effects, is
an important consideration. In consideration of these facts, the
present study reports the cases of a series of patients with brain
metastasis who were treated in Beth Israel Deaconess Medical Center
(Harvard Medical School, Boston, MA, USA) with stereotactic
radiosurgery (SRS).
Patients and methods
Patients
From a database of >1,045 patients with brain
metastases who were treated with SRS in Beth Israel Deaconess
Medical Center between January 2006 and February 2013, 8 patients
with 20 lesions treated by SRS for brain metastasis from
gynecological malignancies were identified. The medical records,
including radiology and pathology records, were reviewed
retrospectively for the study. The present study was approved by
the Dana-Farber/Harvard Cancer Center Institutional Review
Board.
Examination and treatment of
patients
All patients were reviewed in the multidisciplinary
brain tumor clinic and underwent diagnostic gadolinium-enhanced
magnetic resonance imaging (MRI; GE Healthcare Bio-Sciences,
Pittsburgh, PA, USA). The Cyberknife™ robotic SRS system (version
8.5, Accuray, Inc., Sunnyvale, CA, USA) was used to treat these
patients. Prior to treatment, the patients were simulated with
aquaplast (QFix, Avondale, PA, USA) mask immobilization and
contrast enhanced computed tomography (CT; Toshiba American Medical
Systems, Glen Mills, PA, USA) of 1-mm thickness were performed. CT
MRI fusion was obtained using Multiplan™ (version 3.5; Accuray,
Inc.) and the treating radiation oncologist and neurosurgeon
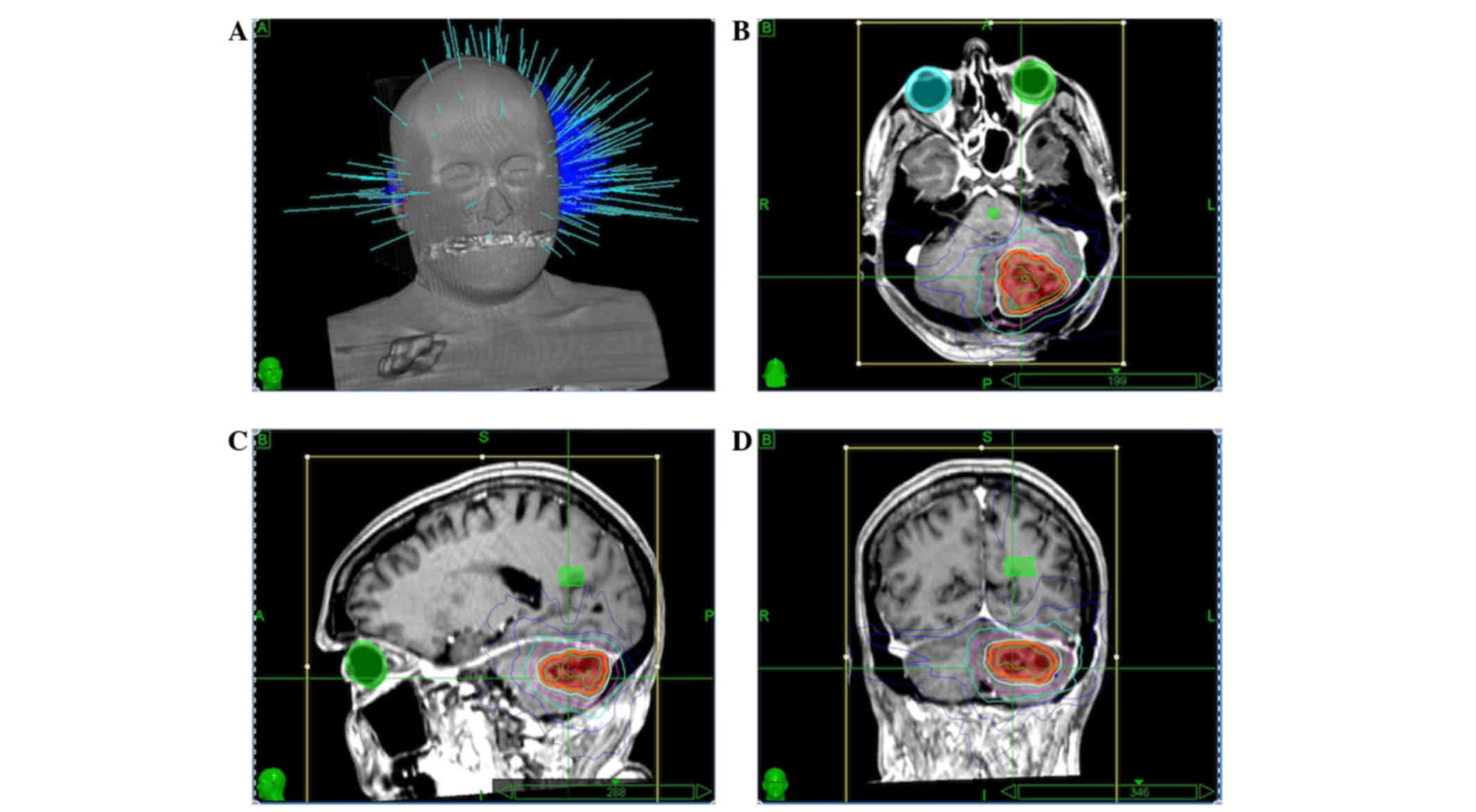
delineated all target volumes. A representative treatment plan is
shown in Fig. 1.
The enhancing tumor or resection cavity was
identified as the target volume. A total 2-mm expansion for the
planning target volume was awarded to resection cavities. Doses of
16, 18 or 22 Gy were prescribed to lesions measuring >3, 2–3 and
<2 cm, respectively. Lesions >5 cm or resection cavities
received fractions of 8 Gy 3 times, meaning a total of 24 Gy
delivered on 3 consecutive days. Similarly, patients who had
received prior SRS were re-irradiated with 5 Gy 5 times, meaning a
total of 25 Gy. The prescription isodose line that covered at least
95% of the target volume was chosen. All patients were
pre-medicated with 4 mg dexamethasone twice a day, starting on the
day of treatment, which was tapered off after treatment by 2 mg
every 3 days, and patients with supratentorial lesions received
seizure prophylaxis with leviteractam at a dose of 1,000 mg twice a
day for 1 week from the day of treatment.
Follow-up
All patients underwent neurological and radiological
follow-up at 1 month post-treatment and every 2 months thereafter.
Gadolinium-enhanced MRI was performed at these visits. Each patient
also maintained follow-up with their medical oncologists.
Results
Between January 2006 and February 2013, 8 patients
with 20 lesions were treated with SRS for single or oligo brain
metastases. The mean age was 61 years (range, 41–78 years) and the
median Karnofsky Performance Status score was 70. There were no
metastatic cervical cancer patients in this cohort. While 1 patient
presented with metastatic endometrial cancer, the remainder
presented with metastatic ovarian cancer. Other potential, more
common, primary sites (including co-existing lung or breast cancer)
were ruled out by full staging scans. All patients had previously
received systemic therapy and 1 patient had undergone whole brain
radiation therapy (WBRT) 5 years earlier. While there were 11
treatment sessions for solitary lesions, 2 patients had 2 lesions
each treated in 1 session and 1 patient had 5 lesions treated in 1
session. A total of 3 patients underwent a surgical resection and 1
patient was provided with re-irradiation twice. A total of 11
lesions were infratentorial, including 2 in the cerebellopontine
angle.
Local recurrence occurred and was re-irradiated
twice in the cerebellum of the same patient, which eventually
resulted in local control at the last follow-up. There were no
other local failures. A total of 6 patients otherwise demonstrated
treatment failure by exhibiting metastatic disease in the rest of
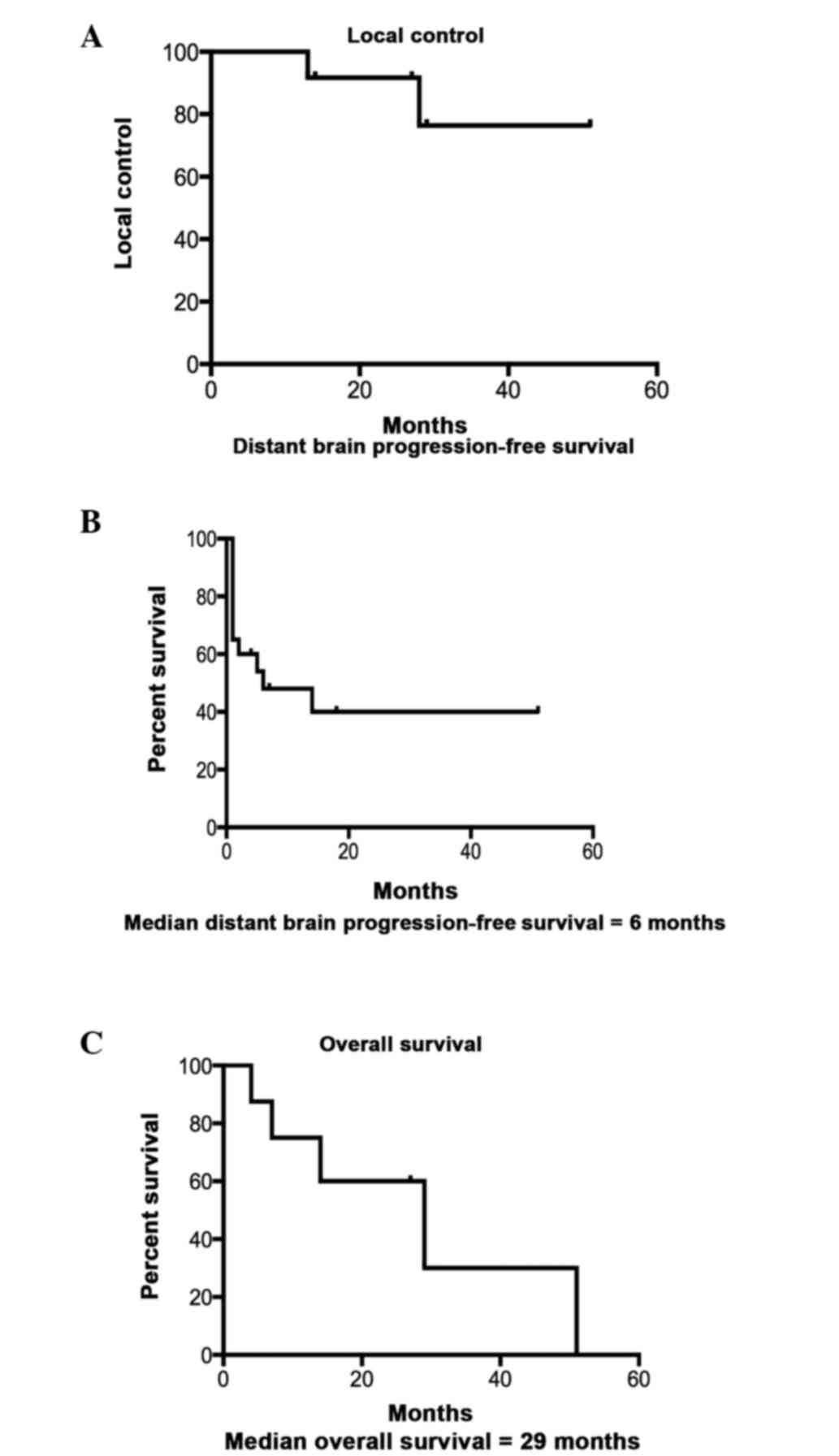
the brain and therefore required repeat SRS. The 1-, 2- and 3-year
actuarial local control rate was 91, 76 and 76%, respectively.
There were 6 distant failures in 4 patients (2 patients
demonstrated failures in the distant non-treated brain twice);
salvage WBRT was administered to 1 patient for leptomeningeal
failure, posterior fossa radiotherapy was administered to 1 patient
and the other patients received further SRS. The median distant
brain progression-free survival time was 6 months. The median
overall survival time in this population was 29 months. The
Kaplan-Meier survival curves for local control, distant brain
progression-free survival and overall survival are shown in
Fig. 2.
Discussion
Brain metastases from gynecological cancers,
particularly endometrial and ovarian cancers, are extremely rare
(4,18), hence, there are no strong guidelines
for their management (19,20). Local therapy with SRS for smaller
lesions and surgery for symptomatic space occupying lesions, with
or without WBRT, has been the standard treatment. The management of
oligometastatic brain disease in general is controversial and there
is an increasing trend to use SRS in this setting. This is
partially driven by worries of neurocognitive effects following
WBRT (21). By contrast, in patients
with widespread metastasis or leptomeningeal disease, WBRT remains
the standard of care. In the present study, it was shown that
patients with brain metastases from gynecological cancers,
particularly ovarian cancers, can live a long time and that
WBRT-sparing therapies such as SRS may be appropriate. SRS provides
excellent local control and can be successfully used for salvage
therapy, with limited recurrences.
With successful systemic therapy, patients with
gynecological cancers may live for a long period of time (11–13).
Prolonged survival rendered by effective modern systemic therapy
may have led to the presumptive increase in the detection of brain
metastasis (14–17) due to the inability of systemic therapy
to breach the blood brain barrier (10). Randomized trials have shown no
survival improvements with the addition of WBRT to surgery or
radiation (22,23) for brain metastasis in general. In
fact, avoiding WBRT can preserve or improve neurocognitive outcomes
without the expense of decreased survival (21). While long-term neurocognitive sequelae
remain a concern in all patients with limited brain metastasis, it
becomes particularly relevant in this group of patients who survive
longer. The median overall survival time of 29 months in the
present study validates this hypothesis. Other groups have reported
similar overall survival times in such patients. Kastritis et
al (10) reported long-term
survivors, and Anupol et al (24) reported a mean survival time of 22
months in patients with brain metastasis from ovarian cancer.
Similarly other studies have reported excellent local control and
survival following SRS for endometrial cancer (25,26). By
contrast, patients with brain metastasis from cervical cancer
appear to do relatively poorly (6,27–29). This could reflect the inherent poor
biological behavior of squamous cervical cancer.
Tumor markers are often unreliable in screening for
brain metastasis (30), but cancer
antigen 125 elevation can occasionally precede the clinical
detection of brain metastasis (24),
and elevated marker levels with no other signs of metastasis in the
presence of neurological symptoms should arouse suspicion.
Surgical resection when appropriate (31) plus WBRT has been previously used in
patients with brain metastasis from gynecological malignancies.
Certain studies have reported improved outcomes with multimodality
treatments that include surgical resection (8,14).
However, the majority of series have used WBRT to treat these
patients. In fact, the Hellinic Oncology Group reported poor
outcomes primarily with the use of WBRT or supportive care
(32).
Prior studies have reported the use of SRS in
patients with brain metastasis from gynecological malignancies.
(27,33–35).
Improved outcomes after SRS compared with WBRT (25,33,35) could
reflect selection bias. Patients with isolated or limited CNS
disease, controlled systemic disease and reasonable performance
status appear to do well overall (19,24), and
are those who could potentially benefit from WBRT-sparing
approaches such as SRS. In the present study, it was shown that
patients with a reasonable performance status and controlled
systemic disease can achieve excellent local control rates and
achieve long overall survival times with SRS treatment alone. This
is particularly true due to the high efficacy of SRS for isolated
brain relapses, with WBRT reserved for widespread and
leptomeningeal metastases.
In conclusion, brain metastasis from gynecological
malignancies is rare. Systemic therapy is effective in patients
with metastatic cancer of the female genital tract, who can
subsequently survive for a long period of time. In this setting,
when these patients present with limited brain metastasis, surgery
(when appropriate) and/or SRS is effective in controlling the brain
disease.
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jemal A, Center MM, DeSantis C and Ward
EM: Global patterns of cancer incidence and mortality rates and
trends. Cancer Epidemiol Biomarkers Prev. 19:1893–1907. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Aalders JG, Abeler V and Kolstad P:
Recurrent adenocarcinoma of the endometrium: A clinical and
histopathological study of 379 patients. Gynecol Oncol. 17:85–103.
1984. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chura JC, Marushin R, Boyd A, Ghebre R,
Geller MA and Argenta PA: Multimodal therapy improves survival in
patients with CNS metastasis from uterine cancer: A retrospective
analysis and literature review. Gynecol Oncol. 107:79–85. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ikeda S, Yamada T, Katsumata N, Hida K,
Tanemura K, Tsunematu R, Ohmi K, Sonoda T, Ikeda H and Nomura K:
Cerebral metastasis in patients with uterine cervical cancer. Jpn J
Clin Oncol. 28:27–29. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cordeiro JG, Prevedello DM, da Silva
Ditzel LF, Pereira CU and Araújo JC: Cerebral metastasis of
cervical uterine cancer: Report of three cases. Arq Neuropsiquiatr.
64:300–302. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cormio G, Lissoni A, Losa G, Zanetta G,
Pellegrino A and Mangioni C: Brain metastases from endometrial
carcinoma. Gynecol Oncol. 61:40–43. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Root K and Armaghany T: Solitary brain
metastasis in a patient with ovarian cancer with BRCA2 mutation. J
Clin Oncol. 30:e239–e240. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kastritis E, Efstathiou E, Gika D, Bozas
G, Koutsoukou V, Papadimitriou C, Pissakas G, Dimopoulos MA and
Bamias A: Brain metastases as isolated site of relapse in patients
with epithelial ovarian cancer previously treated with platinum and
paclitaxel-based chemotherapy. Int J Gynecol Cancer. 16:994–999.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Leath CA III and Straughn JM Jr:
Chemotherapy for advanced and recurrent cervical carcinoma: Results
from cooperative group trials. Gynecol Oncol. 129:251–257. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Vale CL, Tierney J, Bull SJ and Symonds
PR: Chemotherapy for advanced, recurrent or metastatic endometrial
carcinoma. Cochrane Database Syst Rev. 8:CD0039152012.
|
|
13
|
Foley OW, Rauh-Hain JA and del Carmen MG:
Recurrent epithelial ovarian cancer: An update on treatment.
Oncology. 27:288–294. 2013.PubMed/NCBI
|
|
14
|
Rodriguez GC, Soper JT, Berchuck A, Oleson
J, Dodge R, Montana G and Clarke-Pearson DL: Improved palliation of
cerebral metastases in epithelial ovarian cancer using a combined
modality approach including radiation therapy, chemotherapy, and
surgery. J Clin Oncol. 10:1553–1560. 1992.PubMed/NCBI
|
|
15
|
Bruzzone M, Campora E, Chiara S, Giudici
S, Merlini L, Simoni C, Mammoliti S, Rubagotti A and Rosso R:
Cerebral metastases secondary to ovarian cancer: Still an unusual
event. Gynecol Oncol. 49:37–40. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Stein M, Steiner M, Klein B, Beck D, Atad
J, Kuten A, Robinson E and Goldsher D: Involvement of the central
nervous system by ovarian carcinoma. Cancer. 58:2066–2069. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hardy JR and Harvey VJ: Cerebral
metastases in patients with ovarian cancer treated with
chemotherapy. Gynecol Oncol. 33:296–300. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen YL, Cheng WF, Hsieh CY and Chen CA:
Brain metastasis as a late manifestation of ovarian carcinoma. Eur
J Cancer Care (Engl). 20:44–49. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
McMeekin DS, Kamelle SA, Vasilev SA,
Tillmanns TD, Gould NS, Scribner DR, Gold MA, Guruswamy S and
Mannel RS: Ovarian cancer metastatic to the brain: What is the
optimal management? J Surg Oncol. 78:194–200. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hu XQ, Imitola J, Kim RY, Mahta A and
Kesari S: Brain metastasis from ovarian cancer: Case report and
review of the literature. Med Oncol. 29:1250–1252. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chang EL, Wefel JS, Hess KR, Allen PK,
Lang FF, Kornguth DG, Arbuckle RB, Swint JM, Shiu AS, Maor MH and
Meyers CA: Neurocognition in patients with brain metastases treated
with radiosurgery or radiosurgery plus whole-brain irradiation: A
randomised controlled trial. Lancet Oncol. 10:1037–1044. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Patchell RA, Tibbs PA, Regine WF, Dempsey
RJ, Mohiuddin M, Kryscio RJ, Markesbery WR, Foon KA and Young B:
Postoperative radiotherapy in the treatment of single metastases to
the brain: A randomized trial. JAMA. 280:1485–1489. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Aoyama H, Shirato H, Tago M, Nakagawa K,
Toyoda T, Hatano K, Kenjyo M, Oya N, Hirota S, Shioura H, et al:
Stereotactic radiosurgery plus whole-brain radiation therapy vs
stereotactic radiosurgery alone for treatment of brain metastases:
A randomized controlled trial. JAMA. 295:2483–2491. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Anupol N, Ghamande S, Odunsi K, Driscoll D
and Lele S: Evaluation of prognostic factors and treatment
modalities in ovarian cancer patients with brain metastases.
Gynecol Oncol. 85:487–492. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shiohara S, Ohara M, Itoh K, Shiozawa T
and Konishi I: Successful treatment with stereotactic radiosurgery
for brain metastases of endometrial carcinoma: A case report and
review of the literature. Int J Gynecol Cancer. 13:71–76. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Elliott KS, Borowsky ME, Lee YC, Rao C and
Abulafia O: Prolonged survival in recurrent endometrial carcinoma
to the brain. Gynecol Oncol. 95:247–251. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chung SB, Jo KI, Seol HJ, Nam DH and Lee
JI: Radiosurgery to palliate symptoms in brain metastases from
uterine cervix cancer. Acta Neurochir (Wien). 155:399–405. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Agrawal A, Kumar A, Sinha AK, Kumar M,
Pandey SR and Khaniya S: Intracranial metastases from carcinoma of
the cervix. Singapore Med J. 48:e154–e156. 2007.PubMed/NCBI
|
|
29
|
Park SH, Ro DY, Park BJ, Kim YW, Kim TE,
Jung JK, Lee JW, Kim JY and Han CW: Brain metastasis from uterine
cervical cancer. J Obstet Gynaecol Res. 36:701–704. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cooper KG, Kitchener HC and Parkin DE:
Cerebral metastases from epithelial ovarian carcinoma treated with
carboplatin. Gynecol Oncol. 55:318–323. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Pothuri B, Chi DS, Reid T, Aghajanian C,
Venkatraman E, Alektiar K, Bilsky M and Barakat RR: Craniotomy for
central nervous system metastases in epithelial ovarian carcinoma.
Gynecol Oncol. 87:133–137. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Pectasides D, Aravantinos G, Fountzilas G,
Kalofonos C, Efstathiou E, Karina M, Pavlidis N, Farmakis D,
Economopoulos T and Dimopoulos MA: Brain metastases from epithelial
ovarian cancer. The Hellenic Cooperative Oncology Group (HeCOG)
experience and review of the literature. Anticancer Res.
25:3553–3558. 2005.PubMed/NCBI
|
|
33
|
Lee YK, Park NH, Kim JW, Song YS, Kang SB
and Lee HP: Gamma-knife radiosurgery as an optimal treatment
modality for brain metastases from epithelial ovarian cancer.
Gynecol Oncol. 108:505–509. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Monaco E III, Kondziolka D, Mongia S,
Niranjan A, Flickinger JC and Lunsford LD: Management of brain
metastases from ovarian and endometrial carcinoma with stereotactic
radiosurgery. Cancer. 113:2610–2614. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Niu X, Rajanbabu A, Delisle M, Peng F,
Vijaykumar DK, Pavithran K, Feng Y, Lau S, Gotlieb WH and Press JZ:
Brain metastases in women with epithelial ovarian cancer:
Multimodal treatment including surgery or gamma-knife radiation is
associated with prolonged survival. J Obstet Gynaecol Can.
35:816–822. 2013. View Article : Google Scholar : PubMed/NCBI
|