Introduction
Melanoma is the least common but most serious form
of skin cancer (1). Rich blood supply
affects the growth and metastasis of melanoma (2). A unique form of microcirculation called
vasculogenic mimicry (VM), which is composed strictly of tumor
cells without endothelial cells and efficiently supplies blood to
tumor cells, has been reported (3).
VM is associated with poor prognosis for patients with certain
aggressive malignant tumors, including melanoma (4), hepatocellular carcinoma (5) and breast cancer (6). It was reported that the expression and
secretion of matrix metalloproteinase (MMP)-2 is important in VM
formation (7). The sole application
of angiogenic inhibitors proved to be ineffective on VM due to the
different molecular mechanisms that exist in endothelium-dependent
angiogenesis and VM (8,9). Thus, it is important to develop new
angiogenic inhibitors that target tumor VM or to combine anti-VM
drugs with conventional chemotherapies. Traditional Chinese
medicines are reported to have multifunctional antitumor activities
(10).
Norcantharidin (NCTD), a demethylated analog of
cantharidin, is a 7-oxabicyclo heptane-2,3-dicarboxylic acid
derivative isolated from natural blister beetles that has antitumor
properties in a variety of tumors such as primary hepatocellular
(11) and bladder cancer (12), and the mechanism of NCTD against
bladder cancer may be attributable to its anti-VM activity. NCTD
also induces cell apoptosis in melanoma in vitro (13). However, whether NCTD can inhibit the
tumor growth of melanoma in vivo and its underlying
mechanisms remain unclear.
The current study aims to investigate the antitumor
activity of NCTD as a VM inhibitor for human melanoma and its
underlying mechanisms. The results indicate that NCTD inhibits
tumor growth and VM of human melanoma by suppressing MMP-2
expression in vitro and in vivo. Thus, the present
study reveals that NCTD may be a potential anti-VM agent for human
melanoma.
Materials and methods
Cell culture
The A375 human melanoma cell line was obtained from
the Cell Resource Center (Beijing, China) and was maintained in
RPMI 1640 medium (Beijing Neuronbc Laboratories, Co. Ltd., Beijing,
China) supplemented with 10% fetal bovine serum (FBS; HyClone; GE
Healthcare Life Sciences, Logan, UT, USA) in an incubator with
high-efficiency particulate arrestance class 100 filter (Forma™
Series II; Thermo Fisher Scientific, Inc., Waltham, MA, USA) at
37°C with 5% CO2.
Invasion assays
Transwell membranes (8-µm pore size; Costar;
Corning, Life Sciences, Cambridge, UK) were coated with Matrigel (1
mg/ml; BD Biosciences, Franklin Lakes, NJ, USA) prior to cell
passage. A375 cells (1×105) were seeded into the upper
wells in RPMI 1640 medium supplemented with 0.2% FBS. Cells were
untreated (control group) or treated with 28 µg/ml (1/2 half
maximal inhibitory concentration) NCTD (NCTD group). NCTD was
purchased from Jiangsu Kangxi Pharmaceutical Works (Jiangsu, China)
in fresh culture medium. Lower wells contained RPMI 1640 medium
supplemented with 20% FBS. After 24 h of incubation at 37°C,
non-invading cells were removed from the upper surface of the
membrane, and the cells that invaded each membrane were stained
with a crystal violet solution and counted as described previously
(7).
Three-dimensional (3-D) cultures
Matrigel was thawed at 4°C, and 20 µl was quickly
added to each well of a 96-well plate and allowed to solidify for
40 min at 37°C in a humidified 5% CO2 incubator. Tumor
cells were seeded in complete RPMI 1640 medium onto the plate and
incubated with or without NCTD (28 µg/ml) at 37°C for 12 and 24 h,
respectively. Vasculogenic-like structure formation was filmed
under an inverted phase-contrast light microscope.
Tumor xenografts
Mice (4–6 week-old BALB/c nu/nu male mice; Shanghai
Laboratory Animal Center, Shanghai, China) weighing 18–24 g were
randomly divided into the control group and the NCTD group
(n=10/group). The mice were housed according to the official
recommendations of the Chinese Community Guidelines (14). Tumors were established by inoculation
of 1×106 A375 cells suspended in 100 µl normal saline
into the right back of the mice by subcutaneous injection. After
the tumors had grown to a size of ~100 mm3, NCTD was
administered by intraperitoneal injection at 28 mg⁄kg (1/5 half
maximal lethal dose) in 0.1 ml normal saline for 13 consecutive
days. Control animals were administered 0.1 ml normal saline as a
vehicle control. The maximum diameter (a) and the minimum diameter
(b) were measured with calipers every 2 days. The tumor volume (V)
was calculated by the following formula: V = ab2 x 0.5.
The tumor weight was also evaluated once the mice were sacrificed.
All animal procedures were performed according to an approved
protocol by the Animal Ethical Committee of the Tianjin
International Joint Academy of Biotechnology and Medicine (Tianjin,
China).
Immunohistochemical analysis
Xenografted tumors from the mice were excised, fixed
in 4% paraformaldehyde for 24 h, and then embedded in paraffin for
histological studies. Paraffin-embedded tissues were sectioned into
slices of 4-µm thickness for histological studies. Dewaxed and
rehydrated tissue sections were subjected to antigen retrieval
processes. Upon blocking, the sections were incubated overnight at
4°C with anti-cluster of differentiation (CD) 31 antibody (dilution
1:50; catalogue number JC70; Neomarkers, Fremont, CA, USA) or
anti-MMP-2 antibody (dilution 1:200; catalogue number ab37150;
Abcam, Cambridge, UK). Negative controls were prepared using PBS
instead of the primary antibodies. Upon washing with PBS, the
sections were incubated with a goat anti-mouse EnVision kit
(Genentech, South San Francisco, CA, USA) for 40 min at 37°C, and
then incubated with 3,3′-diaminobenzidine chromogen for 10 min. The
slides for CD31-periodic acid-Schiff (PAS; Beijing Zhongshan
Jinqiao Biotechnology Co. Ltd., Beijing, China) double staining
were then exposed to a 0.5% periodic acid solution for 15 min and
subsequently incubated in Schiff solution for 20 min in a dark
chamber. Subsequently, the slides were washed with distilled water
for 3 min and counterstained with hematoxylin. Multiplication of
intensity and percentage scores was utilized to determine the
staining index result.
Zymography assays and MMP-2 protein
concentration determination
Gelatin zymography was used to examine the levels of
MMP-2 activity in A375 cells that were either untreated (control
group) or treated with 28 µg/ml NTCD for 48 h. The culture media
were collected and subjected to 10% SDS-PAGE using 0.01% w/v
gelatin. The gel was washed twice in 2.5% (w/v) Triton X-100
solution and incubated overnight at 37°C in developing buffer [50
mmol/l Tris/HCl (pH 7.4), 10 mmol/l CaCl2, 5 mmol/l
ZnCl2 and 0.05% Brij™ 35 Surfact-Amps™ Detergent
Solution (Thermo Fisher Scientific, Inc.)]. The gels were
subsequently stained with Coomassie Brilliant Blue R250 and
destained until the wash remained clear and cleared zones
associated with MMP activity were apparent. The concentrations of
exogenous pro-MMP-2 and active MMP-2 in cell culture supernatants
were assessed using the MMP-2 Biotrak Activity Assay (GE Healthcare
Life Sciences, Chalfont, UK) according to the manufacturer's
guidelines.
Statistical analysis
All data were reported as the mean ± standard error
of the mean and evaluated using SPSS 17.0 (SPSS Inc., Chicago, IL,
USA). Student-Newman-Keuls t tests were used to evaluate
differences between two groups. P<0.05 was considered to
indicate a statistically significant difference.
Results
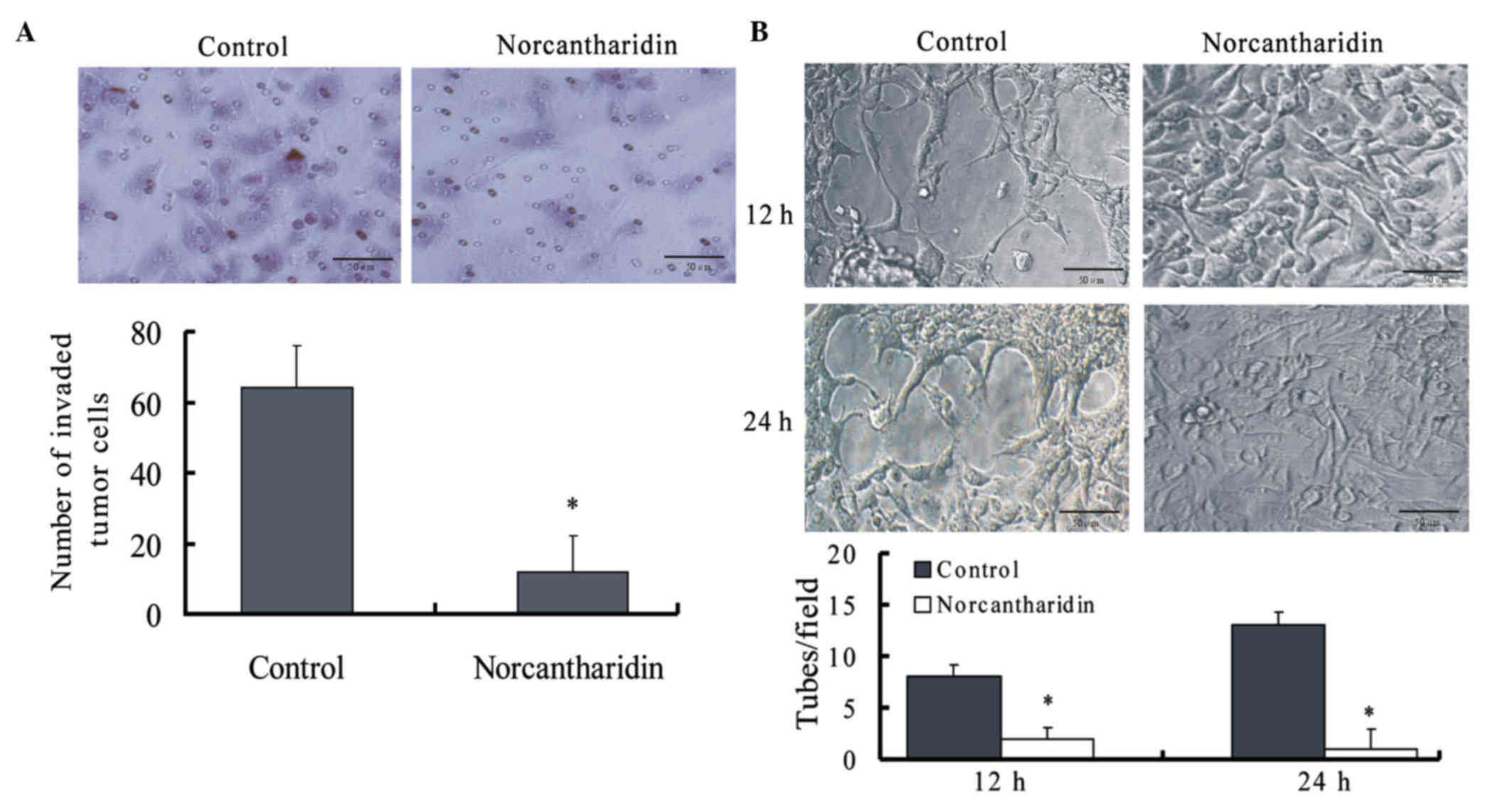
NCTD inhibits the invasion of A375
cells in vitro
Matrigel-coated transwell plates were used to assess
the effects of NCTD on the ability of A375 melanoma cells to invade
a basement membrane matrix. The results revealed that NCTD
significantly reduced the number of cells that invaded through the
Matrigel matrix after 24 h of treatment (P= 0.005) (Fig. 1A).
NCTD inhibits tube formation by A375
cells in vitro
The vasculogenic-like network formation ability of
melanoma cells was assessed in vitro by seeding the cells
onto Matrigel-coated plates and then observing the cells under an
inverted phase-contrast light microscope. As shown in Fig. 1B, the formation of VM networks by A375
melanoma cells was disrupted by the addition of NCTD for 12 or 24
h.
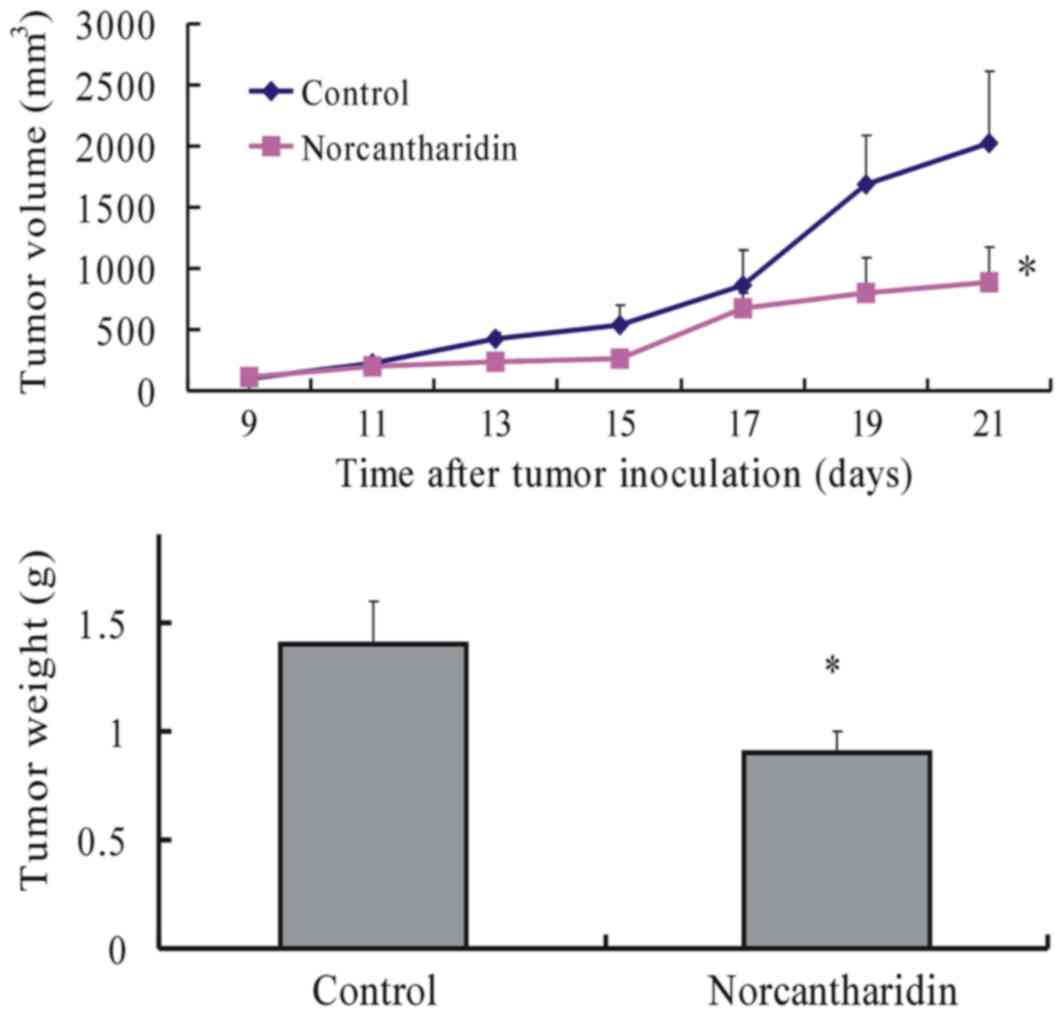
NCTD inhibits melanoma growth and VM
formation in mice
To investigate the efficacy of NCTD in inhibiting
melanoma growth, the tumor sizes of melanoma-bearing mice were
measured once every 2 days throughout the experiment. At the end of
the experiment, the volume and weight of the xenografts in the NCTD
group decreased significantly with increased tumor inhibition in
comparison with those of the control group (Fig. 2). CD31-PAS double staining was used to
identify VM in the xenografts on day 21 of tumor inoculation.
Microscopically, the xenografts in the control group exhibited
tumor cell-lined channels containing red blood cells (Fig. 3) without any evidence of tumor
necrosis. The channels consisted of tumor cells negative for CD31
and positive for PAS. By contrast, VM could hardly be observed in
the tumor tissues treated with NCTD, while large areas of necrosis
were easily detected, suggesting that NCTD inhibits the VM
formation of melanoma xenografts in vivo.
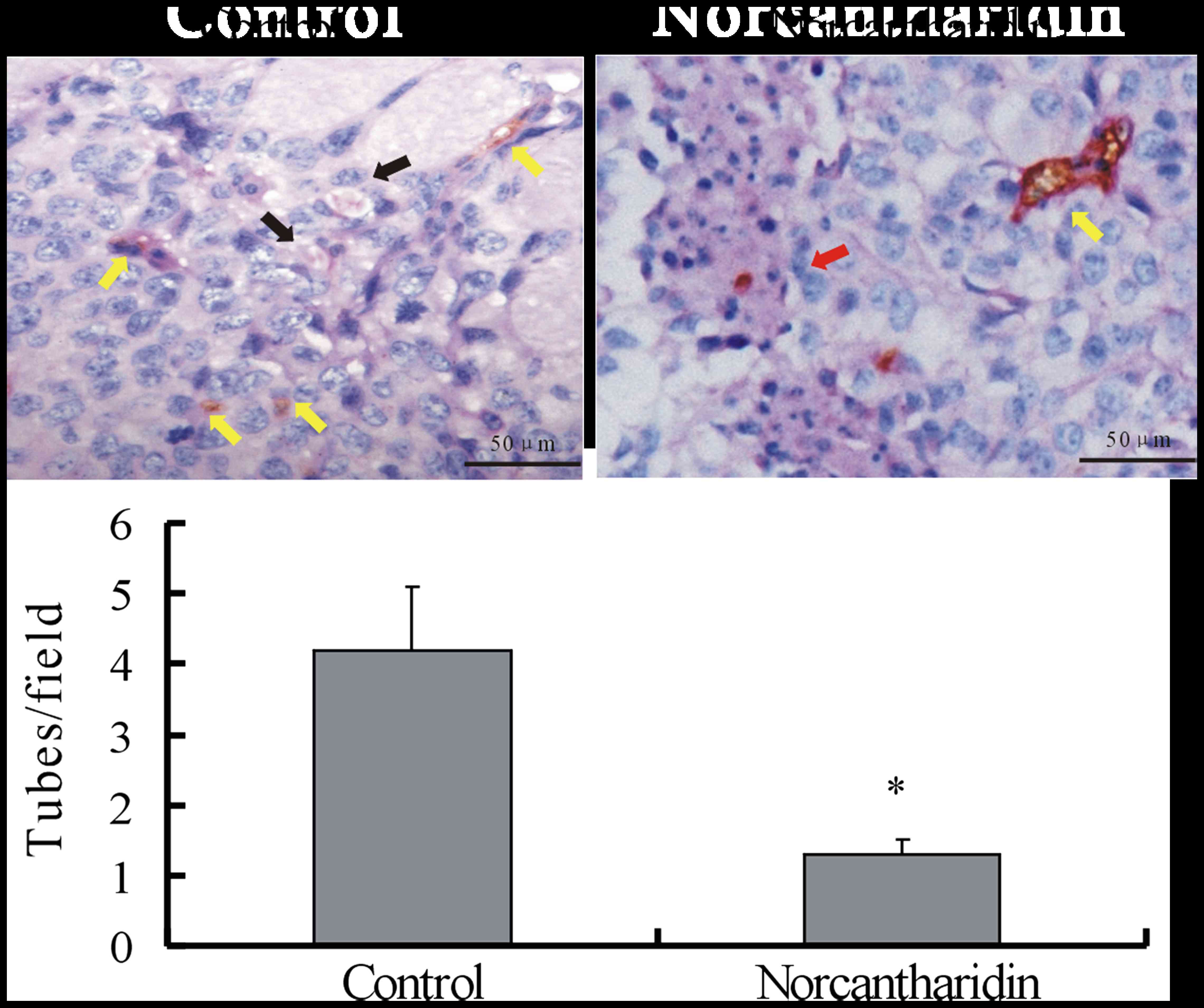 | Figure 3.NCTD decreases the capillary-like tube
formation ability of A375 melanoma xenografts in vivo. Upper panel,
representative images of the presence of VM in tissue samples of
A375 melanoma xenografts with or without treatment with NCTD. In
the control group, CD31-periodic acid-Schiff staining was used for
the morphological observations of VM formed by tumor cells without
CD31-positively stained endothelial cells (black arrow). Red blood
cells were present in the center of the channels. The yellow arrow
points to CD31-positively stained endothelium-dependent vessels in
the same field. In the NCTD-treated group, VM was not observed,
while large areas of necrosis were easily noticed (red arrow).
Yellow arrow, endothelium-dependent vessels. Magnification, ×400.
Lower panel, quantitative analysis indicating that less
capillary-like tubes were formed upon NCTD treatment in the treated
group compared with those in the control group. *P<0.05 compared
with the control group. NCTD, norcantharidin; CD, cluster of
differentiation; VM, vasculogenic mimicry. |
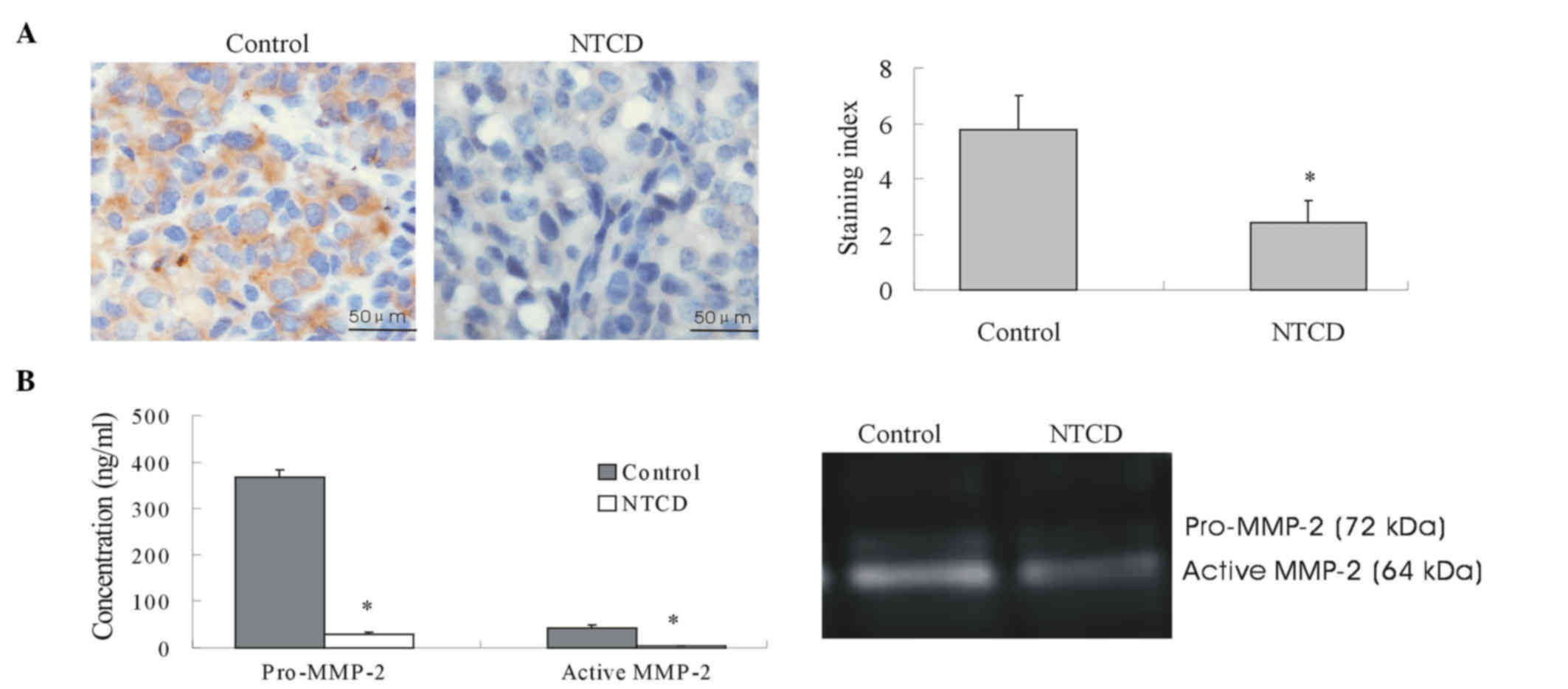
NCTD downregulates the expression and
activity of MMP-2
MMP-2 is a key player in VM formation (15). Thus, to explore the possible
mechanisms of NCTD effects on tumor growth and VM of human melanoma
in vitro and in vivo, in the present study, the
expression and activity of MMP-2 protein from sections of melanoma
xenografts and supernatants of 3-D culture samples were determined.
The results indicated that MMP-2 expression in the in vivo
xenografts of the NCTD group was significantly lower than that of
the control group (Fig. 4A).
Furthermore, the addition of NCTD decreased both the expression and
activity of MMP-2 in the 3-D culture samples (Fig. 4B and Table
I).
 | Table I.Concentration and activity state of
MMP-2 in serum-free conditioned medium from melanoma cells cultured
on Matrigel. |
Table I.
Concentration and activity state of
MMP-2 in serum-free conditioned medium from melanoma cells cultured
on Matrigel.
| Serum-free
conditioned medium | MMP-2 (ng/ml) | MMP-2 activity
(ng/ml) |
|---|
| Control | 367.82±14.8 | 28.09±5.64 |
| NCTD |
43.15±5.67a |
2.29±0.18a |
Discussion
The present study demonstrates that NCTD inhibits
tumor growth and VM of melanoma by suppressing MMP-2
expression.
Widespread metastasis caused by increased cell
motility and a rich blood supply of tumor cells is the main cause
of the poor prognosis of melanoma patients (16). Traditional anti-angiogenic drugs,
including bevacizumab, sunitinib, angiostatin and endostatin, have
yielded disappointing results on the management of melanoma, since
VM exists as a particular microcirculation pattern, and the sole
blockage of angiogenesis may not be effective (9,17–20). Thus, the development of anti-VM drugs
for the treatment of melanoma with VM is an urgent concern. NCTD is
a demethylated and low-cytotoxic derivative of cantharidin
(12). It has antitumor properties,
hypotoxicity in a variety of tumor and apoptosis-promoting effects
in melanoma in vitro (13).
NCTD also inhibits VM formation in human gallbladder carcinomas
(21). The present study further
investigated the anti-VM activity of NCTD as a VM inhibitor for
human melanoma. The results indicate that NCTD inhibits the growth
and VM formation of melanoma both in vitro and in
vivo, thus suggesting that NCTD may be a potential therapeutic
agent targeting VM in melanoma.
We also sought to determine the possible mechanism
of the inhibitory effects of NCTD on the growth and VM formation of
melanoma. The MMP-2 protein is considered to play a key role in VM
formation in melanoma via the cleavage of the laminin (Ln)-5γ2
chain into two segments (Ln-5γ2x and Ln-5γ2′), which results in VM
formation (15). Zhang et al
reported that NCTD inhibits tumor growth and VM of human
gallbladder carcinomas by suppressing the
phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3-K)/MMP-2/Ln-5γ2
signaling pathway (12). Thus, in the
current study, the effects of NCTD on MMP-2 protein expression and
activity in melanoma were determined both in vivo and in
vitro. The results demonstrate that NCTD not only inhibits VM
formation of melanoma cells and xenografts, but also downregulates
MMP-2 expression in vitro and in vivo. These results
suggest that the PI3-K/MMP-2/Ln-5γ2 signaling pathway may also be
the underlying molecular mechanism of the inhibitory effects of
NCTD on the growth and VM formation of melanoma. Therefore, NCTD
could be used as a potential anti-VM inhibitor in melanoma
treatment.
In conclusion, the present study demonstrated that
NCTD inhibits the growth and VM formation of melanoma by
suppressing MMP-2 expression. NCTD may be used as a potential
therapeutic agent targeting VM in melanoma. Further investigations
are necessary to verify other molecular mechanisms of the
inhibitory effects of NCTD on the VM formation of melanoma.
References
|
1
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Neitzel LT, Neitzel CD, Magee KL and
Malafa MP: Angiogenesis correlates with metastasis in melanoma. Ann
Surg Oncol. 6:70–74. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Maniotis AJ, Folberg R, Hess A, Seftor EA,
Gardner LM, Pe'er J, Trent JM, Meltzer PS and Hendrix MJ: Vascular
channel formation by human melanoma cells in vivo and in vitro:
Vasculogenic mimicry. Am J Pathol. 155:739–752. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Warso MA, Maniotis AJ, Chen X, Majumdar D,
Patel MK, Shilkaitis A, Gupta TK and Folberg R: Prognostic
significance of periodic acid-Schiff-positive patterns in primary
cutaneous melanoma. Clin Cancer Res. 7:473–477. 2001.PubMed/NCBI
|
|
5
|
Liu WB, Xu GL, Jia WD, Li JS, Ma JL, Chen
K, Wang ZH, Ge YS, Ren WH, Yu JH, et al: Prognostic significance
and mechanisms of patterned matrix vasculogenic mimicry in
hepatocellular carcinoma. Med Oncol. 28:(Suppl 1). S228–S238. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shirakawa K, Wakasugi H, Heike Y, Watanabe
I, Yamada S, Saito K and Konishi F: Vasculogenic mimicry and
pseudo-comedo formation in breast cancer. Int J Cancer. 99:821–828.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Seftor EA, Meltzer PS, Kirschmann DA,
Pe'er J, Maniotis AJ, Trent JM, Folberg R and Hendrix MJ: Molecular
determinants of human uveal melanoma invasion and metastasis. Clin
Exp Metastasis. 19:233–246. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
van der Schaft DW, Seftor RE, Seftor EA,
Hess AR, Gruman LM, Kirschmann DA, Yokoyama Y, Griffioen AW and
Hendrix MJ: Effects of angiogenesis inhibitors on vascular network
formation by human endothelial and melanoma cells. J Natl Cancer
Inst. 96:1473–1477. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rice C and Huang LE: From antiangiogenesis
to hypoxia: Current research and future directions. Cancer Manag
Res. 3:9–16. 2010.PubMed/NCBI
|
|
10
|
Sun M, Han J, Duan J, Cui Y, Wang T, Zhang
W, Liu W, Hong J, Yao M, Xiong S and Yan X: Novel antitumor
activities of Kushen flavonoids in vitro and in vivo. Phytother
Res. 21:269–277. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yeh CB, Hsieh MJ, Hsieh YH, Chien MH,
Chiou HL and Yang SF: Antimetastatic effects of norcantharidin on
hepatocellular carcinoma by transcriptional inhibition of MMP-9
through modulation of NF-kB activity. PLoS One. 7:e310552012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang JT, Sun W, Zhang WZ, Ge CY, Liu ZY,
Zhao ZM, Lu XS and Fan YZ: Norcantharidin inhibits tumor growth and
vasculogenic mimicry of human gallbladder carcinomas by suppression
of the PI3-K/MMPs/Ln-5γ2 signaling pathway. BMC Cancer. 14:1932014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
An WW, Wang MW, Tashiro S, Onodera S and
Ikejima T: Norcantharidin induces human melanoma A375-S2 cell
apoptosis through mitochondrial and caspase pathways. J Korean Med
Sci. 19:560–566. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Qin Y, Zhang Q, Lee S, Zhong WL, Liu YR,
Liu HJ, Zhao D, Chen S, Xiao T, Meng J, et al: Doxycycline reverses
epithelial-to-mesenchymal transition and suppresses the
proliferation and metastasis of lung cancer cells. Oncotarget.
6:40667–40679. 2015.PubMed/NCBI
|
|
15
|
Hess AR, Seftor EA, Seftor RE and Hendrix
MJ: Phosphoinositide 3-kinase regulates membrane Type 1-matrix
metalloproteinase (MMP) and MMP-2 activity during melanoma cell
vasculogenic mimicry. Cancer Res. 63:4757–4762. 2003.PubMed/NCBI
|
|
16
|
Hofmann-Wellenhof R, Woltsche-Kahr I,
Smolle J and Kerl H: Clinical and histological features of poor
prognosis in cutaneous metastatic melanomas. J Cutan Pathol.
23:199–204. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen HX and Cleck JN: Adverse effects of
anticancer agents that target the VEGF pathway. Nat Rev Clin Oncol.
6:465–477. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Higa GM and Abraham J: Biological
mechanisms of bevacizumab-associated adverse events. Expert Rev
Anticancer Ther. 9:999–1007. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gille J: Antiangiogenic cancer therapies
get their act together: Current developments and future prospects
of growth factor- and growth factor receptor-targeted approaches.
Exp Dermatol. 15:175–186. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Grothey A and Galanis E: Targeting
angiogenesis: Progress with anti-VEGF treatment with large
molecules. Nat Rev Clin Oncol. 6:507–518. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang JT, Fan YZ, Chen CQ, Zhao ZM and Sun
W: Norcantharidin: A potential antiangiogenic agent for gallbladder
cancers in vitro and in vivo. Int J Oncol. 40:1501–1514.
2012.PubMed/NCBI
|