Introduction
In recent years, the incidence of non-alcoholic
fatty liver disease (NAFLD) has increased (1). Patients with fatty liver degeneration
have twice the risk of complications following liver resection
compared with patients without liver disease; these complications
may include infective, wound-related (seroma, infection and
hernia), hepatobiliary (cholangitis, biliary obstruction, liver
failure, hepatic artery infusion pump failure, ascites, perihepatic
fluid collection and perihepatic abscess) and gastrointestinal
complications (gastrointestinal hemorrhage, bowel obstruction,
paralytic ileus, infectious diarrhea, fistula, pancreatitis and
esophagitis). Additionally, the risk of mortality for patients with
severe steatosis following liver resection is almost three-times
greater than that for patients without liver steatosis (2). Compared to a normal liver, a liver with
fatty degeneration more easily suffers from ischemia reperfusion
(IR) injury. Treatment with omega-3 fatty acids significantly
reduces hepatic steatosis, resulting in significantly reduced IR
injury (3). Proanthocyanidins from
grape seeds have a protective effect against liver IR injury,
particularly in mice with diet-induced obesity (4). However, the use of drugs to treat fatty
liver degeneration is an indirect strategy for reducing injury; the
treatment can enhance liver function and thus increase the
tolerance of the liver to IR damage, but not reduce IR injury
directly.
Increasing evidence indicates that remote ischemic
preconditioning (RIPC) represents a strategy for harnessing the
body's endogenous protective capabilities to counter the injury
that is incurred by IR in various organs (5–13),
including the normal liver (14–17). The
protective mechanism of RIPC is highly complex (18–22) and
involves the improvement of hepatic oxygenation (23), reduction of serum cytokine-induced
neutrophil chemoattractant-1 levels (24), modulation of hepatic microcirculation
(15) and activation of the soluble
guanylate cyclase-cyclic guanosine monophosphate pathway (20), resulting in protective effects against
IR injury. Notably, Abu-Amara et al (25) suggested that nitric oxide (NO) is an
essential mediator of the protection that is afforded by hind-limb
RIPC against liver IR injury. The mechanisms underlying this
protective effect involve the preservation of the sinusoidal
structure and the maintenance of blood flow through the hepatic
microcirculation (25). However, the
mechanism by which RIPC increases NO, and the role and mechanism of
RIPC in NAFLD liver IR injury remain unclear.
Therefore, in the present study, a NAFLD rat model
was utilized in a series of different surgical procedures and
molecular experiments. The data indicate that RIPC has a protective
effect on NAFLD liver IR injury. RIPC may exert this effect by
reducing expression levels of the microRNAs (miRNAs) miR-29a/b/c in
the skeletal muscle, subsequently increasing inducible NO synthase
(iNOS) and thus increasing NO. miR-29a/b/c targets iNOS, which
plays an important role in the protective effect of RIPC in NAFLD
liver IR injury.
Materials and methods
Cell cultures and tissue
collections
The skeletal muscle cell line C2C12 was purchased
from the Shanghai Cell Bank (Shanghai, China) and cultured in
Dulbecco's modified Eagle's medium (DMEM) (Invitrogen; Thermo
Fisher Scientific, Inc., Carlsbad, CA, USA) that was supplemented
with 10% fetal bovine serum (FBS) (Sigma-Aldrich; Merck Millipore,
Bedford, MA, USA). The rats were purchased from The Model Animal
Research Center of Nanjing University (Nanjing, China). The animal
studies were approved by the Ethics Committee of Soochow
University.
Establishment of the animal
models
To establish the NAFLD rat model, specific
pathogen-free-grade Sprague Dawley male rats weighing ~200 g were
fed a high-fat diet containing 2% cholesterol, 0.5% sodium cholate,
0.2% propylthiouracil, 5% sugar, 10% lard, and 82.3% basic feed.
The rats were maintained in a temperature-controlled environment
with 40–70% humidity and fed for 5 weeks.
To establish the NAFLD/liver IR rat model, NAFLD
rats were anesthetized with 10% chloral hydrate by intraperitoneal
injection (350 mg/kg). Laparotomy was subsequently performed with a
median incision. The perihepatic ligament was separated, and the
blood supply to the hepatic left lateral lobe, left interior lobe
and middle lobe was blocked using a metal microvascular clamp,
resulting in 70% liver ischemia.
To establish the NAFLD/RIPC rat model, the right
hind limb of an NAFLD rat was tied up with a tourniquet such that
the right femoral artery was pulseless for 5 min. The tourniquet
was then released to restore the blood flow for 5 min. These two
procedures were repeated 6 times.
Rats were sacrificed by spinal dislocation
immediately at the end of experimental process.
Experimental groups
In the control group, the liver was prodded
following a median-incision laparotomy. In the RIPC group, the hind
limb was ischemic for 5 min, followed by reperfusion for 5 min.
After 6 cycles, the hind limb underwent reperfusion for 160 min. In
the IR group, the blood supply to the hepatic left lateral lobe,
left interior lobe and middle lobe was blocked for 40 min, followed
by reperfusion for 120 min. In the RIPC+IR group, following 6
cycles of hind limb ischemia and reperfusion, liver ischemia was
performed for 40 min, and reperfusion was given for 120 min. A
total of 6 rats were allocated to each group in the study.
Pathological examination
The left lobe of the liver was resected and frozen
in liquid nitrogen following the surgical procedure. For the
pathological examination, 4-µm-thick tissue sections were fixed,
rinsed, stained with hematoxylin and eosin (HE), dehydrated through
increasing concentrations of ethanol and xylene, and blocked by
neutral gum. Pathological observation was performed by two
pathologists.
Liver function test
Hepatic venous blood was drawn from the rats
following the surgical procedure. The serum alanine transaminase
(ALT) and aspartate transaminase (AST) levels were measured by
biochemical analysis using ALT and AST assay kits (Beckman Coulter,
Inc., Brea, CA, USA) with a Beckman Coulter Chemistry Analyzer
AU5800 series (Beckman Coulter K.K., Tokyo, Japan).
Cell apoptosis analysis
Fresh liver tissue was minced with scissors and
digested with 0.02% protease and 0.05% collagenase P (Roche, Basel,
Switzerland) in a balanced salt solution for 30 min at 37°C. The
suspension containing the cells and tissues was filtered through a
200-nm nylon mesh. The cell pellets were collected, washed with
PBS, suspended in 100 µl of 1X binding buffer, and stained with 5
µl of fluorescein isothiocyanate (FITC)-Annexin V and a 1-µl
working solution of propidium iodide (PI) (100 µg/ml) at room
temperature for 15 min in the dark. The stained cells were
immediately analyzed by flow cytometry.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from skeletal muscle and
cell lines using TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc.). For tissue RNA extraction, 100 mg of frozen
muscle in liquid nitrogen was ground and suspended in 1 ml of
TRIzol. For total RNA extraction from cells, 1×107 cell
pellets were collected, washed and resuspended in 1 ml of
TRIzol.
To analyze mRNA, cDNA was synthesized using the
PrimeScript RT Kit (Takara, Dalian, China). qPCR was performed with
FastStart Universal SYBR Green Master Mix (ROX) (Roche Diagnostics,
Indianapolis, IN, USA) with an ABI 7500 Real-Time PCR System
(Applied Biosystems; Thermo Fisher Scientific, Inc.). The reaction
mixture contained 12.5 µl FastStart Universal SYBR Green Master Mix
(ROX), 0.5 µl forward primer (30 µM), 0.5 µl reverse primer (30
µM), 9.5 µl PCR-grade water and 2.5 µl of template cDNA (25 ng).
Thermal cycling conditions for qPCR were 50°C for 2 min and 95°C
for 10 min, followed by 95°C for 15 sec and 58°C for 30 sec for 40
cycles. Each reaction was analyzed in triplicate. Relative mRNA
expression was examined as the inverse log of the ΔΔCq and
normalized to the expression of the reference gene GAPDH (26). The primers for qPCR were synthesized
by Invitrogen (Thermo Fisher Scientific, Inc., Shanghai, China),
and the sequences were as follows: iNOS sense,
5′-AGGACGAGAAGCGGAGACC-3′; iNOS antisense,
5′-CATGAGCAAAGGCGCAGAA-3′; GAPDH sense,
5′-TCACCCACACTGTGCCCATCTACGA-3′; and GAPDH antisense,
5′-CAGCGGAACCGCTCATTGCCAATGG-3′.
For miRNA analysis, samples of total RNA (500 ng
each) were reverse-transcribed into cDNA with miR-29a/b/c reverse
transcriptase primers using TaqMan MicroRNA Reverse Transcription
Kits [#4366596 (miR-29a/002112, miR-29b/000413, miR-29c/000587);
Applied Biosystems; Thermo Fisher Scientific, Inc.]. The RT master
mix included the following: 5 µl RNA samples (10 ng), 0.15 µl
dNTPs, 1 µl reverse transcriptase, 1.5 µl RT buffer, 0.19 µl RNase
inhibitor, 3.16 µl nuclease-free water and 4 µl of RT primers for
miR-29a/b/c and U6 (1 µl each) up to a total volume of 15 µl. The
final mixture was centrifuged and incubated on ice for 5 minutes.
The thermal program for RT was 30 min at 16°C, 30 min at 42°C, 5
min at 85°C and hold at 4°C. The levels of miR-29a/b/c and U6
expression were determined by qPCR with TaqMan MicroRNA Assays
[#4427975 (miR-29a/002112, miR-29b/000413, miR-29c/000587); Applied
Biosystems; Thermo Fisher Scientific, Inc.]. The qPCR reaction
mixture contained 1 µl TaqMan MicroRNA Assays (20X; including
primers), 2 µl product from RT reaction, 10 µl TaqMan Universal PCR
Master Mix II (2X) and 7 µl nuclease-free water up to a total
volume of 20 µl. The final mix was subjected to the following
program of heating using an ABI 7500 Real-Time PCR System: 2 min at
50°C; 10 min at 95°C; then 15 sec at 95°C and 60 sec at 60°C for 40
cycles. The levels of mature miR-29a/b/c expression were then
normalized to U6 and calculated as the inverse log of the ΔΔCq.
Western blotting
Skeletal muscle tissue (100 mg) or cells
(1×107) were lysed using radioimmunoprecipitation assay
buffer with 1% phenylmethane sulfonyl fluoride on ice. The total
protein concentration was determined using a bicinchoninic acid
assay kit (Keygen, Nanjing, China). Equal amounts of protein (30
µg) were resolved by 10% SDS-PAGE and transferred to polyvinylidene
difluoride membranes (EMD Millipore, Billerica, MA, USA) using a
Mini Trans-Blot apparatus (Bio-Rad Laboratories, Inc., Hercules,
CA, USA). The membranes were blocked in Tris-buffered saline with
Tween-20 (TBST) containing 5% non-fat milk and probed with rabbit
anti-rat monoclonal iNOS primary antibodies (1:10; #ab15323; Abcam,
Cambridge, MA, USA) overnight at 4°C. An anti-GAPDH antibody
(1:1,000; AG1019; Beyotime, Nantong, China) was used as an internal
control. Subsequently, the membranes were washed with 3X TBST prior
to incubation with horseradish peroxidase-conjugated goat
anti-rabbit secondary antibodies (1:1,000; #A0192; Beyotime,
Nantong, China) for 2 h at room temperature. The membranes were
then washed with 3X TBST and 1X TBS. For image development,
Immobilon Western Chemiluminescent HRP Substrate (#WBKLS0500; EMD
Millipore) was used. Bands on the membrane were developed using a
ChemiImager 5500 Imaging System (Alpha Innotech Co., San Leandro,
CA, USA).
miRNA transfection
miRNA transfection was performed using Lipofectamine
2000 (Invitrogen; Thermo Fisher Scientific, Inc., Carlsbad, CA,
USA). The miR-29a/b/c mimic, inhibitor and control were designed
and synthesized by GenePharma, Co., Ltd. (Shanghai, China). The
sequences were as follows: miR-29a-mimic, 5′-
UAGCACCAUCUGAAAUCGGUUA-3′; miR-29b-mimic,
5′-UAGCACCAUUUGAAAUCAGUGUU-3′; miR-29c-mimic, 5′-
UAGCACCAUUUGAAAUCGGUUA-3′; mimic control, 5′-
UUCUCCGAACGUGUCACGUTT-3′; miR-29a inhibitor,
5′-UAACCGAUUUCAGAUGGUGCUA-3′; miR-29b inhibitor,
5′-AACACUGAUUUCAAAUGGUGCUA-3′; miR-29c inhibitor,
5′-UAACCGAUUUCAAAUGGUGCUA-3′; inhibitor control,
5′-CAGUACUUUUGUGUAGUACAA-3′.
For transfection, C2C12 skeletal muscle cells were
plated at 1.0×106 cells/well in 6-well plates.
Lipofectamine 2000 (5 µl) was added into 250 µl DMEM at room
temperature and stood for 5 min. In addition, miRNA-mimics or
inhibitors for miR-29a, −29b or −29c, respectively (5 µl), were
added into 250 µl DMEM at room temperature and stood for 5 min.
Subsequently, the two solutions were mixed and allowed to incubate
for 20 min. Prior to transfection, cell culture medium was removed
and the cells were incubated in 2 ml DMEM. The aforementioned
510-µl mixture was added into the corresponding wells. The
transfected cells were incubated in a humidified chamber at 37°C
with 5% CO2 for 24 h. Total RNA and protein were
extracted at 24 h post-transfection and used for RT-qPCR and
western blot analyses.
Luciferase reporter assay
Luciferase reporter constructs were generated by
ligating 60-bp synthetic oligonucleotides (Invitrogen; Thermo
Fisher Scientific, Inc., Shanghai, China) containing putative miRNA
binding sites (or their mutant versions) from the 3′-untranslated
region (3′-UTR) of iNOS to the XbaI-FseI sites of the
pGL3-control vector (Promega Corporation, Madison, WI, USA).
Successful cloning was verified by sequencing. At 24 h prior to
transfection, C2C12 skeletal muscle cells were plated at
1.5×105 cells/well in 24-well plates, and 200 ng of each
independent luciferase reporter plasmid plus 80 ng of pRL-TK
(Promega Corporation) were transfected, together with 60 pmol of
miR-29a/b/c mimics, inhibitors or control miRNAs using
Lipofectamine 2000. The luciferase activity was measured at 48 h
following transfection using the Dual-Luciferase Reporter Assay
System (Promega Corporation). Firefly luciferase activity was
normalized to Renilla luciferase activity in each
transfected well.
Statistical analysis
All experiments were repeated in triplicate and the
values presented are the mean ± standard deviation. Statistical
significance was determined with Student's t-test using SPSS 15.0
software (SPSS, Inc., Chicago, IL, USA) P<0.05 was considered to
indicate a statistically significant difference.
Results
Establishment of the NAFLD rat
model
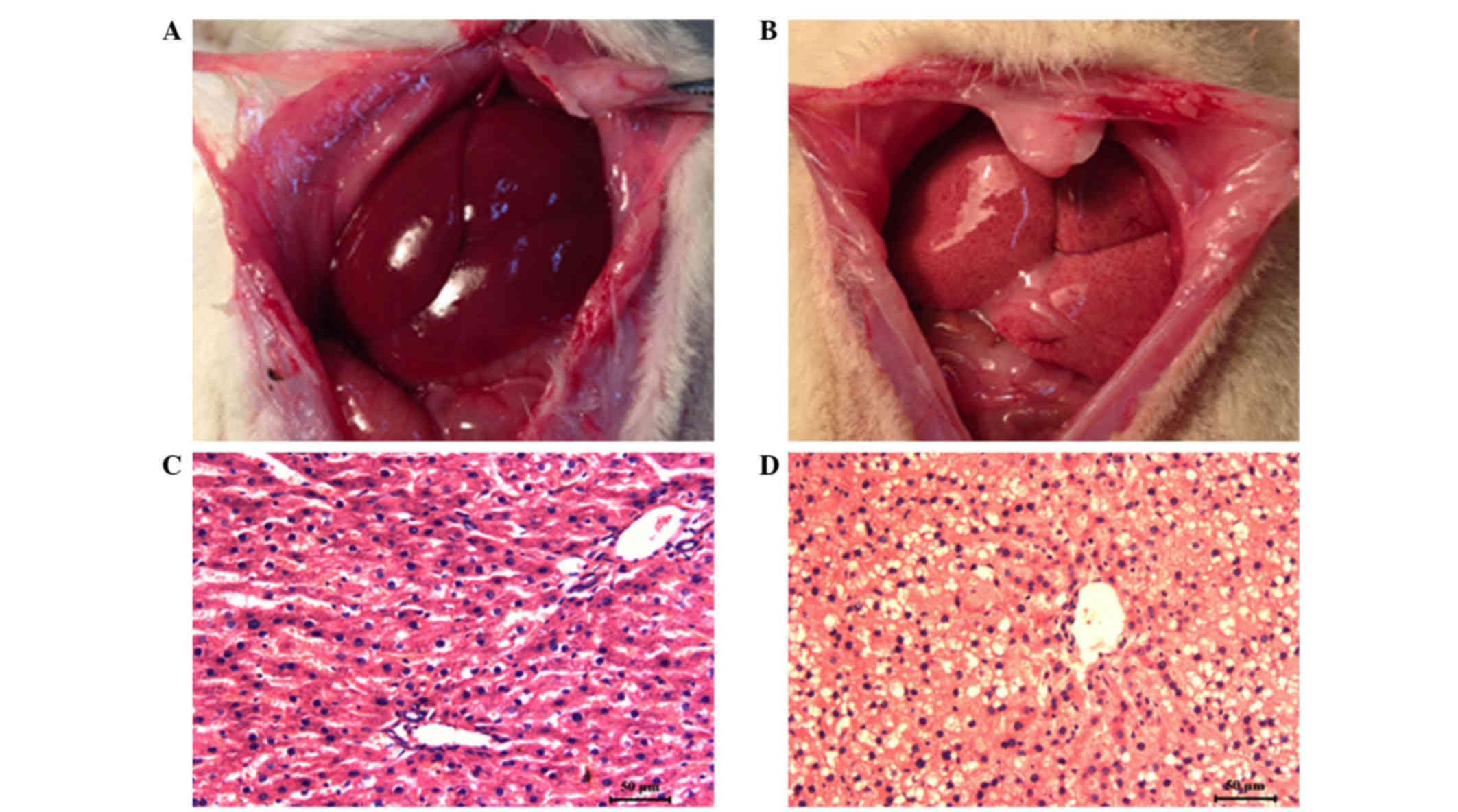
As shown in Fig. 1, in
contrast to the livers of the rats that were fed a normal diet, the
livers of the rats that were fed a high-fat diet for five weeks
were pale yellow, soft and smooth on the surface; however, no focal
nodule formation was observed (Fig. 1A
and B). HE staining revealed that the rats that were fed the
high-fat diet developed hepatic steatosis, particularly around the
liver lobes. These liver cells were swollen and round, with large
vacuoles in the cytoplasm. These morphological changes indicated
that the NAFLD rat model had been successfully established
(Fig. 1C and D).
RIPC has a protective effect on liver
IR
The gross and microscopic appearances of the livers
of rats following different surgical treatments are shown in
Fig. 2A and B. The gross appearance
indicated that the injuries incurred in rats of the IR group were
more severe than those incurred in rats of the RIPC+IR group. There
was no obvious difference between the RIPC group and the control
group. Upon pathological observation, the control group and the
RIPC group exhibited hepatic steatosis. The IR group exhibited
liver cell steatosis with a few inflammatory cells infiltrating the
portal area and severe edema in the liver cells. The RIPC+IR group
exhibited liver steatosis in the hepatic lobule, a small amount of
liver cell necrosis in the portal area, and mild edema of the liver
cells. Based on the gross and microscopic appearances of the
livers, rats of the IR group and the RIPC+IR group experienced more
severe injuries than the rats of the control group. However, the
liver condition of the RIPC+IR group was better than that of the IR
group. There was no clear difference between the RIPC group and the
control group.
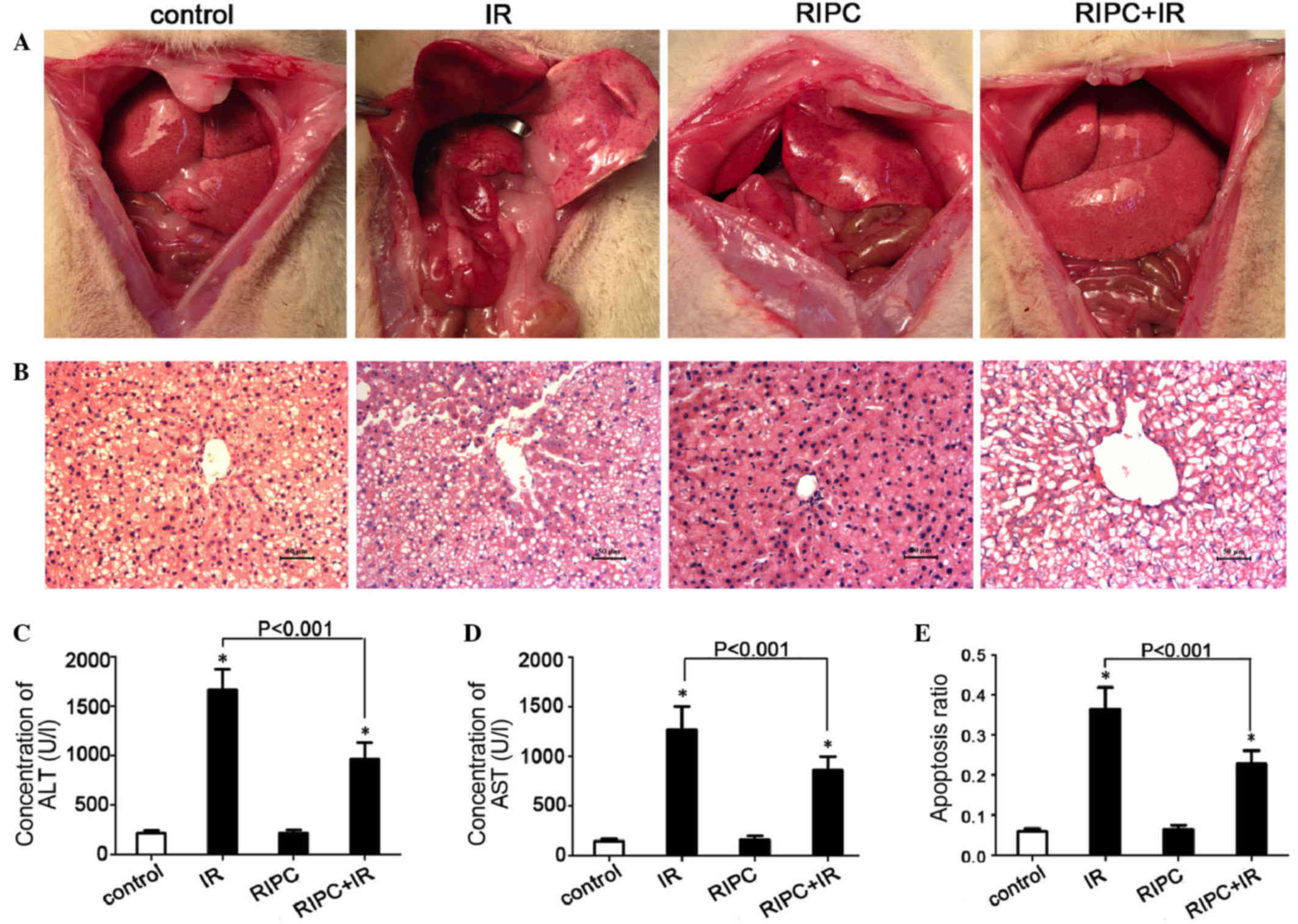 | Figure 2.RIPC has a protective effect against
liver IR. (A) Gross appearance of livers from rats of the control,
IR, RIPC, and RIPC+IR groups. (B) Microscopic view of livers from
rats of the control, IR, RIPC, RIPC+IR groups (hematoxylin and
eosin staining; scale bar, 50 µm). (C) Serum concentration of ALT
in each group. (D) Serum concentration of AST in each group. (E)
Cell apoptosis ratio in each group. Data are presented as the mean
+ standard deviation (n=6; *P<0.001 vs. control group). RIPC,
remote ischemic preconditioning; IR, ischemia-reperfusion; ALT,
alanine transaminase; AST, aspartate transaminase. |
As shown in Fig. 2C and
D, the levels of ALT and AST were lower in the RIPC+IR group
compared with the IR group (P<0.001). In addition, compared with
the control group, the ALT and AST levels were significantly higher
in the RIPC+IR and IR groups (both P<0.001). However, there were
no significant differences between the RIPC group and the control
group (ALT, P=0.9246; AST, P=0.5268).
Flow cytometry revealed that the cell apoptosis
ratio was significantly lower in the RIPC+IR group as compared with
the IR group (P<0.001; Fig. 2E).
The ratio of hepatocyte apoptosis was also significantly increased
in the RIPC+IR group and the IR group (both P<0.001) compared
with the control group. No significant difference was identified
between the RIPC group and the control group (P=0.4334).
Collectively, these results indicate that RIPC has a protective
effect on liver IR.
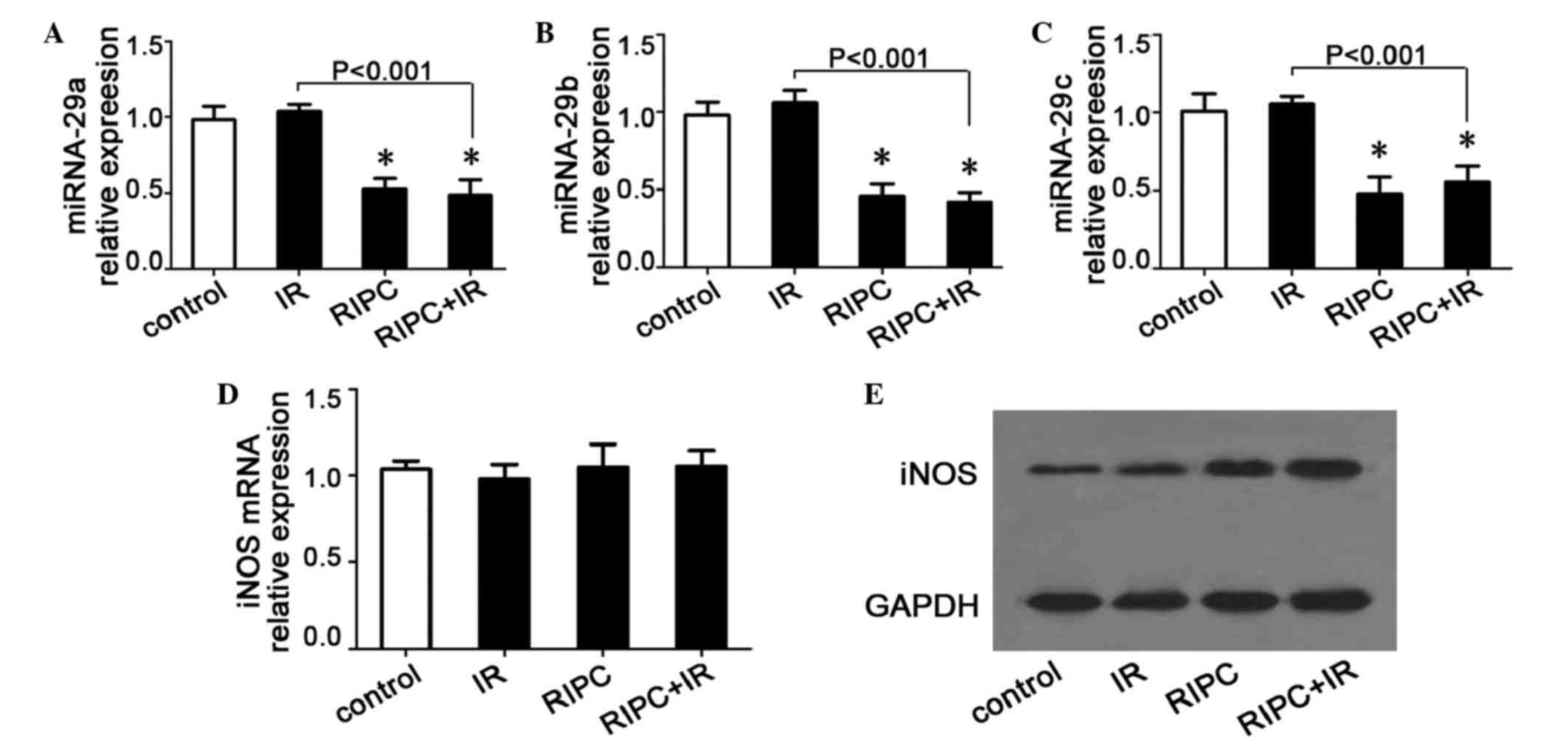
RIPC decreases the miR-29a/b/c levels
in skeletal muscle and increases the iNOS protein level
miR-29a/b/c levels in skeletal muscle were examined
by RT-qPCR. Compared with the control group, the miR-29a/b/c levels
were found to be significantly decreased in the RIPC and RIPC+IR
groups (P<0.05), whereas there was no variation in the IR group
(miR-29a vs. control, P=0.4021; miR-29b vs. control, P=0.3001;
miR-29c vs. control, P=0.5516; Fig.
3A-C). No significant differences between the RIPC group and
the RIPC+IR group were observed (miR-29a, P=0.6075; miR-29b,
P=0.5322; miR-29c, P=0.4414; Fig.
3A-C). The iNOS mRNA levels were not significantly different in
the IR (P=0.3661), RIPC (P=0.8991) or RIPC+IR (P=0.8077) groups
compared with the control group (Fig.
3D). However, the iNOS protein levels were increased
significantly in the RIPC group and the RIPC+IR group compared with
the control group, while no marked changes were observed in the IR
group (Fig. 3E). There was no evident
difference between the RIPC group and the RIPC+IR group. These
results indicate that RIPC may decrease the level of miR-29a/b/c in
skeletal muscle cells, thereby increasing the iNOS protein level,
which may be the molecular mechanism by which RIPC protects against
liver IR.
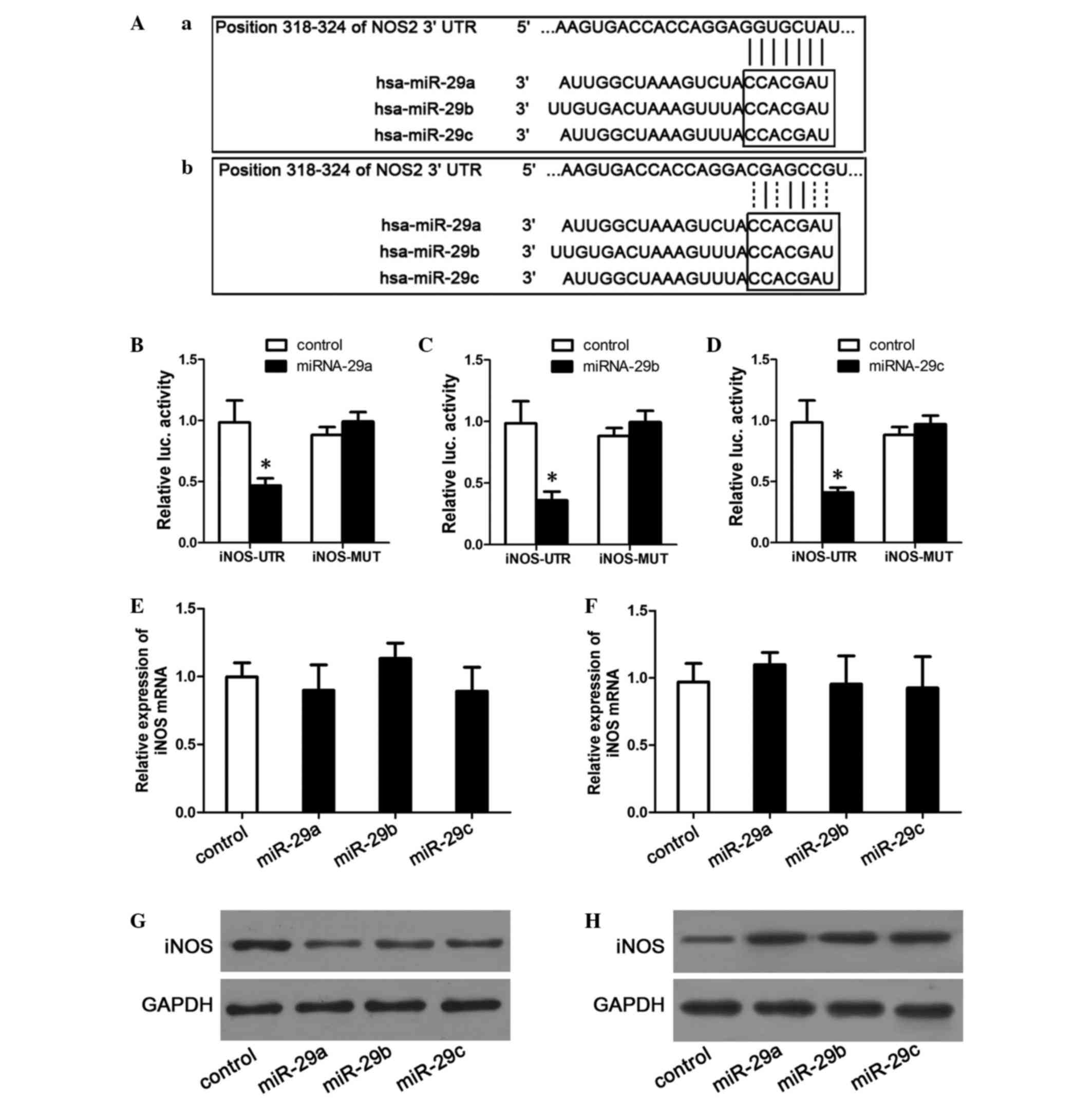
miR-29a/b/c targeting of iNOS
iNOS was hypothesized to be a target gene of
miR-29a/b/c, and the regulatory mechanism of miR-29a/b/c was
therefore explored. Potential miR-29a/b/c recognition sites in the
iNOS promoter were identified using the TargetScan website
(www.targetscan.org), and plasmids
containing a matching site or mutant site were subsequently
constructed. The sequences of these sites are listed in Fig. 4A. To establish a direct molecular link
between miR-29a/b/c and iNOS, a luciferase reporter assay was
performed. The data indicated that, compared to control plasmids,
transfection with miR-29a/b/c mimics significantly decreased the
luciferase activities of plasmids containing the wild-type iNOS
3′-UTR (P<0.001), whereas the activities of plasmids containing
the mutant iNOS 3′-UTR sequence were not markedly affected
[relative fluorescence intensities: miR-29a, 0.99±0.08 (P=0.1349);
miR-29b, 0.99±0.09 (P=0.1607); miR-29c, 0.97±0.07 (P=0.1824)]
(Fig. 4B-D).
To examine the effect of miR-29a/b/c on endogenous
iNOS mRNA expression, RT-qPCR was used to detect changes in the
mRNA expression levels of iNOS in miR-29a/b/c mimic-transfected or
inhibitor-transfected cells. The results revealed no significant
differences between the miRNA-mimic-transfected groups and the
control groups (P=0.4662 for miR-29a; P=0.1999 for miR-29b; and
P=0.4183 for miR-29c; Fig. 4E). In
addition, no significant differences were observed when comparing
the miR-inhibitor-transfected groups and the control groups
(P=0.2472 for miR-29a; P=0.9159 for miR-29b; P=0.7879 for miR-29c;
Fig. 4F). Finally, the effects of
miR-29a/b/c on the iNOS protein levels were examined. As shown in
Fig. 4G and H, transfection with the
miR-29a/b/c mimics downregulated iNOS protein expression levels,
whereas transfection with the miR-29a/b/c inhibitors increased the
iNOS protein levels. These results indicate that miR-29a, b and c
target and downregulate the iNOS protein, but not iNOS mRNA.
Discussion
The present study demonstrated that RIPC has a
protective effect on NAFLD liver IR injury. In addition, the data
revealed that RIPC reduced the miR-29a/b/c expression levels in
skeletal muscle and increased the protein levels of iNOS; these
results suggested that miR-29a/b/c targets iNOS, which plays an
important role in the protective effect of RIPC during NAFLD liver
IR injury.
In recent decades, IR injury has become a topic of
particular interest in the context of liver surgery and research.
Studies have predominantly focused on RIPC, which is a strategy for
harnessing the body's endogenous protective capabilities against
the injury that is incurred during IR (5–17). NO is
an essential mediator of the protection that is afforded by
hind-limb RIPC against liver IR injury (25). The mechanisms underlying this
protection involve the preservation of the sinusoidal structure and
the maintenance of blood flow through the hepatic microcirculation
(25). The NO level is predominantly
influenced by iNOS (27). NO is a
volatile gas, and it is possible to monitor its release by
measuring the mRNA and protein levels of iNOS (28). High expression of iNOS plays a
protective role in IR (29).
In the current study, the liver condition of the
NAFLD rats of the RIPC+IR group was better than that of NAFLD rats
of the IR group. In addition, the liver cell apoptosis ratio was
significantly lower in the RIPC+IR group compared with the IR
group, supporting the concept of a protective role of RIPC in NAFLD
liver IR injury. The iNOS protein level was significantly increased
in the RIPC and RIPC+IR groups compared with the control group,
suggesting that NO is an important mediator of the protective
effect of RIPC during NAFLD liver IR injury.
RIPC can increase the NO level (25). However, the specific mechanism
underlying this increase is not clear. In the present study,
miR-29a/b/c levels were determined to be decreased in skeletal
muscle cells of animals subjected to RIPC. miR-29 may serve
tumor-suppressive and tumorigenic roles in cancer; thus, the
expression of miR-29 may depend on the tissue and cellular context
(30). It has been reported that RIPC
protects the ischemic tissue from IR injury via a reactive oxygen
species (ROS)-dependent pathway (11,31,32), and
that oxidative stress alters miRNA expression (33,34). In
the present study, oxidative stress or ROS were not measured.
However, combining our data with that of previous reports, we
speculate that the decrease in the miR-29a/b/c levels may be a
result of oxidative stress due to the generation of ROS during
RIPC.
To investigate the association between the increased
iNOS and decreased miR-29a/b/c levels, a dual luciferase reporter
assay was performed, which indicated that miR-29a/b/c directly
targets iNOS. Notably, in a previous study, a significant increase
in miR-29b and decrease in miR-29c expression was observed in the
spleen following Cryptosporidium parvum-induced inflammation
in C57BL mice, as compared with the controls. However, no
significant change was observed in the expression of miR-29b/c in
iNOS-knockout mice, suggesting that iNOS is required for the
Cryptosporidium parvum-induced increase in miR-29b and
decrease in miR-29c (35); this
indicates that the regulatory mechanism between iNOS and
miR-29a/b/c is complex and may involve a feedback loop. Further
studies are therefore required to investigate the association
between iNOS and miR-29a/b/c.
In conclusion, the current study demonstrated that
RIPC has a protective effect against NAFLD liver IR injury. This
effect may occur due to a reduction of miR-29a/b/c levels in
skeletal muscle, leading to increases in iNOS and, subsequently,
NO. RIPC should be investigated further as a potential alternative
protective strategy during NAFLD liver IR injury.
Acknowledgements
This work was supported by grants from the National
Natural Science Foundation of China (no. 81502002), Applied Basic
Research Project of Changzhou (no. CJ20140023) and Changzhou
High-Level Medical Talents Training Project (no. 2016CZBJ044).
References
|
1
|
Lomonaco R, Sunny NE, Bril F and Cusi K:
Nonalcoholic fatty liver disease: Current issues and novel
treatment approaches. Drugs. 73:1–14. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
de Meijer VE, Kalish BT, Puder M and
Ijzermans JN: Systematic review and meta-analysis of steatosis as a
risk factor in major hepatic resection. Br J Surg. 97:1331–1339.
2010. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Marsman HA, Heger M, Kloek JJ, Nienhuis
SL, ten Kate FJ and van Gulik TM: Omega-3 fatty acids reduce
hepatic steatosis and consequently attenuate ischemia-reperfusion
injury following partial hepatectomy in rats. Dig Liver Dis.
43:984–990. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Song X, Xu H, Feng Y, Li X, Lin M and Cao
L: Protective effect of grape seed proanthocyanidins against liver
ischemic reperfusion injury: Particularly in diet-induced obese
mice. Int J Biol Sci. 8:1345–1362. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gassanov N, Nia AM, Caglayan E and Er F:
Remote ischemic preconditioning and renoprotection: From myth to a
novel therapeutic option. J Am Soc Nephrol. 25:216–224. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Manchurov V, Ryazankina N, Khmara T,
Skrypnik D, Reztsov R, Vasilieva E and Shpektor A: Remote ischemic
preconditioning and endothelial function in patients with acute
myocardial infarction and primary PCI. Am J Med. 127:670–673. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Schmidt MR, Støttrup NB, Michelsen MM,
Contractor H, Sørensen KE, Kharbanda RK, Redington AN and Bøtker
HE: Remote ischemic preconditioning impairs ventricular function
and increases infarct size after prolonged ischemia in the isolated
neonatal rabbit heart. J Thorac Cardiovasc Surg. 147:1049–1055.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Crimi G, Pica S, Raineri C, Bramucci E, De
Ferrari GM, Klersy C, Ferlini M, Marinoni B, Repetto A, Romeo M, et
al: Remote ischemic post-conditioning of the lower limb during
primary percutaneous coronary intervention safely reduces enzymatic
infarct size in anterior myocardial infarction: A randomized
controlled trial. JACC Cardiovasc Interv. 6:1055–1063. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cai ZP, Parajuli N, Zheng X and Becker L:
Remote ischemic preconditioning confers late protection against
myocardial ischemia-reperfusion injury in mice by upregulating
interleukin-10. Basic Res Cardiol. 107:2772012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wever KE, Warlé MC, Wagener FA, van der
Hoorn JW, Masereeuw R, van der Vliet JA and Rongen GA: Remote
ischaemic preconditioning by brief hind limb ischaemia protects
against renal ischaemia-reperfusion injury: The role of adenosine.
Nephrol Dial Transplant. 26:3108–3117. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dong HL, Zhang Y, Su BX, Zhu ZH, Gu QH,
Sang HF and Xiong L: Limb remote ischemic preconditioning protects
the spinal cord from ischemia-reperfusion injury: A newly
identified nonneuronal but reactive oxygen species-dependent
pathway. Anesthesiology. 112:881–891. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhou W, Zeng D, Chen R, Liu J, Yang G, Liu
P and Zhou X: Limb ischemic preconditioning reduces heart and lung
injury after an open heart operation in infants. Pediatr Cardiol.
31:22–29. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rentoukas I, Giannopoulos G, Kaoukis A,
Kossyvakis C, Raisakis K, Driva M, Panagopoulou V, Tsarouchas K,
Vavetsi S, Pyrgakis V and Deftereos S: Cardioprotective role of
remote ischemic periconditioning in primary percutaneous coronary
intervention: Enhancement by opioid action. JACC Cardiovasc Interv.
3:49–55. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kanoria S, Jalan R, Davies NA, Seifalian
AM, Williams R and Davidson BR: Remote ischaemic preconditioning of
the hind limb reduces experimental liver warm ischaemia-reperfusion
injury. Br J Surg. 93:762–768. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tapuria N, Junnarkar SP, Dutt N, Abu-Amara
M, Fuller B, Seifalian AM and Davidson BR: Effect of remote
ischemic preconditioning on hepatic microcirculation and function
in a rat model of hepatic ischemia reperfusion injury. HPB
(Oxford). 11:108–117. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang M, Shen J, Feng B, Gui L, Chen Q,
Zhang B, Tang J and Li X: Remote ischemic preconditioning promotes
early liver cell proliferation in a rat model of small-for-size
liver transplantation. J Surg Res. 179:e245–e253. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Abu-Amara M, Yang SY, Quaglia A, Rowley P,
Tapuria N, Seifalian AM, Fuller BJ and Davidson BR: Effect of
remote ischemic preconditioning on liver ischemia/reperfusion
injury using a new mouse model. Liver Transpl. 17:70–82. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jensen RV, Zachara NE, Nielsen PH, Kimose
HH, Kristiansen SB and Bøtker HE: Impact of O-GlcNAc on
cardioprotection by remote ischaemic preconditioning in
non-diabetic and diabetic patients. Cardiovasc Res. 97:369–378.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Contractor H, Støttrup NB, Cunnington C,
Manlhiot C, Diesch J, Ormerod JO, Jensen R, Bøtker HE, Redington A,
Schmidt MR, et al: Aldehyde dehydrogenase-2 inhibition blocks
remote preconditioning in experimental and human models. Basic Res
Cardiol. 108:3432013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Abu-Amara M, Yang SY, Quaglia A, Rowley P,
Tapuria N, Fuller B, Davidson B and Seifalian A: The hepatic
soluble guanylyl cyclase-cyclic guanosine monophosphate pathway
mediates the protection of remote ischemic preconditioning on the
microcirculation in liver ischemia-reperfusion injury.
Transplantation. 93:880–886. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zitta K, Meybohm P, Bein B, Heinrich C,
Renner J, Cremer J, Steinfath M, Scholz J and Albrecht M: Serum
from patients undergoing remote ischemic preconditioning protects
cultured human intestinal cells from hypoxia-induced damage:
Involvement of matrixmetalloproteinase-2 and −9. Mol Med. 18:29–37.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li J, Xuan W, Yan R, Tropak MB,
Jean-St-Michel E, Liang W, Gladstone R, Backx PH, Kharbanda RK and
Redington AN: Remote preconditioning provides potent
cardioprotection via PI3K/Akt activation and is associated with
nuclear accumulation of β-catenin. Clin Sci (Lond). 120:451–462.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kanoria S, Glantzounis G, Quaglia A,
Dinesh S, Fusai G, Davidson BR and Seifalian AM: Remote
preconditioning improves hepatic oxygenation after ischaemia
reperfusion injury. Transpl Int. 25:783–791. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tapuria N, Junnarkar S, Abu-Amara M,
Fuller B, Seifalian AM and Davidson BR: Modulation of
microcirculatory changes in the late phase of hepatic
ischaemia-reperfusion injury by remote ischaemic preconditioning.
HPB (Oxford). 14:87–97. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Abu-Amara M, Yang SY, Quaglia A, Rowley P,
de Mel A, Tapuria N, Seifalian A, Davidson B and Fuller B: Nitric
oxide is an essential mediator of the protective effects of remote
ischaemic preconditioning in a mouse model of liver
ischaemia/reperfusion injury. Clin Sci (Lond). 121:257–266. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Michel T and Feron O: Nitric oxide
synthases: which, where, how, and why? J Clin Invest.
100:2146–2152. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Alderton WK, Cooper CE and Knowles RG:
Nitric oxide synthases: structure, function and inhibition. Biochem
J. 357:593–615. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Phillips L, Toledo AH, Lopez-Neblina F,
Anaya-Prado R and Toledo-Pereyra LH: Nitric oxide mechanism of
protection in ischemia and reperfusion injury. J Invest Surg.
22:46–55. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jiang H, Zhang G, Wu JH and Jiang CP:
Diverse roles of miR-29 in cancer (review). Oncol Rep.
31:1509–1516. 2014.PubMed/NCBI
|
|
31
|
Galagudza MM, Sonin DL, Vlasov TD,
Kurapeev DI and Shlyakhto EV: Remote vs. local ischaemic
preconditioning in the rat heart: infarct limitation, suppression
of ischaemic arrhythmia and the role of reactive oxygen species.
Int J Exp Pathol. 97:66–74. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Totzeck M, Hendgen-Cotta U and Rassaf T:
Concepts of hypoxic NO signaling in remote ischemic
preconditioning. World J Cardiol. 7:645–651. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Fatemi N, Sanati MH, Shamsara M, Moayer F,
Zavarehei MJ, Pouya A, Sayyahpour F, Ayat H and Gourabi H:
TBHP-induced oxidative stress alters microRNAs expression in mouse
testis. J Assist Reprod Genet. 31:1287–1293. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang Z, Liu Y, Han N, Chen X, Yu W, Zhang
W and Zou F: Profiles of oxidative stress-related microRNA and mRNA
expression in auditory cells. Brain Res. 1346:14–25. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Mathé E, Nguyen GH, Funamizu N, He P,
Moake M, Croce CM and Hussain SP: Inflammation regulates microRNA
expression in cooperation with p53 and nitric oxide. Int J Cancer.
131:760–765. 2012. View Article : Google Scholar : PubMed/NCBI
|