Introduction
The incidence of esophageal squamous cell carcinoma
(ESCC) shows geographical variation and the highest rates have been
reported in China (1). A number of
risk factors have been reported for ESCC, including dietary
factors, bad behavioral habits, socioeconomic status and genetics
(1,2).
Research has identified numerous molecular markers, particularly
for the early stage of ESCC (1,2).
Therefore, it is important to investigate molecular alterations in
tumors and precancerous lesions for the early detection or
classification of ESCC (1).
The vascular endothelial growth factor (VEGF)-A gene
may be an important marker in the development and progression of
ESCC in Chinese populations (3). The
aberrant methylation of tumor related-genes, such as fragile
histidine triad, E-cadherin and integrin-α4, has been reported to
be an independent adverse prognostic factor in ESCC (4,5).
Clinicopathological and molecular investigations of early squamous
cell carcinoma in precancerous lesions and early-stage ESCCs have
identified effective biomarkers to predict the risk of patients
with dysplasia, the infiltration depth of tumors, and lymph node
metastasis and vascular invasion (4–6).
Reversion-inducing cysteine-rich protein with kazal
motifs (RECK) is a novel tumor suppressor gene that negatively
regulates matrix metalloproteinases (MMPs). RECK is expressed in
various normal human tissues, but is downregulated in several types
of human tumors and has been positively correlated with the
survival of patients with cancer (7–10).
Furthermore, RECK is hypothesized to be involved in the maturation
of blood vessels, and RECK and cluster of differentiation (CD) 34
(a known vessel marker) exhibited a strong positive correlation in
glioma (11). In addition, there is a
significant correlation between RECK gene expression and the
formation of new blood vessels by VEGF (12), and the expression of RECK was
associated with VEGF and CD105, which have an important role in
esophageal carcinoma (13).
A previous study reported that hypoxia was able to
induce RECK silenced by histone deacetylase (HDAC) 1 and the
interaction of hypoxia-inducible factor (HIF)-1α with the reverse
HRE2 site in the promoter, and that downregulation of RECK may be a
therapeutic and preventive target for tumorigenesis (14). The inhibition of HDAC by small
interfering (si)RNA-targeting RECK and trichostatin A successfully
restored RECK expression under hypoxic conditions and inhibited the
migration and invasion of cancer cells (15). It has been suggested that the binding
activity of HIF-1α is important for the activation of the RECK
promoter under hypoxic conditions (16). Furthermore, the results of a previous
study supported the notion that RECK dsRNA formation in the
promoter region has the potential to upregulate RECK gene
expression (17).
Gene promoter hypermethylation has been associated
with the silencing of tumor-related genes, which is considered the
most important epigenetic disruption in numerous tumors (18,19). In a
previous study, hypermethylation of the RECK gene was partially
reversed by epigallocatechin gallate treatment, and the mRNA
expression level of RECK was significantly enhanced in oral
squamous cell carcinoma cell lines (20). RAS was able to increase the binding of
DNA methyltransferase (DNMT) 3B to the RECK promoter and induce
promoter methylation, while treatment with 5′-azacytidine and
DNMT3B-targeting siRNA restored RECK expression and potently
suppressed cell invasion (21). These
results suggested that RECK methylation may be an important
regulatory mechanism of RECK expression in ESCC. However, the
molecular mechanism underlying this downregulation and its
biological significance in ESCC remain unclear. Furthermore, no
studies have investigated RECK methylation and RECK mRNA expression
in ESCC. The present study aimed to investigate the relationship
between RECK mRNA expression and promoter methylation in ESCC.
Materials and methods
Patients and tissue samples
Tumor and non-tumor samples were obtained by
surgical resection from 310 ESCC patients between May 2001 and June
2014. These specimens were collected from Changzhou Cancer Hospital
and Nanyang Center Hospital in China. The matched non-tumor tissues
were obtained from >3 cm away from the tumors and were confirmed
to be tumor-free by microscopic examination. The tissues were
maintained at −196°C until processing for RNA/DNA extraction. The
patients included 180 men and 130 women, and ranged in age from
36–82 years (mean age, 52.47±12.43 years). In total, 88 cases were
well-differentiated, 142 were moderately-differentiated and 80 were
poorly-differentiated. The present study was approved by the
committee on Human Experimentation of Soochow University
(Changzhou, China). Written informed consent was obtained from all
patients.
RECK promoter methylation
analysis
Tissue DNA was isolated using the Commercial QIAamp
DNA Mini kit (Qiagen GmbH, Hilden, Germany). Bisulfite modification
of the DNA was performed using the EpiSeeker DNA Purification and
Modification kit (Abcam, Cambridge, MA, USA) as described
previously (22), and RECK
methylation was measured by methylation-specific polymerase chain
reaction (PCR) (19). Unmethylated
RECK primers were as follows: Forward,
5′-TAAAGAGTTTTGGTATGGGGTATGT-3′ and reverse,
5′-CTCCAAACCACAAAATACTCAAA-3′. Methylated RECK primers were:
Forward, 5′-AATAAAGAGTTTTGGTACGGGGTAC-3′ and reverse,
5′-AAAACCGCGAAATACTCGAA-3′. Modified DNA samples (2 µl) were used
for PCR amplification. PCR was performed in a thermal cycler for 40
cycles. The conditions for PCR were denaturation at 95°C for 30
sec, annealing at 58°C for 30 sec and extension at 72°C for 30 sec.
The PCR products were separated on 2% agarose gels and visualized
by ethidium bromide staining. The methylation index (MI) of RECK
was calculated using the following formula: MI = 100 × methylated
reaction / (unmethylated reaction + methylated reaction) (23). ΔMI was defined as MIESCC -
MINon-tumor.
RECK mRNA quantitative analysis
Total RNA was isolated from 310 ESCC and adjacent
normal tissues using TRIzol reagent (Takara Biotechnology Co.,
Ltd., Dalian, China). First-strand cDNA was synthesized from 2 µg
total RNA using PrimeScript™ RT Reagent kit (Takara Biotechnology
Co., Ltd.). RECK mRNA primers were as follows: Forward,
5′-CCTCAGTGAGCACAGTTCAGA-3′ and reverse, 5′-GCAGCACACACACTGCTGTA-3′
(19). Quantitative PCR (qPCR) was
performed using 20 µl SYBR Premix Ex Taq (Takara Biotechnology Co.,
Ltd.) on the Mx3000P QPCR System (Agilent Technologies, Inc., Santa
Clara, CA, USA). qPCR was performed for 40 cycles of 95°C for 30
sec, 60°C for 30 sec and 72°C for 1 min. β-actin mRNA was amplified
from the same cDNA samples as an internal control. All results were
normalized to β-actin. The relative RECK expression level was
determined using the comparative Cq method (24), using average Cq values for RECK and
β-actin.
Statistical analysis
The clinical data, MI and mRNA expression levels
were analyzed using SPSS 18.0 software (SPSS, Inc., Chicago, IL,
USA). Comparisons were performed using Pearson's χ2
test, or unpaired or paired t-tests. All P-values are two-sided,
and a P-value of <0.05 was considered statistically significant.
Survival curves were based on Kaplan-Meier estimates.
Results
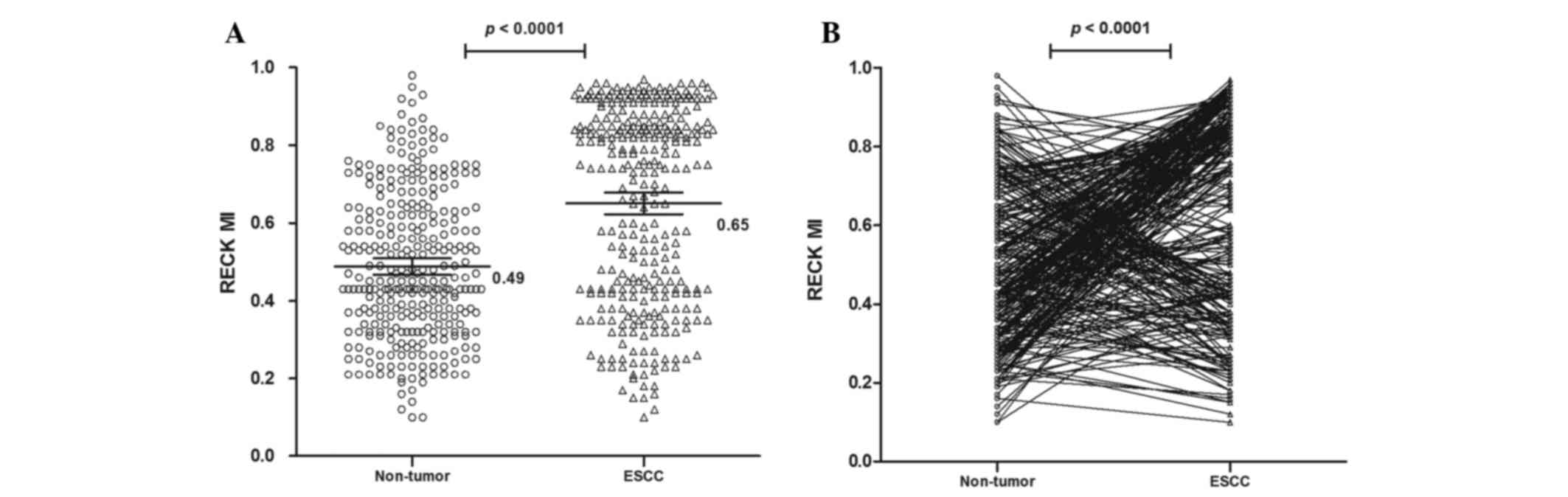
MI of the RECK gene in tissues
The methylation status of the RECK gene was analyzed
in 310 ESCC and non-tumor tissues. The methylated PCR product
contains methylated and/or unmethylated PCR products (25). It was observed that the mean MI of the
RECK gene was 0.65 [95% confidence interval (CI)=0.62 to 0.68] in
ESCC and 0.49 (95% CI=0.47 to 0.51) in non-tumor samples, which was
significantly different (P<0.0001; Fig. 1). These results suggest that the RECK
promoter is hypermethylated in ESCC compared with non-tumor
samples.
RECK methylation and
clinicopathological features of ESCC
Associations between the RECK methylation level and
the clinical characteristics of ESCC patients, including age,
gender, American Joint Committee on Cancer (AJCC) stage,
differentiation and others, were analyzed (Table I). A cutoff value of 0.16 was set for
ΔMI and the patients were classified according to the mean MI of
RECK in ESCC and non-tumor tissues. There was a significant
difference in the AJCC stage (P<0.0001) and the stage of lymph
node metastasis (P=0.001) between cases where ΔMI ≤0.16 and ΔMI
>0.16. These results suggest that RECK hypermethylation may
occur more frequently in ESCC patients with advanced tumors and
lymph node metastasis.
 | Table I.Correlation of clinicopathological
variables with RECK MI in esophageal squamous cell carcinoma. |
Table I.
Correlation of clinicopathological
variables with RECK MI in esophageal squamous cell carcinoma.
| Variable | n | ΔMI ≤0.16
(n=151) | ΔMI >0.16
(n=159) | Odds ratio (95%
CI) | P-valuea |
|---|
| Gender |
|
|
|
| 0.092 |
| Male | 180 | 95 | 85 | 1.48 (0.94–2.33) |
|
|
Female | 130 | 56 | 74 |
|
|
| Age (years) |
|
|
|
| 0.965 |
| ≥60 | 170 | 83 | 87 | 1.01 (0.65–1.58) |
|
|
<60 | 140 | 68 | 72 |
|
|
| Size (cm) |
|
|
|
| 0.387 |
|
<3 | 172 | 80 | 92 | 0.82 (0.52–1.29) |
|
| ≥3 | 138 | 71 | 67 |
|
|
| Tobacco |
|
|
|
| 0.062 |
|
Yes | 168 | 90 | 78 | 1.53
(0.98–2.40) |
|
| No | 142 | 61 | 81 |
|
|
| Alcohol |
|
|
|
| 0.056 |
|
Yes | 147 | 80 | 67 | 1.55
(0.99–2.42) |
|
| No | 163 | 71 | 92 |
|
|
| Depth of
invasion |
|
|
|
| 0.079 |
|
T1–2 | 224 | 108 | 116 | 0.93
(0.57–1.53) |
|
|
T3–4 | 86 | 43 | 43 |
|
|
| AJCC stage |
|
|
|
|
<0.0001b |
|
I–II | 125 | 78 | 47 | 2.55
(1.60–4.06) |
|
|
III–IV | 185 | 73 | 112 |
|
|
| Lymph node
metastasis |
|
|
|
| 0.001b |
|
N0–1 | 148 | 87 | 61 | 2.18
(1.39–3.44) |
|
|
N2–3 | 162 | 64 | 98 |
|
|
| Distant
metastasis |
|
|
|
| 0.513 |
|
M0 | 251 | 120 | 131 | 0.83
(0.47–1.46) |
|
|
M1 | 59 | 31 | 28 |
|
|
|
Differentiation |
|
|
|
| 0.806 |
|
G1 | 88 | 41 | 47 |
|
|
|
G2 | 142 | 72 | 70 |
|
|
|
G3 | 80 | 38 | 42 |
|
|
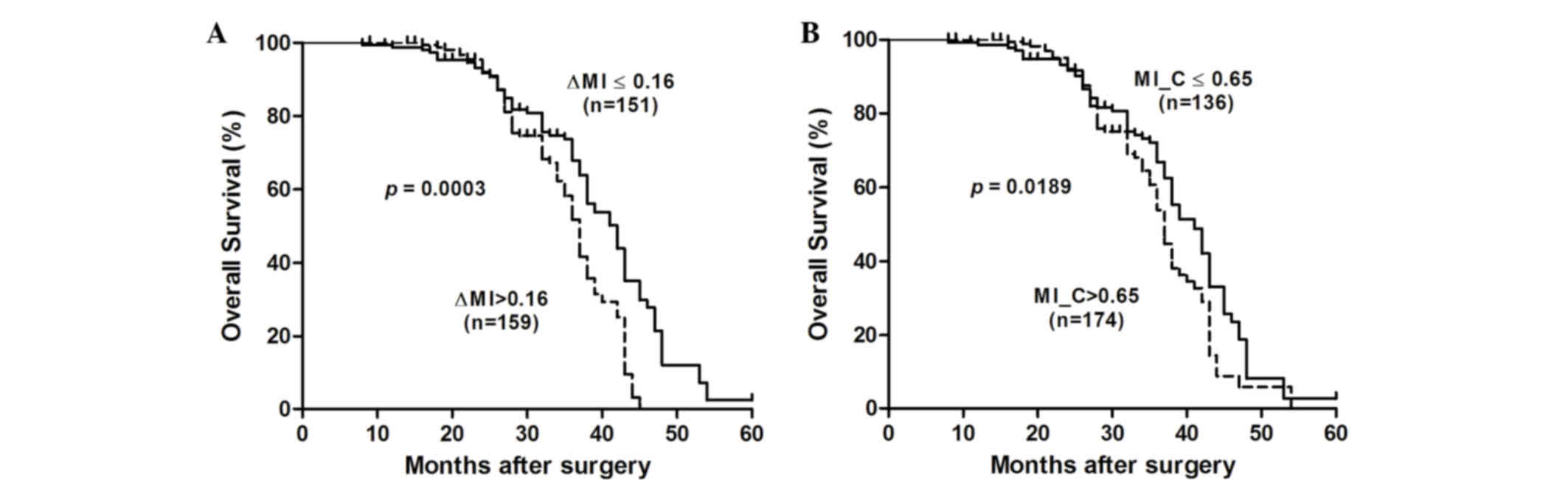
Subsequently, associations between the RECK MI and
postoperative outcomes were assessed using the Kaplan-Meier method
and log-rank test (Fig. 2). The
results suggested that the median cumulative survival time was
significantly shorter for ESCC patients with ΔMI >0.16 compared
with those with ΔMI ≤0.16 [37 vs. 42 months; log-rank P=0.0003;
hazard ratio (HR)=1.896 (95% CI=1.34 to 2.68)]. Furthermore, it was
observed that ESCC patients with an MI of >0.65 had a
significantly shorter median cumulative survival time compared with
those with an MI of ≤0.65 [37 vs. 41 months; log-rank P=0.0189;
HR=1.484 (95% CI=1.07 to 2.06)]. These results suggest that the
RECK MI may be a good prognostic marker in ESCC.
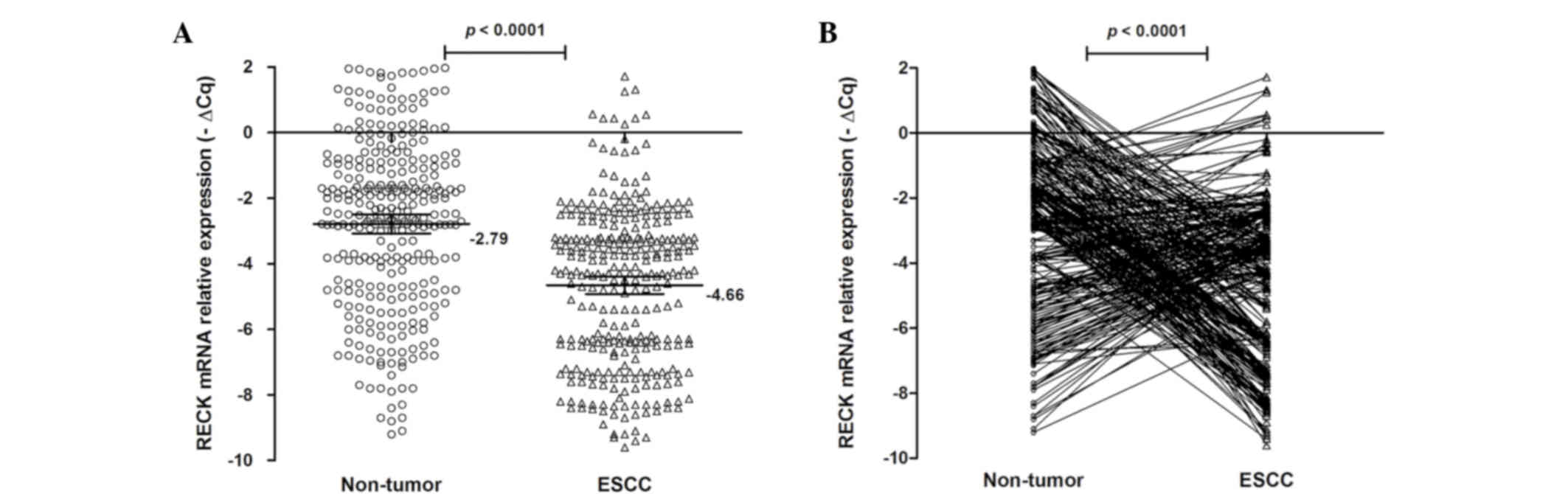
RECK mRNA expression level in
tissues
qPCR was performed to quantify the relative mRNA
expression levels of RECK in 310 ESCC and matched non-tumor
tissues. The RECK mRNA expression levels are shown in Fig. 3. The mean-∆Cq RECK mRNA
expression level was −4.66 (95% CI=−4.92 to −4.39) in ESCC tissues
and −2.79 (95% CI=−3.08 to −2.50) in non-tumor tissues. There was a
significant difference between ESCC tissues and matching non-tumor
tissues (P<0.0001; Fig. 3).
Furthermore, the RECK mRNA expression level was increased (−∆∆Cq
>0) in 130 patients (41.94%), but decreased (−∆∆Cq ≤0) in 150
patients (58.06%). These results suggest that RECK mRNA expression
may be an important factor for ESCC.
RECK mRNA is associated with the
clinical features of ESCC
The relationship between the mRNA expression level
of RECK and clinical factors was evaluated for 310 tumor tissues.
These analyses are summarized in Table
II. There was a significant difference in the mRNA expression
levels of RECK between ESCC patients with N0–1 and
N2–3 stages of lymph node metastasis. A lower mRNA
expression level of RECK (−ΔΔCq ≤0) was observed in 67/148 (43.24%)
ESCC patients with N0–1 lymph node metastasis and
113/162 (69.75%) ESCC patients with N2–3 lymph node
metastasis (P<0.0001).
 | Table II.Correlation of clinicopathological
variables with RECK mRNA expression in esophageal squamous cell
carcinoma. |
Table II.
Correlation of clinicopathological
variables with RECK mRNA expression in esophageal squamous cell
carcinoma.
| Variable | n |
−ΔΔCqRECK >0 (n=130) |
−ΔΔCqRECK ≤0 (n=180) | Odds ratio (95%
CI) |
P-valuea |
|---|
| Gender |
|
|
|
| 0.292 |
|
Male | 180 | 80 | 100 | 1.28
(0.81–2.03) |
|
|
Female | 130 | 50 | 80 |
|
|
| Age (years) |
|
|
|
| 0.221 |
|
≥60 | 170 | 66 | 104 | 0.75
(0.48–1.19) |
|
|
<60 | 140 | 64 | 76 |
|
|
| Size (cm) |
|
|
|
| 0.012 |
|
<3 | 172 | 83 | 89 | 1.81
(1.14–2.87) |
|
| ≥3 | 138 | 47 | 91 |
|
|
| Tobacco |
|
|
|
| 0.571 |
|
Yes | 168 | 68 | 100 | 0.88
(0.59–1.38) |
|
| No | 142 | 62 | 80 |
|
|
| Alcohol |
|
|
|
| 1.000 |
|
Yes | 147 | 65 | 82 | 1.0
(0.64–1.56) |
|
| No | 163 | 65 | 98 |
|
|
| Depth of
invasion |
|
|
|
| 0.296 |
|
T1–2 | 224 | 98 | 126 | 1.31
(0.79–2.19) |
|
|
T3–4 | 86 | 32 | 54 |
|
|
| AJCC stage |
|
|
|
| 0.545 |
|
I–II | 125 | 55 | 70 | 1.15
(0.73–1.82) |
|
|
III–IV | 185 | 75 | 110 |
|
|
| Lymph node
metastasis |
|
|
|
|
<0.0001b |
|
N0–1 | 148 | 85 | 67 | 3.19
(1.99–5.10) |
|
|
N2–3 | 162 | 45 | 113 |
|
|
| Distant
metastasis |
|
|
|
| 0.421 |
|
M0 | 251 | 108 | 143 | 1.27
(0.71–2.28) |
|
|
M1 | 59 | 22 | 37 |
|
|
|
Differentiation |
|
|
|
| 0.533 |
|
G1 | 88 | 36 | 52 |
|
|
|
G2 | 142 | 64 | 78 |
|
|
|
G3 | 80 | 30 | 50 |
|
|
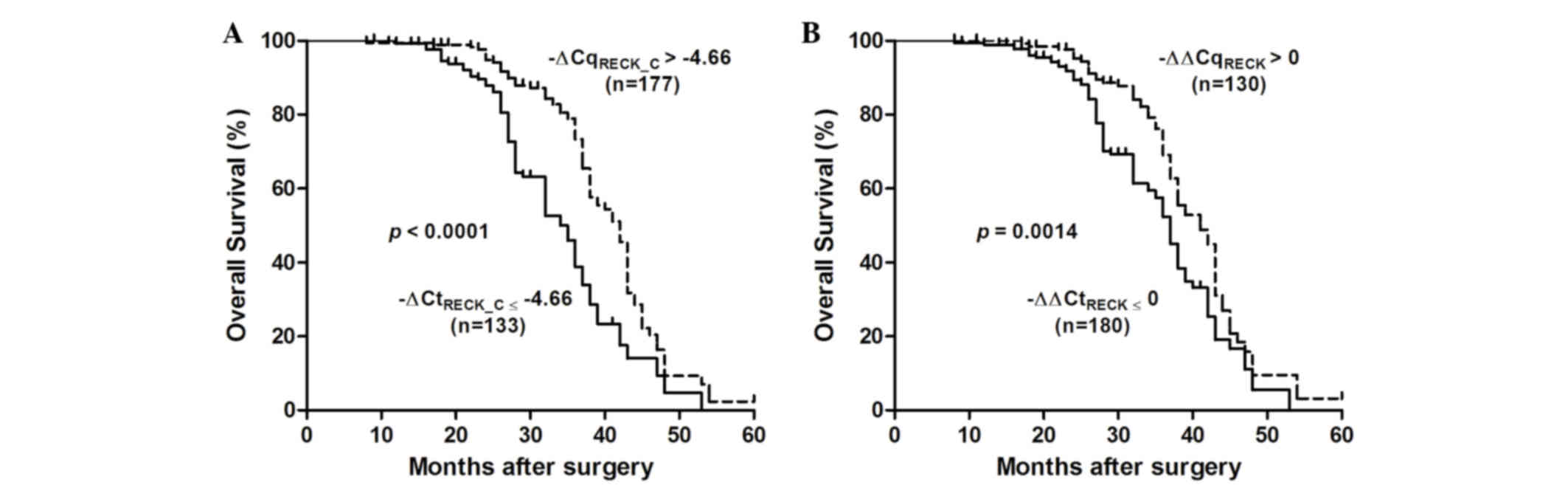
The association between RECK mRNA expression and
survival in patients with ESCC was assessed using the Kaplan-Meier
method and log rank test (Fig. 4).
Decreased RECK mRNA expression (−∆∆Cq <0) was significantly
correlated with a poor overall survival [37 vs. 41 months;
P=0.0014; HR=0.586 (95% CI=0.42 to 0.81)]. In addition, ESCC
patients with -∆CqRECK ≤-4.66 had a shorter median
cumulative survival time compared with those with
-∆CqRECK >-4.66 [35 vs. 42 months; log-rank
P<0.0001; HR=0.379 (95% CI=0.26 to 0.55)]. These results suggest
that RECK mRNA silencing may have an important role in the poor
survival of patients with ESCC.
Decreased RECK mRNA expression and
RECK hypermethylation in ESCC
Subsequently, the association between the MI and
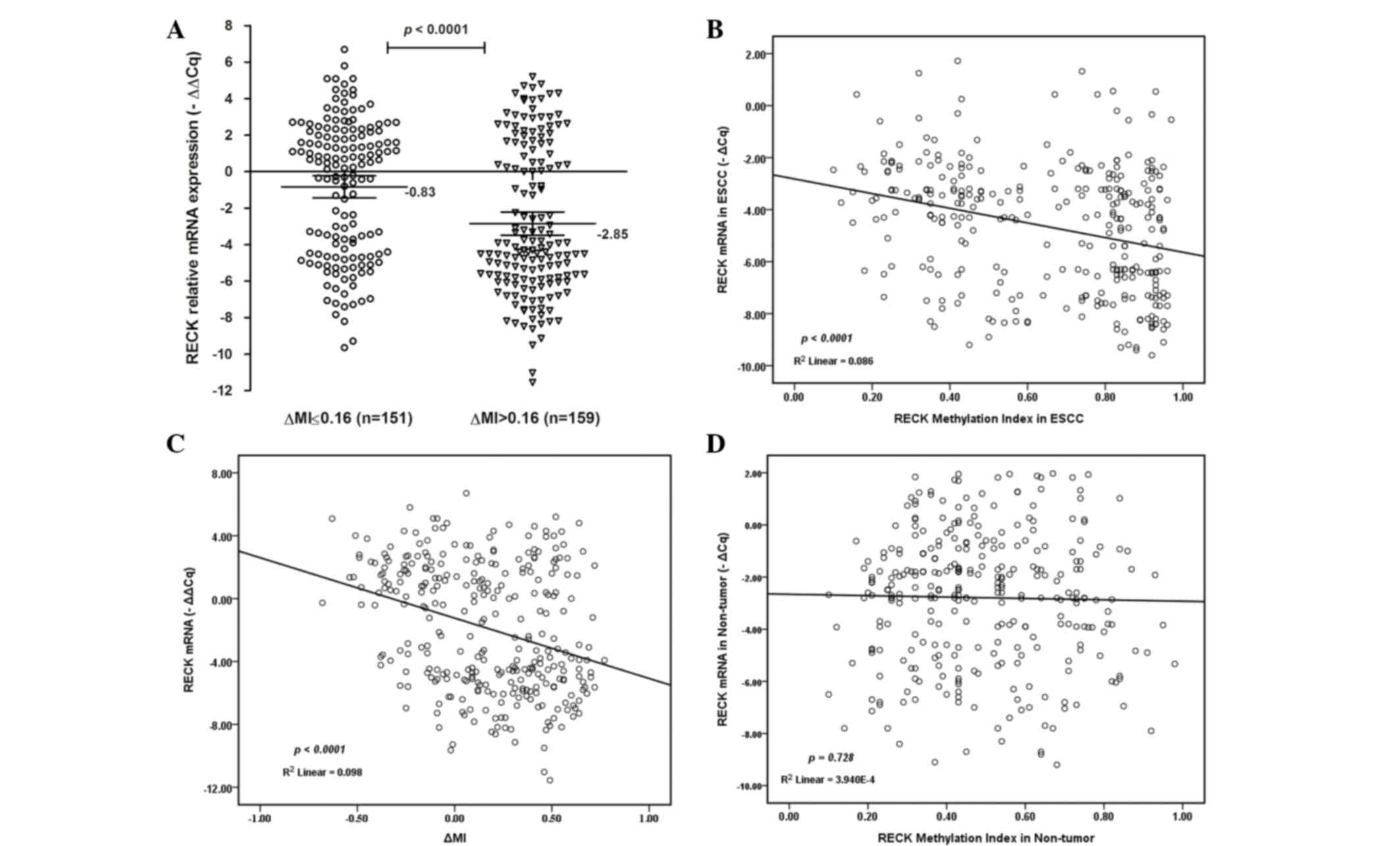
mRNA expression of RECK was investigated (Fig. 5). Notably, the mRNA expression levels
of RECK were lower in ESCC tissues with ΔMI >0.16
[mean-∆∆Cq =−2.85; 95% CI=−3.48 to 2.21) compared with
ESCC tissues with ΔMI ≤0.16 [mean-∆∆Cq =−0.83; 95%
CI=−1.43 to −0.23; P<0.0001; Fig.
5A]. There was a decreasing tendency for RECK mRNA expression
from promoter hypermethylation to hypomethylation in ESCC tissues.
A decreased RECK mRNA expression level was significantly associated
with the demethylation status of RECK in ESCC patients
(R2=0.086; P<0.0001; Fig.
5B; R2=0.098; P<0.0001; Fig. 5C). However, there was no significant
association between the demethylation status of RECK and mRNA
expression in non-tumor tissues (R2=0.00039; P=0.728;
Fig. 5D). These results suggest that
promoter hypermethylation may be an important factor for RECK
silencing in ESCC.
Discussion
RECK, as a tumor-related gene, has an important role
in regulating the invasion and metastasis of tumor cells. RECK is
widely expressed in normal tissues, but is significantly reduced in
tumor tissues (26). In a previous
study, the RECK expression level was inversely correlated with the
MMP9 expression level in nasopharyngeal carcinoma (27). Furthermore, there was a significant
association between the positive expression of RECK and that of
MMP2 in adenoid cystic carcinoma (28). These findings suggest that RECK has an
important role in regulating the proliferation and migration of
normal epithelial cells and carcinoma cells (29). Therefore, understanding RECK
expression is useful for delineating the molecular basis of
malignancies.
A previous study detected RECK methylation in 27.5%
of adjacent normal mucosa samples and 47.5% of gastric cancer
samples (30). Non-small cell lung
cancer (NSCLC) patients with RECK methylation exhibited lower RECK
mRNA expression compared with patients with promoter
hypomethylation (31). RECK
methylation was detected in 63.6% (35/55) of lung cancer specimens,
and the methyltransferase inhibitor 5′-azacytidine was shown to
upregulate RECK expression and reduce the invasive ability of NSCLC
cells (17). In the present study,
the mean MI of the RECK promoter was 0.65 in ESCC and 0.49 in
non-tumor samples. Furthermore, there was a significant association
between the MI of RECK and AJCC stage and lymph node metastasis in
ESCC patients, with ESCC patients with RECK hypomethylation tending
to show better survival.
The present study demonstrated that RECK mRNA
expression was lower in ESCC tissues compared with non-tumor
tissues. In addition, patients with lymph node metastasis showed a
lower RECK mRNA expression level, and ESCC patients with high RECK
mRNA expression showed better survival. Therefore, RECK silencing
may have an important role in the pathogenesis of ESCC. In the
present study, the mRNA expression level of RECK was lower in ESCC
patients with hypermethylation (∆MI >0.16; mean-∆∆Cq
=−2.85) compared with those with hypomethylation (∆MI ≤0.16;
mean-∆∆Cq=−0.83), and there was a decreased tendency for
RECK mRNA expression in ESCC patients with promoter
hypermethylation (P<0.0001). These results suggested that
hypermethylation of RECK gene may lead to RECK silencing in ESCC,
and RECK expression could be regulated by DNA methylation in
ESCC.
In conclusion, RECK methylation was frequently
observed in ESCC and was associated with the downregulation of its
mRNA expression, which was significantly correlated with a poor
survival in ESCC. Further studies are required to elucidate the
detailed mechanism of promoter methylation and RECK mRNA expression
in ESCC.
Acknowledgements
This study was supported by the Natural Science
Foundation of Jiangsu (grant nos. BL2013012 and BE2016656), the
Changzhou Sci&Tech Program, China (grant nos. CE20155043 and
CJ20159023), the High-level Health Talents of Changzhou City grant
(nos. 2016CZLJ021 and 2016CZLJ009) and the Health Talents Project
for Jiangsu, China (grant no. RC2016038).
References
|
1
|
Shang L and Wang M: Molecular alterations
and clinical relevance in esophageal squamous cell carcinoma. Front
Med. 7:401–410. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Aghcheli K, Marjani HA, Nasrollahzadeh D,
Islami F, Shakeri R, Sotoudeh M, Abedi-Ardekani B, Ghavamnasiri MR,
Razaei E, Khalilipour E, et al: Prognostic factors for esophageal
squamous cell carcinoma-a population-based study in Golestan
Province, Iran, a high incidence area. PloS one. 6:e221522011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Golozar A, Beaty TH, Gravitt PE, Ruczinski
I, Qiao YL, Fan JH, Ding T, Tang ZZ, Etemadi A, Hu N, et al:
Oesophageal squamous cell carcinoma in high-risk Chinese
populations: Possible role for vascular epithelial growth factor A.
Eur J Cancer. 50:2855–2865. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lee EJ, Lee BB, Kim JW, Shim YM, Hoseok I,
Han J, Cho EY, Park J and Kim DH: Aberrant methylation of fragile
histidine triad gene is associated with poor prognosis in early
stage esophageal squamous cell carcinoma. Eur J Cancer. 42:972–980.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lee EJ, Lee BB, Han J, Cho EY, Shim YM,
Park J and Kim DH: CpG island hypermethylation of E-cadherin (CDH1)
and integrin alpha4 is associated with recurrence of early stage
esophageal squamous cell carcinoma. Int J Cancer. 123:2073–2079.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shimizu M, Zaninotto G, Nagata K, Graham
DY and Lauwers GY: Esophageal squamous cell carcinoma with special
reference to its early stage. Best Pract Res Clin Gastroenterol.
27:171–186. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen Y and Tseng SH: The potential of RECK
inducers as antitumor agents for glioma. Anticancer Res.
32:2991–2998. 2012.PubMed/NCBI
|
|
8
|
Clark JC, Thomas DM, Choong PF and Dass
CR: RECK-a newly discovered inhibitor of metastasis with prognostic
significance in multiple forms of cancer. Cancer Metastasis Rev.
26:675–683. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Masui T, Doi R, Koshiba T, Fujimoto K,
Tsuji S, Nakajima S, Koizumi M, Toyoda E, Tulachan S, Ito D, et al:
RECK expression in pancreatic cancer: Its correlation with lower
invasiveness and better prognosis. Clin Cancer Res. 9:1779–1784.
2003.PubMed/NCBI
|
|
10
|
Mao X, Liu L, Zhang B and Zhang D:
Reversion-inducing cysteine-rich protein with Kazal motifs gene
expression and its clinical significance in peripheral T-cell
lymphoma. Oncology Lett. 5:1867–1871. 2013.
|
|
11
|
Rahmah NN, Sakai K, Sano K and Hongo K:
Expression of RECK in endothelial cells of glioma: Comparison with
CD34 and VEGF expressions. J Neurooncol. 107:559–564. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Alexius-Lindgren M, Andersson E, Lindstedt
I and Engstrom W: The RECK gene and biological malignancy-its
significance in angiogenesis and inhibition of matrix
metalloproteinases. Anticancer Res. 34:3867–3873. 2014.PubMed/NCBI
|
|
13
|
Li SL, Gao DL, Zhao ZH, Liu ZW, Zhao QM,
Yu JX, Chen KS and Zhang YH: Correlation of matrix
metalloproteinase suppressor genes RECK, VEGF and CD105 with
angiogenesis and biological behavior in esophageal squamous cell
carcinoma. World J Gastroenterol. 13:6076–6081. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lee KJ, Lee KY and Lee YM: Downregulation
of a tumor suppressor RECK by hypoxia through recruitment of HDAC1
and HIF-1alpha to reverse HRE site in the promoter. Biochim Biophys
Acta. 1803:608–616. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jeon HW and Lee YM: Inhibition of histone
deacetylase attenuates hypoxia-induced migration and invasion of
cancer cells via the restoration of RECK expression. Mol Cancer
Ther. 9:1361–1370. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jeon HW, Lee KJ, Lee SH, Kim WH and Lee
YM: Attenuated expression and function of the RECK tumor suppressor
under hypoxic conditions is mediated by the MAPK signaling
pathways. Arch Pharm Res. 34:137–145. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chang HC, Cho CY and Hung WC:
Downregulation of RECK by promoter methylation correlates with
lymph node metastasis in non-small cell lung cancer. Cancer Sci.
98:169–173. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Meng H, Cao Y, Qin J, Song X, Zhang Q, Shi
Y and Cao L: DNA methylation, its mediators and genome integrity.
Int J Biol Sci. 11:604–617. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang C, Ling Y, Zhang C, Xu Y, Gao L, Li
R, Zhu J, Fan L and Wei L: The silencing of RECK gene is associated
with promoter hypermethylation and poor survival in hepatocellular
carcinoma. Int J Biol Sci. 8:451–458. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kato K, Long NK, Makita H, Toida M,
Yamashita T, Hatakeyama D, Hara A, Mori H and Shibata T: Effects of
green tea polyphenol on methylation status of RECK gene and cancer
cell invasion in oral squamous cell carcinoma cells. Br J Cancer.
99:647–654. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chang HC, Cho CY and Hung WC: Silencing of
the metastasis suppressor RECK by RAS oncogene is mediated by DNA
methyltransferase 3b-induced promoter methylation. Cancer Res.
66:8413–8420. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang C, Xu Y, Zhao J, Fan L, Jiang G, Li
R, Ling Y, Wu M and Wei L: Elevated expression of the stem cell
marker CD133 associated with Line-1 demethylation in hepatocellular
carcinoma. Ann Surg Oncol. 18:2373–2380. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bais AJ, Gardner AE, McKenzie OL, Callen
DF, Sutherland GR and Kremmidiotis G: Aberrant CBFA2T3B gene
promoter methylation in breast tumors. Mol Cancer. 3:222004.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang C, Guo X, Zhang L, Lu Z, Ma N, Cheng
Y, Shen F, Zhang B, Wu M and Wei L: Methylation-related silencing
of p14ARF gene correlates with telomerase activity and mRNA
expression of human telomerase reverse transcriptase in
hepatocellular carcinoma. J Surg Oncol. 98:462–468. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Noda M, Takahashi C, Matsuzaki T and
Kitayama H: What we learn from transformation suppressor genes:
Lessons from RECK. Future Oncol. 6:1105–1116. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhou DN, Deng YF, Li RH, Yin P and Ye CS:
Concurrent alterations of RAGE, RECK, and MMP9 protein expression
are relevant to Epstein-Barr virus infection, metastasis and
survival in nasopharyngeal carcinoma. Int J Clin Exp Pathol.
7:3245–3254. 2014.PubMed/NCBI
|
|
28
|
Zhou X, Huang S, Jiang L, Zhang S, Li W,
Chen Z and Zhang D: Expression of RECK and MMP-2 in salivary
adenoid cystic carcinoma: Correlation with tumor progression and
patient prognosis. Oncology Lett. 7:1549–1555. 2014.
|
|
29
|
Yuki K, Yoshida Y, Inagaki R, Hiai H and
Noda M: E-cadherin-downregulation and RECK-upregulation are coupled
in the non-malignant epithelial cell line MCF10A but not in
multiple carcinoma-derived cell lines. Sci Rep. 4:45682014.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Du YY, Dai DQ and Yang Z: Role of RECK
methylation in gastric cancer and its clinical significance. World
J Gastroenterol. 16:904–908. 2010.PubMed/NCBI
|
|
31
|
Pesta M, Kulda V, Topolcan O, Safranek J,
Vrzalova J, Cerny R and Holubec L: Significance of methylation
status and the expression of RECK mRNA in lung tissue of patients
with NSCLC. Anticancer Res. 29:4535–4539. 2009.PubMed/NCBI
|