Introduction
Breast-conserving surgery is a standard treatment
for stage I and II breast cancer; however, 5–10% of patients
treated with breast-conserving surgery are diagnosed with
ipsilateral breast tumor recurrence (IBTR) within 10 years
(1,2).
IBTR following breast-conserving surgery is associated with an
elevated risk of mortality or of developing distant recurrence
(3–7).
The time interval between the initial surgery and
the occurrence of IBTR is defined as the disease-free interval
(DFI), which is a predictor of disease recurrence following IBTR
(3–6,8–12), and patients with early IBTR have a
poorer prognosis, compared with those with late IBTR (8,10–12). However, irrespective of the DFI, the
standard treatment for patients with IBTR is surgery is mastectomy.
This treatment strategy must be modified if a subgroup of patients
with early IBTR, with an equally poor prognosis as that of patients
with regional or distant recurrence, is present (13). Therefore, it is important to estimate
the risk of disease recurrence in such patients, as risk factors
following early IBTR have not yet been elucidated. In the present
study, the risk factors for distant recurrence following early IBTR
were examined.
Patients and methods
Patients
The medical records of 3,793 patients with breast
cancer who underwent breast-conserving surgery between January 1989
and December 2013 at the Osaka Medical Center for Cancer and
Cardiovascular Diseases (Osaka, Japan) were reviewed. Of these
patients (ages 28–89), 180 (4.7%) developed IBTR as the first event
with no evidence of synchronous metastatic disease, and
subsequently underwent salvage surgery. Within this group, the
exclusion criteria were as follows: Patients with non-invasive
tumors present in IBTR tissue specimens and patients who received
neoadjuvant therapy as the initial treatment. A total of 153
patients with IBTR were eligible for the present study. A previous
study examined the same patient group, focusing on patients with
IBTR that occurred 5 years following the initial surgery (14), whereas, in the current study, 40
patients with IBTR that occurred within 3 years of the initial
surgery were analyzed. The present study was approved by the local
ethics committee of the Osaka Medical Center of Cancer and
Cardiovascular Diseases, with waiver of informed patient
consent.
Patients received a physical examination (palpation
for breast, chest wall and regional lymph nodes) every 3–6 months
for 5 years following primary or salvage surgery and annually
thereafter, and also underwent mammograms annually following
primary or salvage surgery. The estrogen receptor (ER) status of
the surgical specimens obtained from patients was determined using
immunohistochemistry (15), and
tumors were classified as positive for ER expression if ≥10% of
cells exhibited positive nuclear staining with monoclonal rabbit
anti-human ERα (clone EP1, Dako; Agilent Technologies, Inc., Santa
Clara, CA, USA). The human epidermal growth factor receptor 2
(HER2) status of patients' tissues was considered positive if the
immunohistochemistry was 3+ or if the fluorescence in situ
hybridization ratio (HER-2/chromosome 17) was >2.0 (16).
Statistical analysis
Distant disease-free survival (DDFS) rate was
defined as the period of time between the date of surgery for
patients with IBTR and the date of the appearance of distant
recurrence, and was calculated using the Kaplan-Meier method.
Log-rank tests were performed to evaluate the differences in DDFS
among various patient subgroups. Univariate and multivariate
analyses were performed using the Cox proportional hazards
model.
All statistical tests were performed using SPSS
version 21.0 (IBM SPSS, Armonk, NY, USA). All statistical tests and
P-values were two tailed, and P<0.05 was considered to indicate
a statistically significant difference.
Results
Patient characteristics
Patients' clinical characteristics are presented in
Table I. Some data was missing (such
as HER2 status of primary tumor and IBTR). Within a median
follow-up period of 2.2 years (range, 0.1–20.8 years) following
salvage surgery for IBTR, distant recurrence occurred in 15/40
patients (37.5%), and the 3-year DDFS rate was 64.3%.
 | Table I.Characteristics of patients. |
Table I.
Characteristics of patients.
| Characteristics of
patients | No. of
patients(n=40) |
|---|
| Median age at initial
diagnosis (range), years | 54 (30–81) |
| p-T stage of primary
tumor |
|
| In
situ | 3 |
| 1 | 7 |
| 2 | 30 |
| Grade of primary
tumor |
|
| 1 | 0 |
| 2 | 18 |
| 3 | 19 |
|
Unknown | 3 |
| Lymphovascular
invasion of primary tumor |
|
|
Negative | 19 |
|
Positive | 20 |
|
Unknown | 1 |
| Histological type of
primary tumor |
|
| DCIS | 3 |
| Invasive
ductal | 35 |
| Invasive
lobular | 1 |
|
Other | 1 |
| No. of positive lymph
nodes of primary tumor |
| 0 | 18 |
| 1–3 | 12 |
| ≥4 | 4 |
|
Unknown | 6 |
| ER status of primary
tumor |
|
|
Positive | 17 |
|
Negative | 22 |
|
Unknown | 1 |
| HER2 status of
primary tumor |
|
|
Positive | 10 |
|
Negative | 18 |
|
Unknown | 12 |
| Adjuvant chemotherapy
following primary |
|
|
surgery |
| Yes | 13 |
| No | 27 |
| Adjuvant hormonal
therapy following primary surgerya |
| Yes | 11 |
| No | 6 |
| Adjuvant trastuzumab
following primary surgeryb |
| Yes | 0 |
| No | 10 |
| Median time interval
between initial surgery and IBTR (range), years | 1.9(0.1–2.1) |
| Median age at IBTR
diagnosis (range), years | 56.5(32.0–82.0) |
| p-T stage of
IBTR |
|
| In
situ | 0 |
| 1 | 26 |
| ≥2 | 13 |
|
Unknown | 1 |
| Grade of IBTR |
|
| 1 | 3 |
| 2 | 10 |
| 3 | 21 |
|
Unknown | 6 |
| Lymphovascular
invasion of IBTR |
|
|
Negative | 19 |
|
Positive | 17 |
|
Unknown | 4 |
| Histological type of
IBTR |
|
| DCIS | 0 |
| Invasive
ductal | 37 |
| Invasive
lobular | 1 |
|
Other | 1 |
|
Unknown | 1 |
| ER status of
IBTR |
|
|
Positive | 17 |
|
Negative | 20 |
|
Unknown | 3 |
| HER2 status of
IBTR |
|
|
Positive | 9 |
|
Negative | 22 |
|
Unknown | 9 |
| Adjuvant chemotherapy
following salvage surgery |
|
| Yes | 15 |
| No | 22 |
|
Unknown | 3 |
| Adjuvant hormonal
therapy following salvage surgerya |
|
| Yes | 9 |
| No | 5 |
|
Unknown | 3 |
| Adjuvant trastuzumab
following salvage surgeryb |
|
|
Yes | 4 |
| No | 5 |
Association with DDFS
Various clinical and pathological factors associated
with DDFS among patients with early IBTR are listed in Table II. The nodal status at primary
surgery and the use of adjuvant chemotherapy treatment following
primary surgery were significantly correlated with DDFS (P=0.001
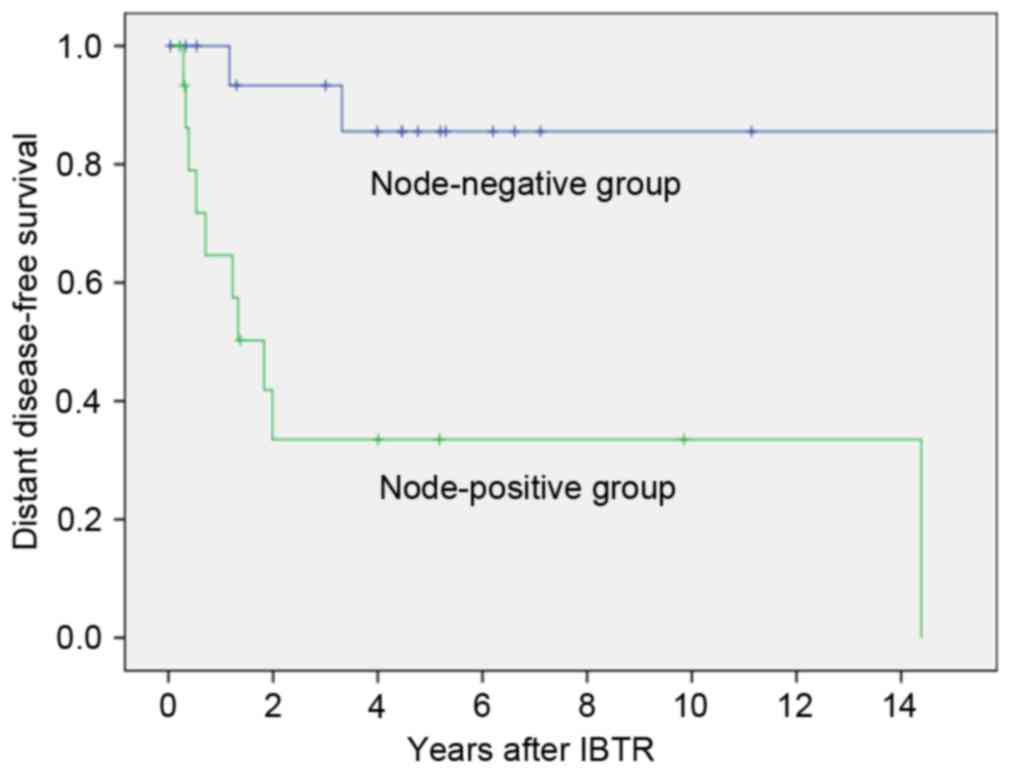
and P=0.002, respectively). Patients who were node-positive at
primary surgery had a significantly poorer DDFS than node-negative
patients (3-year DDFS, 33.5 vs. 93.3%, respectively; P=0.001;
Fig. 1). Patients who received
adjuvant chemotherapy (n=13; mainly anthracycline and/or taxane)
following primary surgery exhibited a significantly poorer DDFS
than those who did not receive chemotherapy (3-year DDFS, 34.4 vs.
77.9%, respectively; P=0.002; Table
II). No significant differences were observed between any of
the following groups: Negative or positive margin at primary
surgery (P=0.58), radiotherapy or no radiotherapy following primary
surgery (P=0.57) and basal (both ER- and HER2-negative) or
non-basal type primary tumors (P=0.27) (Table II). Multivariate analyses
demonstrated that the nodal status at primary surgery was an
independent predictive factor of distant recurrence (P=0.050;
Table III).
 | Table II.Three-year DDFS rates according to
various clinicopathological factors among patients with early IBTR
(n=40). |
Table II.
Three-year DDFS rates according to
various clinicopathological factors among patients with early IBTR
(n=40).
| Characteristics of
patients | 3-year DDFS rates,
% | P-value |
|---|
| Age at initial
diagnosis, years |
|
|
|
<50 | 48.9 | 0.870 |
|
≥50 | 70.3 |
|
| p-T stage of
primary tumor |
|
|
| In
situ or 1 | 80.2 | 0.110 |
| 2 | 50.3 |
|
| Margin of primary
tumor |
|
|
|
Negative | 66.0 | 0.58 |
|
Positive | 53.3 |
|
| Grade of primary
tumor |
|
|
| 1 or
2 | 66.5 | 0.770 |
| 3 | 58.0 |
|
| Lymphovascular
invasion of primary tumor |
|
|
|
Negative | 74.9 | 0.190 |
|
Positive | 51.9 |
|
| Lymph node status
of primary tumor |
|
|
|
Negative | 93.3 | 0.001 |
|
Positive | 33.5 |
|
| ER status of
primary tumor |
|
|
|
Positive | 72.2 | 0.400 |
|
Negative | 55.9 |
|
| HER2 status of
primary tumor |
|
|
|
Positive | 71.1 | 0.220 |
|
Negative | 50.2 |
|
| Basal type of
primary tumor |
|
|
|
Yes | 43.8 | 0.27 |
| No | 66.2 |
|
| Radiotherapy
following primary surgery |
|
|
|
Yes | 66.5 | 0.57 |
| No | 61.4 |
|
| Adjuvant
chemotherapy following primary surgery |
|
|
|
Yes | 34.4 | 0.002 |
| No | 77.9 |
|
| Adjuvant hormonal
therapy following primary surgerya |
|
|
|
Yes | 71.6 | 0.460 |
| No | 75.0 |
|
| Age at IBTR
diagnosis, years |
|
|
|
<50 | 48.9 | 0.870 |
|
≥50 | 70.3 |
|
| p-T stage of
IBTR |
|
|
| 1 | 67.3 | 0.450 |
| ≥2 | 54.9 |
|
| Grade of IBTR |
|
|
| 1 or
2 | 75.0 | 0.490 |
| 3 | 55.1 |
|
| Lymphovascular
invasion of IBTR |
|
|
|
Negative | 69.1 | 0.170 |
|
Positive | 52.1 |
|
| ER status of
IBTR |
|
|
|
Positive | 56.4 | 0.540 |
|
Negative | 64.7 |
|
| HER2 status of
IBTR |
|
|
|
Positive | 77.8 | 0.270 |
|
Negative | 58.0 |
|
| Adjuvant
chemotherapy following salvage surgery |
|
|
|
Yes | 55.8 | 0.210 |
| No | 69.2 |
|
| Adjuvant hormonal
therapy following salvage surgerya |
|
|
|
Yes | 64.8 | 0.071 |
| No | 26.7 |
|
| Adjuvant
trastuzumab following salvage surgeryb |
|
|
|
Yes | 75.0 | 0.800 |
| No | 80.0 |
|
 | Table III.Multivariate analysis of predictors
of distant recurrence following early ipsilateral breast tumor
recurrence. |
Table III.
Multivariate analysis of predictors
of distant recurrence following early ipsilateral breast tumor
recurrence.
| Characteristics of
patients | HR | 95% CI | P-value |
|---|
| Lymph node status
of primary tumor (positive vs. negative) | 5.281 | 1.002–27.002 | 0.050a |
| Adjuvant
chemotherapy following primary surgery (positive vs. negative) | 2.983 | 0.750–11.750 | 0.120 |
Discussion
The present study demonstrated that the nodal status
at the time of primary surgery and the use of adjuvant therapy
subsequent to primary surgery were risk factors for distant
recurrence following early IBTR. It was hypothesized that the nodal
status at primary surgery may interact with adjuvant therapy
following primary surgery. Node-positive breast cancer patients
have poorer prognosis compared with patients with negative lymph
node metastasis. Therefore, patients with positive lymph node
metastasis are more likely to be recommended for adjuvant
chemotherapy compared with those with negative lymph node
metastasis. Therefore, multivariate analysis incorporating these
two factors was performed, which revealed that the nodal status at
primary surgery was an independent prognostic factor in the present
study group. At present, the risk factors that follow IBTR and are
associated with the DFI require further investigation (8–10,14) and, to the best of our knowledge, no
previous studies have been conducted to examine the risk factors
following early IBTR. The nodal status at primary surgery and the
use of adjuvant therapy following primary surgery, which were
demonstrated to be prognostic factors among patients with early
IBTR in the current study, were also associated with primary
surgery, but not with recurrent tumors. By contrast, a previous
study identified that the prognostic factors among patients with
late IBTR were the ER and HER2 status of IBTR tissue specimens,
which were associated with recurrent tumors, but not with primary
surgery (14). Taken together, these
findings suggest that early IBTR is associated with true
recurrence, whereas late IBTR is associated with the presence of
new primary tumors.
The 3-year DDFS rate in the present study was 33.5%
among patients with early IBTR and a positive nodal status at the
time of primary surgery. This DDFS rate is concordant with that
reported by Wapnir et al (5),
in which the 3-year DDFS was 44.9% among patients with early IBTR
and a positive nodal status at the time of primary surgery.
Furthermore, this DDFS rate is similar to that observed in patients
with ipsilateral supraclavicular node recurrence (17) or lung metastases (18). Pergolizzi et al (17) reported that the median time to
progression was 28 months in 44 patients with ipsilateral
supraclavicular node recurrence from breast cancer (as a part of
recurrent regional disease and without distant metastases) who
received combined chemotherapy and radiotherapy treatment. Ludwig
et al (18) observed that,
during a retrospective analysis, the median DDFS following
resection of lung metastatic tumors was 27.6 months.
The results of the current study suggest that
patients with early IBTR and positive axillary nodes at the
diagnosis of the primary tumor possess a high risk of distant
recurrence and, therefore, should potentially receive more
aggressive treatment compared with conventional treatment,
including novel (neo)adjuvant systemic therapy or regional
radiotherapy.
In addition to the DFI, previous studies have
demonstrated that the nodal status at the time of primary surgery
was a prognostic factor among patients with IBTR (4,19). The
association between the DFI and the nodal status of the primary
tumor, and its prognostic relevance among patients with IBTR, has
yet to be elucidated. In addition, the small sample size, short
follow-up period and high frequency of missing data, particularly
for the HER2 status of patients [primary tumor, 30.0% (12/40);
IBTR, 22.5% (9/40)] were limitations of the present study. For
ER-positive tumors, the annual breast cancer mortality rates are
similar during years 0–4 and 5–14 (20).
In conclusion, the nodal status at primary surgery
was demonstrated to be an independent predictive factor of distant
recurrence among patients with early IBTR in the current study;
however, further studies are required to support this
association.
Acknowledgements
The present study was supported in part by the Osaka
Foundation for the Prevention of Cancer and Cardiovascular Diseases
(grant no. 1601079183).
References
|
1
|
Fisher B, Anderson S, Bryant J, Margolese
RG, Deutsch M, Fisher ER, Jeong JH and Wolmark N: Twenty-year
follow-up of a randomized trial comparing total mastectomy,
lumpectomy, and lumpectomy plus irradiation for the treatment of
invasive breast cancer. N Engl J Med. 347:1233–1241. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Veronesi U, Cascinelli N, Mariani L, Greco
M, Saccozzi R, Luini A, Aguilar M and Marubini E: Twenty-year
follow-up of a randomized study comparing breast-conserving surgery
with radical mastectomy for early breast cancer. N Engl J Med.
347:1227–1232. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Haffty BG, Reiss M, Beinfield M, Fischer
D, Ward B and McKhann C: Ipsilateral breast tumor recurrence as a
predictor of distant disease: Implications for systemic therapy at
the time of local relapse. J Clin Oncol. 14:52–57. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Komoike Y, Akiyama F, Iino Y, Ikeda T,
Akashi-Tanaka S, Ohsumi S, Kusama M, Sano M, Shin E, Suemasu K, et
al: Ipsilateral breast tumor recurrence (IBTR) after
breast-conserving treatment for early breast cancer: Risk factors
and impact on distant metastases. Cancer. 106:35–41. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wapnir IL, Anderson SJ, Mamounas EP, Geyer
CE Jr, Jeong JH, Tan-Chiu E, Fisher B and Wolmark N: Prognosis
after ipsilateral breast tumor recurrence and locoregional
recurrences in five national surgical adjuvant breast and bowel
project node-positive adjuvant breast cancer trials. J Clin Oncol.
24:2028–2037. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Anderson SJ, Wapnir I, Dignam JJ, Fisher
B, Mamounas EP, Jeong JH, Geyer CE Jr, Wickerham DL, Costantino JP
and Wolmark N: Prognosis after ipsilateral breast tumor recurrence
and locoregional recurrences in patients treated by
breast-conserving therapy in five national surgical adjuvant breast
and bowel project protocols of node-negative breast cancer. J Clin
Oncol. 27:2466–2473. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ishitobi M, Ohsumi S, Inaji H, Ohno S,
Shigematsu H, Akiyama F, Iwase T, Akashi-Tanaka S, Sato N,
Takahashi K and Oura S: Ipsilateral breast tumor recurrence (IBTR)
in patients with operable breast cancer who undergo
breast-conserving treatment after receiving neoadjuvant
chemotherapy: Risk factors of IBTR and validation of the MD
Anderson prognostic index. Cancer. 118:4385–4393. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kurtz JM, Spitalier JM, Amalric R,
Brandone H, Ayme Y, Jacquemier J, Hans D and Bressac C: The
prognostic significance of late local recurrence after
breast-conserving therapy. Int J Radiat Oncol Biol Phys. 18:87–93.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Elkhuizen PH, Hermans J, Leer JW and van
de Vijver MJ: Isolated late local recurrences with high mitotic
count and early local recurrences following breast-conserving
therapy are associated with increased risk on distant metastasis.
Int J Radiat Oncol Biol Phys. 50:387–396. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
van der Sangen MJ, van de Poll-Franse LV,
Roumen RM, Rutten HJ, Coebergh JW, Vreugdenhil G and Voogd AC: The
prognosis of patients with local recurrence more than five years
after breast conservation therapy for invasive breast carcinoma.
Eur J Surg Oncol. 32:34–38. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
van Laar C, van der Sangen MJ, Poortmans
PM, Nieuwenhuijzen GA, Roukema JA, Roumen RM, Tjan-Heijnen VC and
Voogd AC: Local recurrence following breast-conserving treatment in
women aged 40 years or younger: Trends in risk and the impact on
prognosis in a population-based cohort of 1143 patients. Eur J
Cancer. 49:3093–3101. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ishitobi M, Okumura Y, Arima N, Yoshida A,
Nakatsukasa K, Iwase T, Shien T, Masuda N, Tanaka S, Tanabe M, et
al: Breast cancer subtype and distant recurrence after ipsilateral
breast tumor recurrence. Ann Surg Oncol. 20:1886–1892. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Alkner S, Tang MH, Brueffer C, Dahlgren M,
Chen Y, Olsson E, Winter C, Baker S, Ehinger A, Rydén L, et al:
Contralateral breast cancer can represent a metastatic spread of
the first primary tumor: Determination of clonal relationship
between contralateral breast cancers using next-generation whole
genome sequencing. Breast Cancer Res. 17:1022015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ishitobi M, Okuno J, Kittaka N, Nakayama
T, Koyama H and Tamaki Y: Distant recurrence risk after late
ipsilateral breast tumor recurrence: Results of a retrospective,
single-institution study. Oncology. 89:269–274. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Umemura S, Kurosumi M, Moriya T, Oyama T,
Arihiro K, Yamashita H, Umekita Y, Komoike Y, Shimizu C, Fukushima
H, et al: Immunohistochemical evaluation for hormone receptors in
breast cancer: A practically useful evaluation system and handling
protocol. Breast Cancer. 13:232–235. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wolff AC, Hammond ME, Hicks DG, Dowsett M,
McShane LM, Allison KH, Allred DC, Bartlett JM, Bilous M,
Fitzgibbons P, et al: Recommendations for human epidermal growth
factor receptor 2 testing in breast cancer: American society of
clinical oncology/college of American pathologists clinical
practice guideline update. J Clin Oncol. 31:3997–4013. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pergolizzi S, Adamo V, Russi E,
Santacaterina A, Maisano R, Numico G, Palazzolo C, Ferraù F,
Settineri N, Altavilla G, et al: Prospective multicenter study of
combined treatment with chemotherapy and radiotherapy in breast
cancer women with the rare clinical scenario of ipsilateral
supraclavicular node recurrence without distant metastases. Int J
Radiat Oncol Biol Phys. 65:25–32. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ludwig C, Stoelben E and Hasse J:
Disease-free survival after resection of lung metastases in
patients with breast cancer. Eur J Surg Oncol. 29:532–535. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Voogd AC, van Tienhoven G, Peterse HL,
Crommelin MA, Rutgers EJ, van de Velde CJ, van Geel BN, Slot A,
Rodrigus PT, Jobsen JJ, et al: Local recurrence after breast
conservation therapy for early stage breast carcinoma: Detection,
treatment and outcome in 266 patients. Dutch study group on local
recurrence after breast conservation (BORST). Cancer. 85:437–446.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Early Breast Cancer Trialists'
Collaborative Group (EBCTCG): Effects of chemotherapy and hormonal
therapy for early breast cancer on recurrence and 15-year survival:
An overview of the randomised trials. Lancet. 365:1687–1717. 2005.
View Article : Google Scholar : PubMed/NCBI
|