Introduction
Lung cancer is the most commonly diagnosed cancer
and also the leading cause of cancer-associated mortality (1). Even with more advanced chemotherapeutic
agents and molecularly targeted drugs, the prognosis of this
disease remains poor due to limited treatment efficacy (2,3).
Previously, maintenance therapy has been identified to be an
acceptable treatment paradigm to improve progression free survival
(4). However, data from randomized
clinical trials have demonstrated that the maintenance and
consolidation therapy failed to improve the outcomes of patients
with lung cancer, and in certain cases caused severe side effects
or toxicity-associated mortality (5).
Thus, given the higher recurrence and mortality rates, novel
therapeutic strategies are warranted in order to improve the
outcome of patients with lung cancer.
Aimed at eliminating tumor cells through stimulation
or restoration of a patient's immune system, adoptive cellular
immunotherapies have attracted increasing interests. Among them,
considerable attention has been given to cytokine induced killer
(CIK) cells derived from peripheral blood for treating various
types of cancer (6). Killer cell
lectin like receptor K1 (NKG2D) has been demonstrated to serve an
important role in mediating the elimination of tumor cells by
cytotoxic effectors cells (7).
Previous studies have demonstrated that effector cell recognition
and the lysis of tumor cells are primarily mediated through NKG2D
activating receptor (8–10). NKG2D-mediated cytotoxicity depends on
immune cell surface expression of NKG2D receptors and target cell
expression of NKG2D ligands (11).
Previous studies have demonstrated that increased expression of
NKG2D ligands sensitizes target cells to natural killer (NK)
cell-mediated lysis (12–14). There are two categories of NKG2D
ligands (15), including MHC class I
polypeptide-related sequence (MIC) A and B, and UL16 binding
protein (ULBP) 1, 2 and 3. It was indicated that multiple
malignancies, including primary leukemia, glioma, and melanoma
tumors, expressed ULBP, and the expression of MICA and ULBP1-3 were
identified in almost all primary glioma isolates, but little
expression of MICB on primary glioma was detected (16–18).
Therefore, NKG2D is important in tumor immune surveillance to
prevent tumor initiation and in immunotherapy. In the present
study, the expression of NKG2D ligands in samples from patients
with lung cancer, and in A549 and Q56 cells was investigated. The
cytotoxicity of CIK cells against A549 cell was subsequently
analyzed. The current study aimed to investigate the mechanisms
underlying the effects of CIK cells in tumor cell elimination, in
order to improve the efficacy of CIK cell therapy in the clinical
practice.
Materials and methods
Patients
The present study conformed to The Declaration of
Helsinki and was approved by the Institute Review Committee of the
No. 2 People's Hospital of Changzhou (Changzhou, China). Written
informed consent was obtained from all participants. Patients with
lung cancer were identified from the surgical pathology
biorepository of the Department of Thoracic Surgery at the No. 2
People's Hospital of Changzhou. No other specific inclusion or
exclusion criteria were applied to the present study. Healthy
controls were recruited from individuals who visited the No. 2
People's Hospital of Changzhou for a routine health check-up
without any history of cancer.
Cell lines
The lung cancer cell lines A549 and QG56 were
purchased from The Cell Bank of Type Culture Collection of Chinese
Academy of Sciences (Shanghai, China). Cells were maintained in
Dulbecco's modified Eagle medium (DMEM, HyClone; GE Healthcare Life
Sciences, Logan, UT, USA) supplemented with 100 IU/ml penicillin,
100 µg/ml streptomycin and 10% fetal bovine serum (FBS; HyClone; GE
Healthcare Life Sciences). The cells were incubated at 37°C with 5%
CO2.
Generation of CIK cells
Peripheral blood mononuclear cells (PBMCs) were
isolated using density gradient centrifugation. The blood was
separated with 650 × g for 20 min at 4°C. The CIK cells were
cultured using a previously described method (19). Briefly, PBMCs were cultured in
RPMI-1640 (HyClone; GE Healthcare Life Sciences, Logan, UT, USA
supplemented with 10% FBS and 1,000 U/ml interferon γ
(Prospec-TanyTechnoGene, Ltd., East Brunswick, NJ, USA). Following
24 h, 50 ng/ml humanized anti-cluster of differentiation 3
monoclonal antibody (cat. no. TL-101; dilution, 1:10,000; Wuhan
Institute of Biological Products, Wuhan, China) and 1,000 U/ml
recombinant human interleukin-2 (rhIL-2; Prospec-TanyTechnoGene,
Ltd.) were added to the cell culture. Fresh medium was added to the
culture every 3 days and rhIL-2 every 6–7 days until CIK cells were
harvested following 3 weeks of culture.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
RNA was isolated using TRIzol®
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
according to the manufacturer's protocol. The RT of 1 µg of RNA
into cDNA was performed using SuperScript™ II Reverse Transcriptase
(Invitrogen; Thermo Fisher Scientific, Inc.) and stored at −20°C
until use. RT-qPCR was performed with SYBR Premix Ex Taq (TransGen
Biotech Co., Ltd., Beijing, China) under the following cycling
conditions: one cycle at 94°C for 30 sec, 40 cycles at 94°C for 5
sec and 60°C for 30 sec. The quantification of MHC class I
polypeptide-related sequence A (MICA) and UL16 binding protein 2
(ULBP2), two ligands of NKG2D and GAPDH (housekeeping gene used as
loading control) was performed using specific primers. The relative
amount of MICA ligands' mRNA to GAPDH mRNA (MICA/GAPDH) was used to
represent the expression of the MICA genes. Analysis of relative
gene expression data was analyzed using the 2−ΔΔCq
methods (20). The following primers
were used: MICA forward, 5′-GAGCTCCCAGCATTCTACTAC-3′; reverse,
5′-GGTGTCGTGGCTCAAAGATA-3′; ULBP2 forward,
5′-GAGAGGTGGTGGACATACTTAC-3′; reverse,
5′-CAAGCCATCCTATACAGTCTCC-3′; and GAPDH forward,
5′-CTATTCGATGCCGTGTATGC-3′; reverse,
5′-GCCTGGTCCAGACTTCTTTC-3′.
Western blotting
Proteins from lung cancer and para-carcinoma tissue
samples were extracted with radioimmunoprecipitation assay lysis
buffer for 30 min and centrifuged at 13,400 × g for 10 min at 4°C.
The concentration of proteins was detected using bicinchoninic acid
assay kit (Beyotime Biotechnology Co., Ltd., Shanghai, China). A
total of 40 µg proteins per lane were separated using 12% SDS-PAGE
gel and transferred to a polyvinylidene fluoride membrane at 100 V
for 1 h. The membrane was blocked using 5% non-fat dry milk for 1 h
at room temperature to inhibit non-specific binding. Subsequently,
the membrane was incubated with monoclonal rabbit anti-mouse
primary antibodies directed against MICA (cat. no. ab93170;
dilution, 1:500; Abcam, Cambridge, MA, USA) and β-actin (cat. no.
3700; dilution, 1:10,000; Cell Signaling Technology, Inc., Danvers,
MA, USA) overnight at 4°C. Next, membranes were incubated with
horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG (cat.
no. sc-2004; dilution, 1:6,000; Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA) and peroxidase (HRP)-conjugated goat anti-mouse
IgG (cat. no. sc-2005; dilution, 1:10,000; Santa Cruz
Biotechnology, Inc.) secondary antibodies for 2 h at room
temperature. The secondary antibody was detected using Pierce™ ECL
Western Blotting Substrate (Thermo Fisher Scientific, Inc.).
Cytotoxicity assay
The cytotoxicity of CIK cells against lung cancer
cell lines was analyzed using flow cytometry. CIK cells were used
for cytotoxicity assays following 21 days of culture. Target A549
or QG56 cells were incubated with 0.1 µM calcein acetoxymethyl
ester (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) at 37°C for
15 min in the dark prior to washing twice in ice-cold DMEM
supplemented with 10% FBS. A total of 5,000 cells/well were
re-suspended in DMEM containing 10% FBS and seeded into 96-well
plates. CIK cells were added in at distinct effect or: Target (E:T)
ratios of 1, 5 and 10, in a final volume of 200 µl. The cells were
incubated in the dark for 10 h at 37°C prior to washing with PBS,
then cells were resuspended in 100 µl of 1X binding buffer (10 mM
HEPES/NaOH, 140 mM NaCl, 2.5 mM CaCl2, pH 7.4), and
mixed with 10 µl of 7-amino-actinomycin D (Molecular Probes; Thermo
Fisher Scientific, Inc.). Cells were analyzed using flow cytometry
following 15 min of incubation at room temperature in the dark. The
cytotoxic activity of CIK against the target cells was expressed as
a percentage of specific lysis as calculated using the following
formula: % specific lysis=(CT-TE/CT) ×100, where CT is the
percentage of viable target cells in the absence of CIK cells and
TE is the percentage of viable fluorescent target cells incubated
with CIK cells. In blocking experiments, the CIK cells were
incubated with 10 µg/ml anti-NKG2D antibodies (cat. no. ab203353;
Abcam, Cambridge, MA, USA) at 37°C for 30 min prior to being added
to the wells containing target cells.
Flow cytometry
The expression of NKG2D ligands in lung cancer cell
lines was examined using flow cytometry (BD FACSCanto™ II; BD
Biosciences, Franklin Lakes, NJ, USA) following staining with
monoclonal antibodies against MICA/B (cat. no. 53-5788; dilution,
1:200; eBioscience, Inc., San Diego, CA, USA) and ULBP1, ULBP3 and
ULBP2 (all R&D Systems, Inc., Minneapolis, MN, USA) at 37°C for
1 h. The data was analyzed using Flow Jo software (version 7.6;
Tree Star, Inc., Ashland, OR, USA).
Statistical analysis
All statistical analyses data were presented as the
mean ± standard deviation. Student's t-tests were used to compare
the differences between two groups. P<0.05 was considered to
indicate a statistically significant difference. Statistical
analyses were performed using Prism software (GraphPad Software,
Inc., La Jolla, CA, USA).
Results
Expression of NKG2D ligands and ULBP2
on lung cancer tissue samples
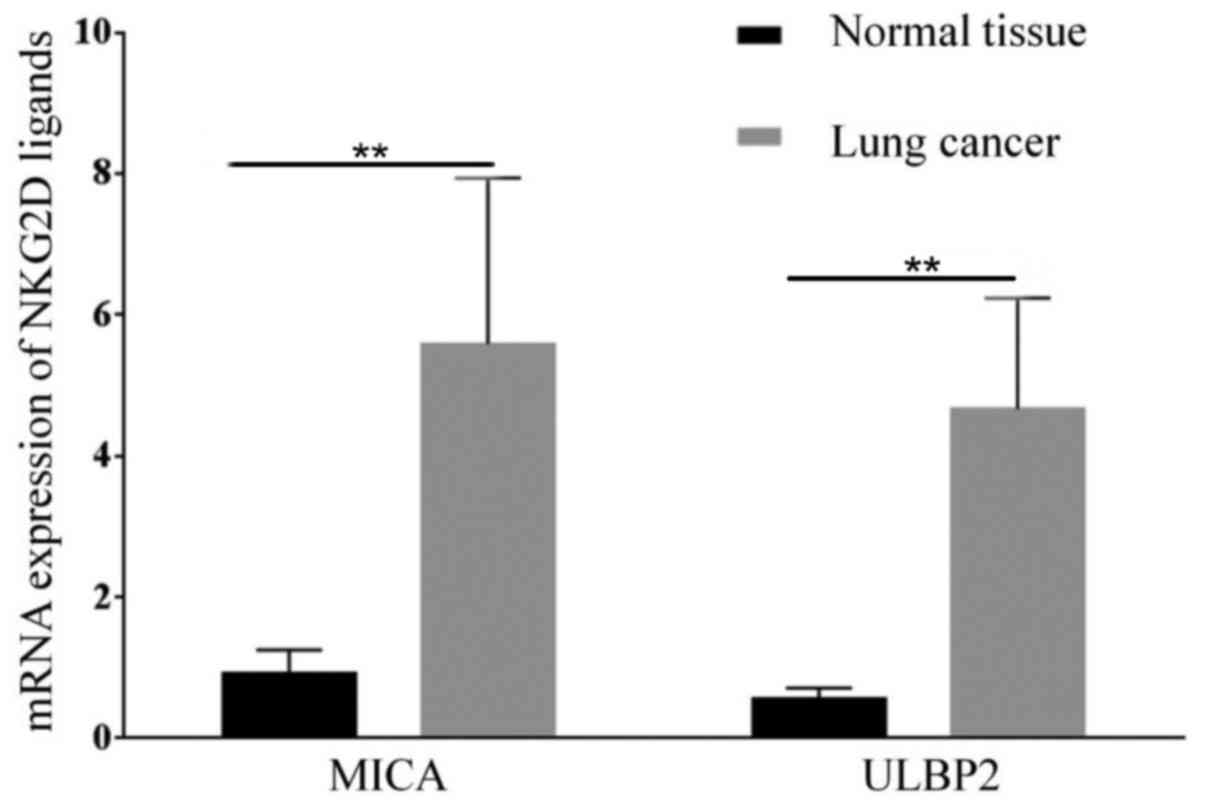
In the present study, the expression of MICA and
ULBP2, which are both ligands of NKG2D was determined in lung
cancer tissue samples. The results suggest that lung cancer tissue
express higher levels of MICA mRNA (P=0.003; n=24) and ULBP2
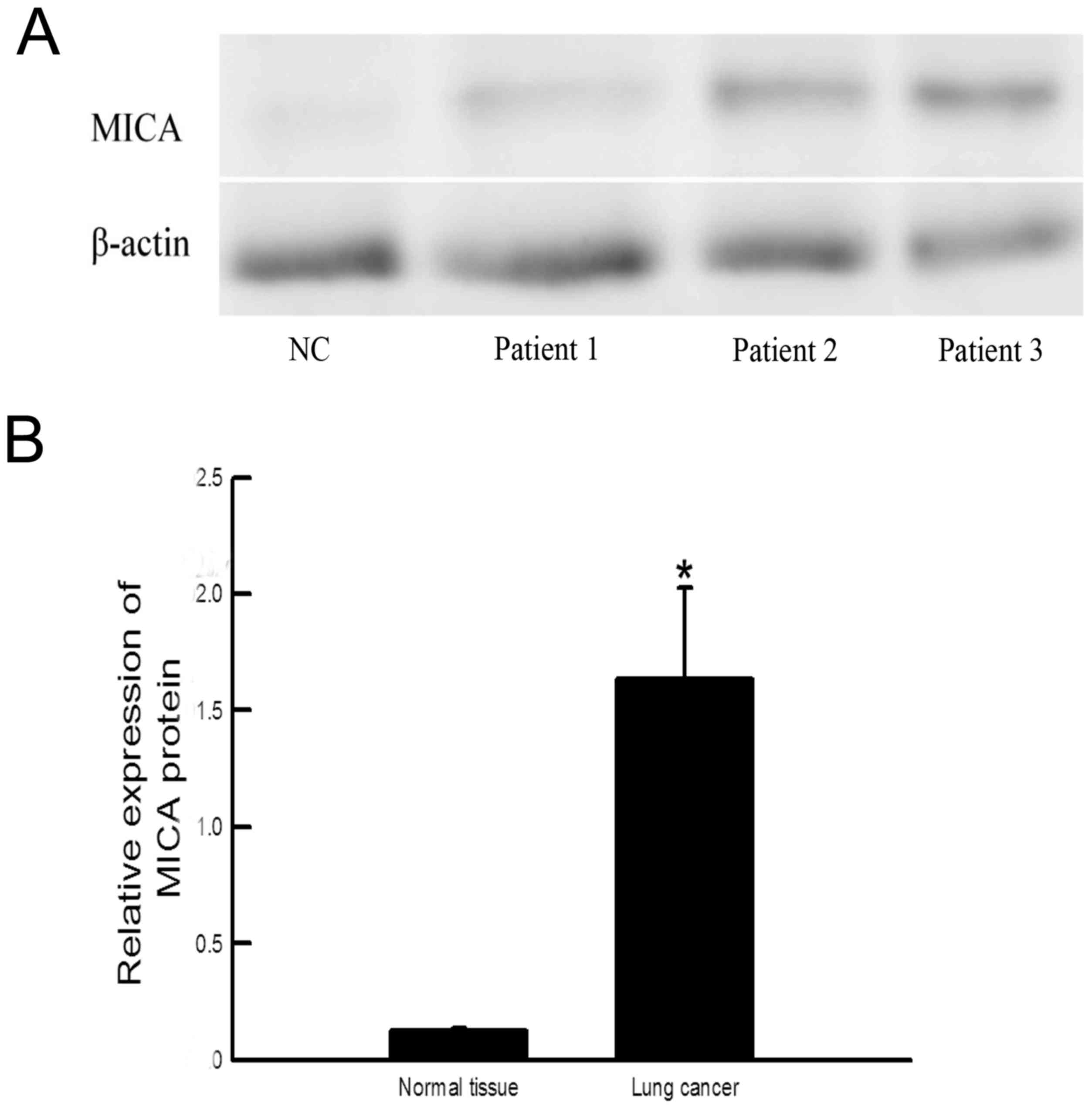
(P=0.00; n=24) compared with healthy tissue (n=5; Fig. 1). The expression of MICA protein in
cancer tissue was also increased compared with healthy tissue
(P=0.034; Fig. 2).
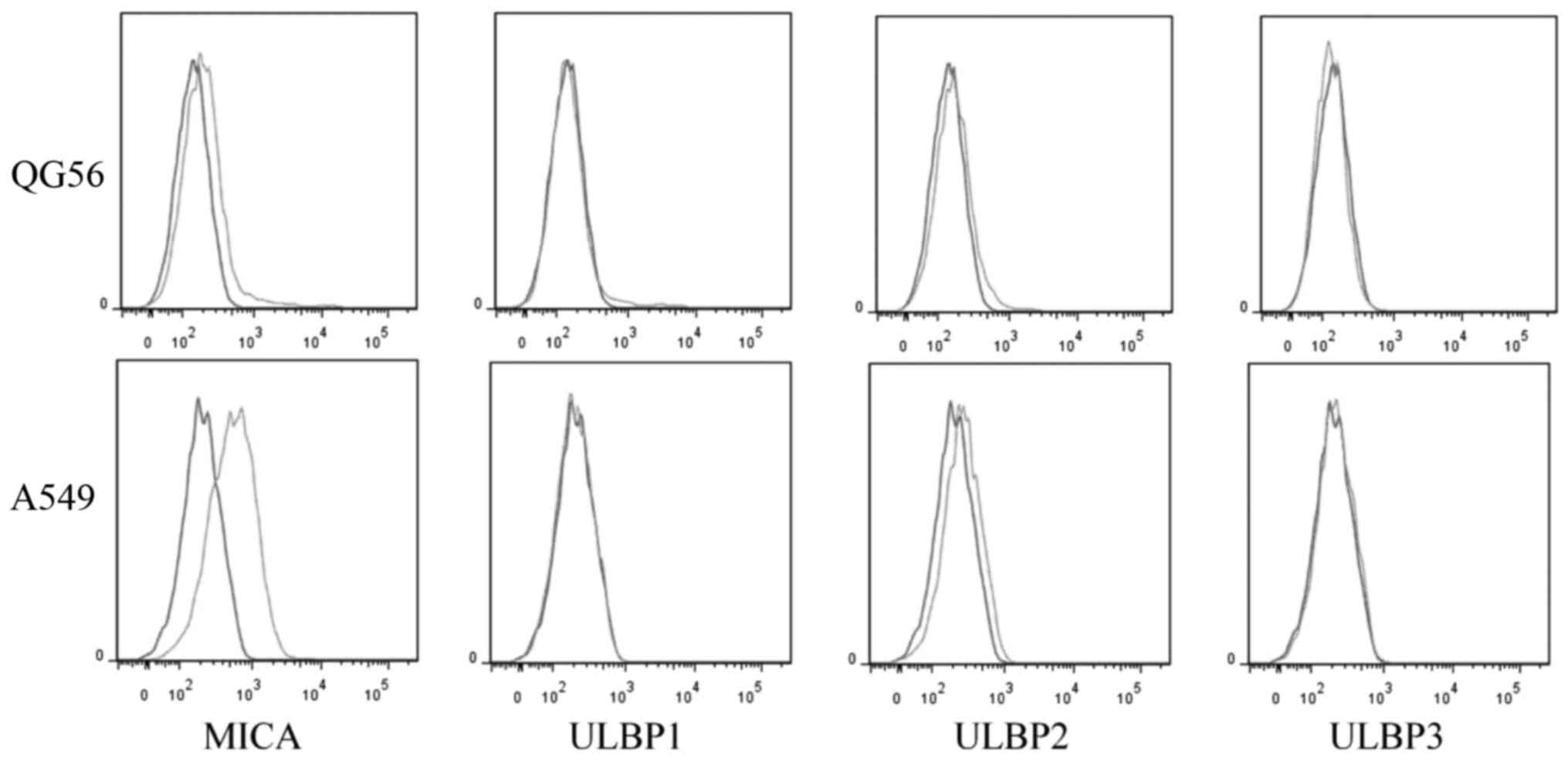
Expression of NKG2D ligands in lung
cancer cell lines
NKG2D ligands expressed in tumor cells have
previously been demonstrated to activate the anti-tumor activity of
lymphocytes, with the cytotoxicity being correlated with the ratio
between NKG2D ligands and (human leukocyte antigen) HLA class I
molecules (11). The expression of
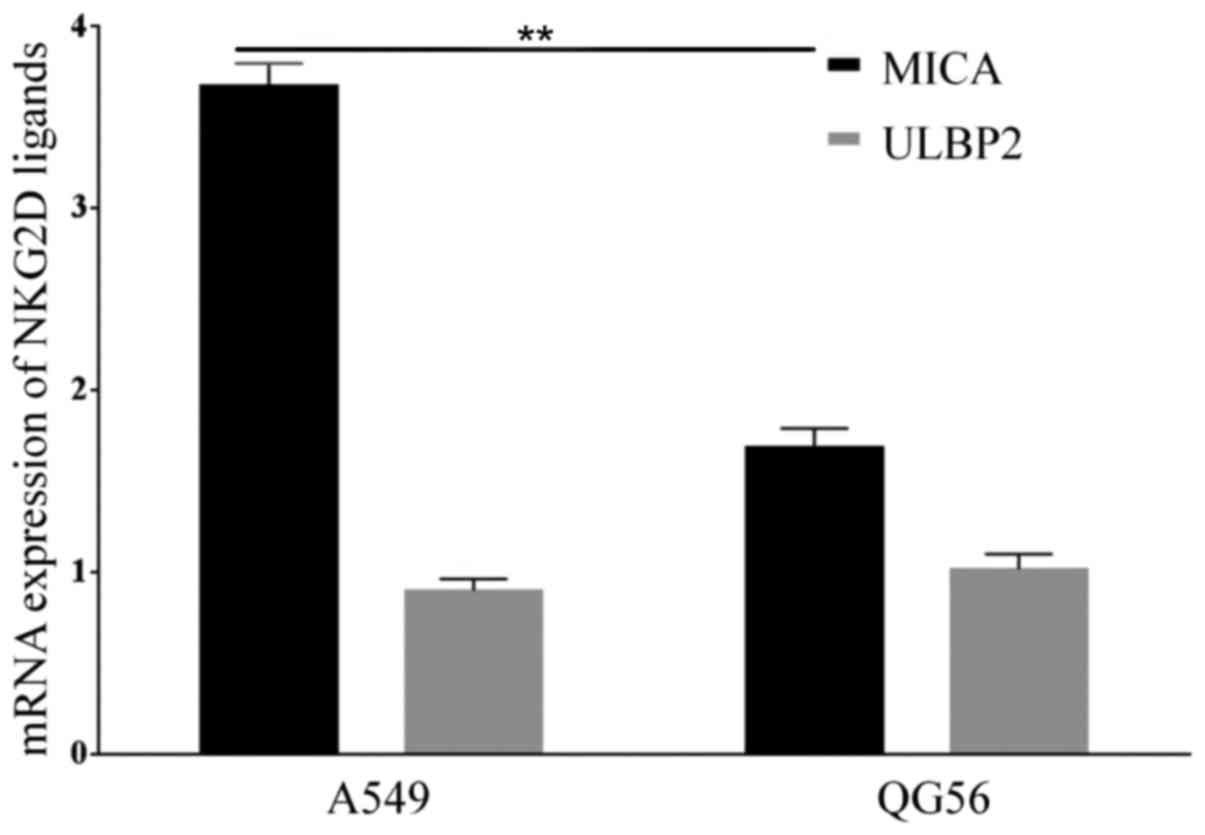
NKG2D ligands MHC Class I molecules and ULBP son lung cancer cell
lines A549 and QG56 was examined in the present study. The results
suggested that both cell lines expressed MICA, but the A549 cells
expressed a higher level of MICA compared with QG56 cells (P=0.01;
n=3; Figs. 3 and 4). However, the expression of ULBP2 between
A549 and QG56 was the same (P=0.8; n=3; Figs. 3 and 4).
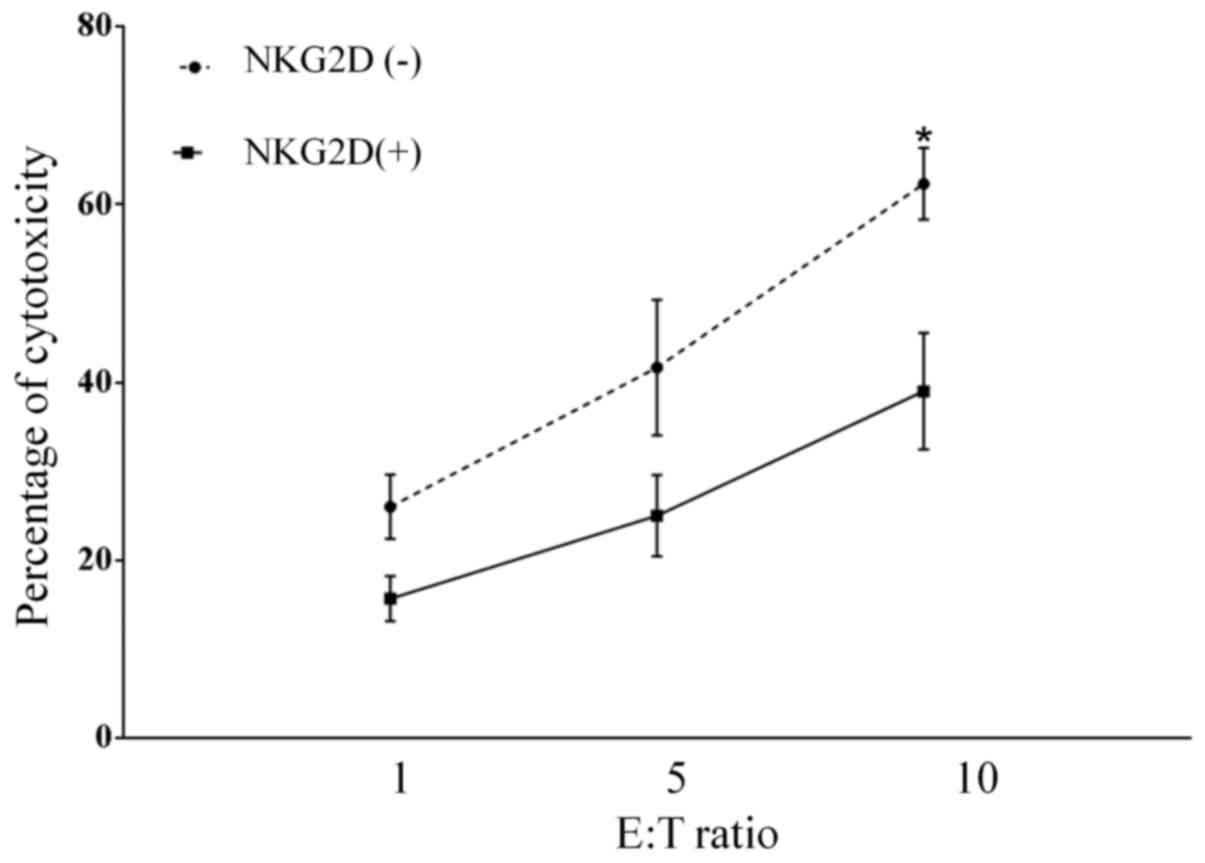
NKG2D is involved in the CIK-mediated
lysis of lung cancer cells
To investigate whether the NKG2D-NKG2D ligand
interaction is involved in CIK cell-mediated recognition and
cytolysis of lung cancer cells, the cytotoxic effect of CIK cells
on the NKG2D ligand-expressing lung cancer A549 cell line was
investigated. The CIK cells caused cytolysis of A549 cells with
60±8.6% specific killing, which decreased (30±3.2%) following
pretreatment of CIK cells with anti-NKG2D antibody (P=0.014;
Fig. 5). This result suggests that
the specific killing of lung cancer cells by CIK cells is partially
mediated by NKG2D-NKG2D ligand interactions.
Discussion
The NKG2D-NKG2D ligand signaling pathway serves an
important role in anti-tumor immunity, with NKG2D as the main
activating receptor of NK cells that induces anti-tumor effects
(15,21). NKG2D ligands are expressed on the
surface of tumor cells. NKG2D has been revealed to trigger natural
immune cell-mediated cytotoxicity against a variety of tumor cells
(22,23), but NKG2D-mediated cytotoxicity
requires NKG2D ligands expressed on target cells and the expression
of NKG2D receptors on the surface of immune cells (11).
CIK cells are heterogeneous cells: NKG2D is
expressed on the surface of all NK cells and certain T cells
(24). CIK cells expressing the NKG2D
receptor may be important in targeting and eliminating malignant
cells. Previous data supports the notion that NKG2D ligands affect
the outcomes of immune cells (11).
The present study demonstrated that NKG2D ligands were expressed on
the surface of lung cancer cell lines. Furthermore, that lung
cancer tissue samples from patients expressed higher level of NKG2D
ligands MICA and UBP2, which may serve an essential role in the
recognition of lung cancer cells by immune cells. CIK cells
demonstrated cytotoxicity against A549 cells in vitro, which
was partially blocked by anti-NKG2D antibodies.
In conclusion, the present study has demonstrated
that NKG2D ligands are expressed in lung cancer tissue samples and
cell lines. The results presented suggest that the interaction
between NKG2D-NKG2D ligand underlies the killing of lung cancer
cells by CIK cells.
Acknowledgements
The present study was supported by Major projects
foundation of Najing Medical University (grant no.,
2012NJMU126).
References
|
1
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Schmittel A, Sebastian M, von Fischer
Weikersthal L, Martus P, Gauler TC, Kaufmann C, Hortig P, Fischer
JR, Link H, Binder D, et al: A German multicenter, randomized phase
III trial comparing irinotecan-carboplatin with
etoposide-carboplatin as first-line therapy for extensive-disease
small-cell lung cancer. Ann Oncol. 22:1798–1804. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jotte R, Conkling P, Reynolds C, Galsky
MD, Klein L, Fitzgibbons JF, McNally R, Renschler MF and Oliver JW:
Randomized phase II trial of single-agent amrubicin or topotecan as
second-line treatment in patients with small-cell lung cancer
sensitive to first-line platinum-based chemotherapy. J Clin Onco.
29:287–293. 2011. View Article : Google Scholar
|
|
4
|
Gerber DE and Schiller JH: Maintenance
chemotherapy for advanced non-small-cell lung cancer: New life for
an old idea. J Clin Oncol. 31:1009–1020. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rossi A, Garassino MC, Cinquini M,
Sburlati P, Di Maio M, Farina G, Gridelli C and Torri V:
Maintenance or consolidation therapy in small-cell lung cancer: A
systematic review and meta-analysis. Lung Cancer. 70:119–128. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mesiano G, Todorovic M, Gammaitoni L,
Leuci V, Diego Giraudo L, Carnevale-Schianca F, Fagioli F,
Piacibello W, Aglietta M and Sangiolo D: Cytokine-induced killer
(CIK) cells as feasible and effective adoptive immunotherapy for
the treatment of solid tumors. Expert Opin Biol Ther. 12:673–684.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Moretta A, Bottino C, Vitale M, Pende D,
Cantoni C, Mingari MC, Biassoni R and Moretta L: Activating
receptors and coreceptors involved in human natural killer
cell-mediated cytolysis. Annu Rev Immunol. 19:197–223. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Carbone E, Neri P, Mesuraca M, Fulciniti
MT, Otsuki T, Pende D, Groh V, Spies T, Pollio G, Cosman D, et al:
HLA class I, NKG2D, and natural cytotoxicity receptors regulate
multiple myeloma cell recognition by natural killer cells. Blood.
105:251–258. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Coudert JD and Held W: The role of the
NKG2D receptor for tumor immunity. Semin Cancer Biol. 16:333–343.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mistry AR and O'Callaghan CA: Regulation
of ligands for the activating receptor NKG2D. Immunology.
121:439–447. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fuertes MB, Girart MV, Molinero LL,
Domaica CI, Rossi LE, Barrio MM, Mordoh J, Rabinovich GA and
Zwirner NW: Intracellular retention of the NKG2D ligand MHC class I
chain-related gene A in human melanomas confers immune privilege
and prevents NK cell-mediated cytotoxicity. J Immunol.
180:4606–4614. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kloss M, Decker P, Baltz KM, Baessler T,
Jung G, Rammensee HG, Steinle A, Krusch M and Salih HR: Interaction
of monocytes with NK cells upon Toll-like receptor-induced
expression of the NKG2D ligand MICA. J Immunol. 181:6711–6719.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lu X, Ohata K, Kondo Y, Espinoza JL, Qi Z
and Nakao S: Hydroxyurea upregulates NKG2D ligand expression in
myeloid leukemia cells synergistically with valproic acid and
potentially enhances susceptibility of leukemic cells to natural
killer cell-mediated cytolysis. Cancer Sci. 101:609–615. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kato N, Tanaka J, Sugita J, Toubai T,
Miura Y, Ibata M, Syono Y, Ota S, Kondo T, Asaka M and Imamura M:
Regulation of the expression of MHC class I-related chain A, B
(MICA, MICB) via chromatin remodeling and its impact on the
susceptibility of leukemic cells to the cytotoxicity of
NKG2D-expressing cells. Leukemia. 21:2103–2108. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bae DS, Hwang YK and Lee JK: Importance of
NKG2D-NKG2D ligands interaction for cytolytic activity of natural
killer cell. Cell Immunol. 276:122–127. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pende D, Rivera P, Marcenaro S, Chang CC,
Biassoni R, Conte R, Kubin M, Cosman D, Ferrone S, Moretta L and
Moretta A: Major histocompatibility complex class I-related chain A
and UL16-binding protein expression on tumor cell lines of
different histotypes: Analysis of tumor susceptibility to
NKG2D-dependent natural killer cell cytotoxicity. Cancer Res.
62:6178–6186. 2002.PubMed/NCBI
|
|
17
|
Friese MA, Platten M, Lutz SZ, Naumann U,
Aulwurm S, Bischof F, Bühring HJ, Dichgans J, Rammensee HG, Steinle
A and Weller M: MICA/NKG2D-mediated immunogene therapy of
experimental gliomas. Cancer Res. 63:8996–9006. 2003.PubMed/NCBI
|
|
18
|
Salih HR, Antropius H, Gieseke F, Lutz SZ,
Kanz L, Rammensee HG and Steinle A: Functional expression and
release of ligands for the activating immunoreceptor NKG2D in
leukemia. Blood. 102:1389–1396. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Linn YC, Lau SK, Liu BH, Ng LH, Yong HX
and Hui KM: Characterization of the recognition and functional
heterogeneity exhibited by cytokine-induced killer cell subsets
against acute myeloid leukaemia target cell. Immunology.
126:423–435. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Morisaki T, Onishi H and Katano M: Cancer
immunotherapy using NKG2D and DNAM-1 systems. Anticancer Res.
32:2241–2247. 2012.PubMed/NCBI
|
|
22
|
Zafirova B, Wensveen FM, Gulin M and Polić
B: Regulation of immune cell function and differentiation by the
NKG2D receptor. Cell Mol Life Sci. 68:3519–3529. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Champsaur M and Lanier LL: Effect of NKG2D
ligand expression on host immune responses. Immunol Rev.
235:267–285. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Spear P, Wu MR, Sentman ML and Sentman CL:
NKG2D ligands as therapeutic targets. Cancer Immun.
13:82013.PubMed/NCBI
|