Introduction
Thymic carcinoma is a rare and invasive mediastinum
neoplasm. The frequency of thymic carcinoma is higher in Asian than
in Caucasian populations (1). It is
difficult to detect these type of tumors in the early stage, since
the symptoms of the disease are not remarkable until it invades or
compromises other organs, including the heart, nerves, bronchus and
blood vessels (2,3). The most favorable treatment is complete
surgical resection (2,3); however, the majority of thymic
carcinomas already invade into other organs and are metastasized
when they are detected (4–6). For advanced and relapsed thymic
carcinoma cases, no optimal chemotherapeutic drug or regimen
exists. Previous studies on chemotherapy for advanced thymic
carcinoma, which were based on the regimens used for advanced
thymoma, lung cancer and germ cell tumors, employed cisplatin-based
chemotherapy (7–10). Furthermore, second-line chemotherapy
regimen has not been established. To establish the effective
regimens for thymic carcinoma, only small-scale clinical trials
have been conducted to date based on a single case report (11–14).
Therefore, an effective and safe drug regimen for advanced and
relapsed thymic carcinoma must be identified.
Clinical trials for thymic carcinoma are difficult
due to its rareness. To solve this problem, the present study aimed
to evaluate thymic cancer patients retrospectively. In order to
examine the predictive factors of the chemotherapeutic agent, the
expression of excision repair cross-complementation group 1 (ERCC1)
and class III β-tubulin (TUBB3) proteins was investigated by
immunohistochemistry (IHC) in thymic carcinoma specimens. Previous
studies reported that ERCC1 disturbs platinum-based chemotherapy
(15–17), while TUBB3 disturbs taxane-based
chemotherapy (18–20). Our group previously demonstrated that
completely resected non-small cell lung cancer (NSCLC) patients,
whose tumors were negative for ERCC1 and TUBB3 expression by IHC,
exhibited better prognosis upon receiving platinum-based plus
paclitaxel chemotherapy than patients whose tumors were positive
for ERCC1 and TUBB3 expression according to IHC (21). The present study is a retrospective
follow-up study that evaluates the protein expression and
effectiveness of carboplatin plus paclitaxel (CP) chemotherapy for
thymic carcinoma patients.
Patients and methods
Patients
The present study examined the expression of ERCC1
and TUBB3 proteins in 40 thymic carcinoma patients who underwent
either surgical resection or core-needle biopsy from June 1986 to
November 2012 at Nagoya City University Hospital (Nagoya, Japan).
The expression of ERCC1 and TUBB3 proteins was also evaluated in 50
patients who underwent curative resection for NSCLC. The present
study was approved by the Institutional Review Board of Nagoya City
University Hospital, and all the patients consented to the use of
their tissues for the present analysis.
Among these patients, 1 was in Masaoka stage I
(2.5%), 8 in stage II (20.0%), 9 in stage III (22.5%), 7 in stage
IVA (17.5%) and 15 in stage IVB (37.5%) of the disease (22). The clinical and pathological
characteristics of these 40 thymic carcinoma patients are as
follows: 24 (60.0%) patients were male and 16 (40.0%) were female.
In total, 31 patients (77.5%) were diagnosed as squamous cell
carcinoma, 5 (12.5%) as neuroendocrine carcinoma (NET), 3 (7.5%) as
adenocarcinoma and 1 (2.5%) as mucoepidermoid carcinoma. A total of
15 patients were treated with complete surgical resection, 9 with
incomplete surgical resection and 16 with biopsy. For all patients,
the median overall survival was 73 months. The present study
investigated whether the expression of ERCC1 and TUBB3 proteins was
associated with the overall survival and clinicopathological
factors of thymic carcinoma patients.
IHC staining
The same IHC method and evaluation for ERCC1 and
TUBB3 as that reported in our previous study on lung cancer
patients was used in the present study (21). The antibody against ERCC1 was an
anti-ERCC1 mouse monoclonal antibody (clone 8F1; Abcam, Cambridge,
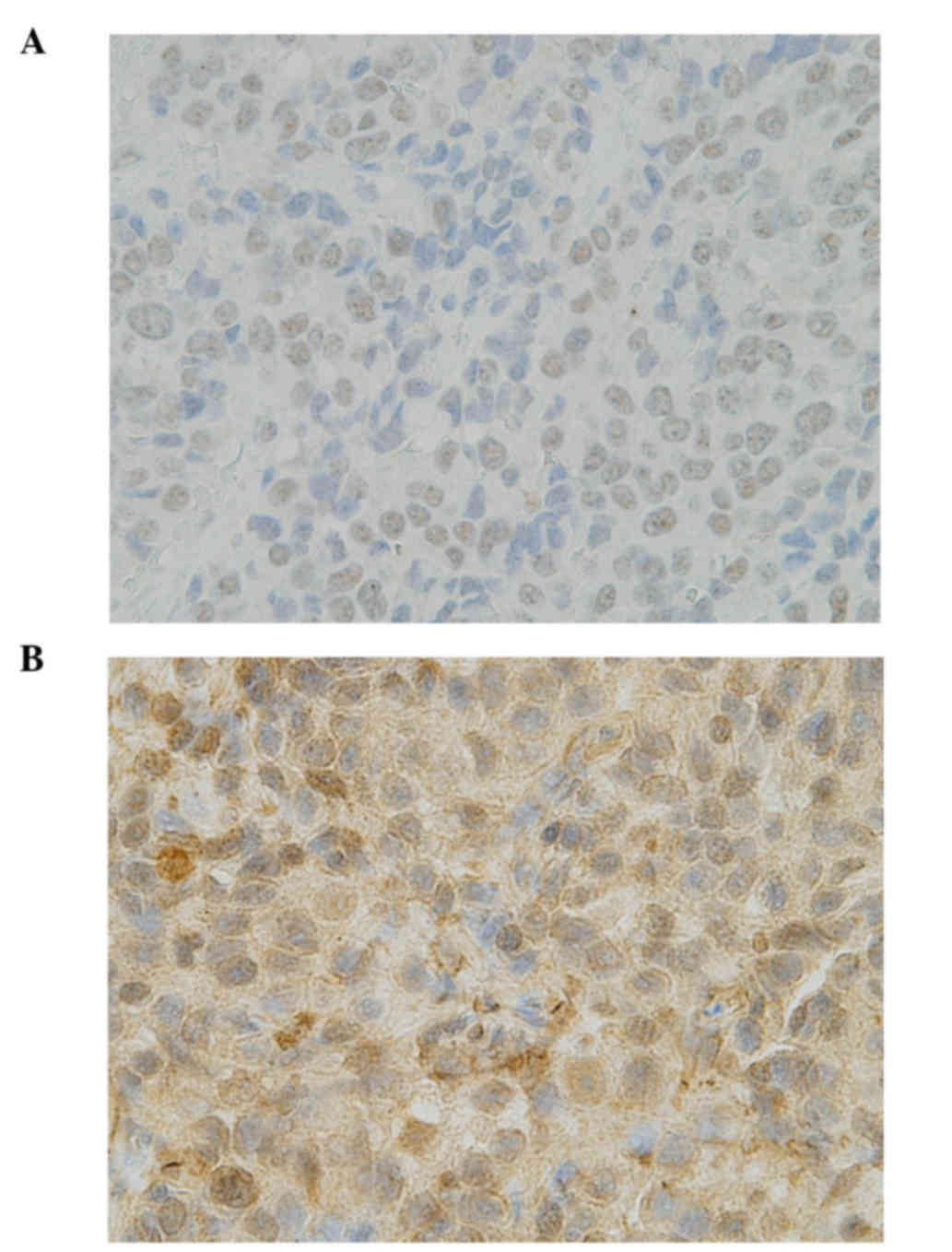
UK). The proportion score of tumor nuclear staining intensity (0–3)
was multiplied by the staining intensity of nuclei (0 if 0%; 0.1 if
1–9%; 0.5 if 10–49%; and 1.0 if ≥50%) in order to obtain a final
semiquantitative H-score. An ERCC1 IHC score of ≥1.0 was considered
as positive (Fig. 1A).
The antibody against TUBB3 was an anti-class III
β-tubulin monoclonal antibody (clone TUJ1; Covance, Inc.,
Princeton, NJ, USA). Over 50% of positive cells with a staining
intensity of 2 was considered as TUBB3 positive (Fig. 1B).
Statistical analysis
Survival curves were generated using the
Kaplan-Meier method, and the log-rank test was used to assess the
statistical significance of the differences between groups. The Cox
proportional hazards model was used to estimate the hazard ratios
and 95% confidence intervals (CIs). Prognostic variables identified
by univariate analysis were further analyzed in a multivariate Cox
model. Of note, the analysis of the present study had limitations
in terms of interpretation due to the small number of patients.
Two-sided P<0.05 was considered to indicate a statistically
significant difference. All data were analyzed with EZR software
version 1.33 (http://www.jichi.ac.jp/saitama-sct/SaitamaHP.files/statmedEN.html)
(23).
Results
ERCC1 expression in 40 thymic
carcinoma and 50 NSCLC patients
ERCC1 IHC staining was negative in 32/40 cases (80%)
of thymic carcinoma. Only 8/40 cases (20%) were positive for ERCC1
IHC staining. No association between the expression of ERCC1 and
clinicopathological factors was identified (Table I). The expression of ERCC1 was
examined by IHC in 50 NSCLC patients who underwent curative
resection in order to compare the expression of the ERCC1 protein
between thymic carcinoma and NSCLC patients. The results revealed
that 29 cases (58%) were negative and 21 cases (42%) were positive
for ERCC1 IHC staining.
 | Table I.Patients' characteristics according to
ERCC1 expression. |
Table I.
Patients' characteristics according to
ERCC1 expression.
|
|
| ERCC1 expression |
|
|---|
|
|
|
|
|
|---|
| Characteristic | No. | Negative (n=32) | Positive (n=8) | P-value |
|---|
| Age (years) |
|
|
| 0.3821 |
|
>60 | 22 | 16 | 6 |
|
| ≤60 | 18 | 16 | 2 |
|
| Sex |
|
|
| 0.8087 |
| Male | 24 | 19 | 5 |
|
|
Female | 16 | 13 | 3 |
|
| Histology |
|
|
| 0.7764 |
| SCC | 31 | 25 | 6 |
|
|
Others | 9 | 7 | 2 |
|
| Masaoka stage |
|
|
| 0.4745 |
| IVA,
IVB | 22 | 19 | 3 |
|
|
I–III | 18 | 13 | 5 |
|
TUBB3 expression in 40 thymic
carcinoma and 50 NSCLC patients
TUBB3 IHC staining was negative in 32/40 cases (80%)
of thymic carcinoma. Only 8/40 cases (20%) were positive for TUBB3
IHC staining. No association was observed between the expression of
TUBB3 and clinicopathological factors, including ERCC1 IHC staining
(Table II). In order to compare the
expression of the TUBB3 protein between thymic carcinoma and NSCLC
patients, TUBB3 protein expression was examined by IHC in 50 NSCLC
patients who underwent curative resection. The results revealed
that 23 cases (46%) were negative and 27 (54%) positive for TUBB3
IHC staining.
 | Table II.Patients' characteristics according to
TUBB3 expression. |
Table II.
Patients' characteristics according to
TUBB3 expression.
|
|
| TUBB3 expression |
|
|---|
|
|
|
|
|
|---|
| Characteristic | No. | Negative (n=32) | Positive (n=8) | P-value |
|---|
| Age (years) |
|
|
| 0.9367 |
|
>60 | 22 | 17 | 5 |
|
| ≤60 | 18 | 15 | 3 |
|
| Sex |
|
|
| 0.2942 |
| Male | 24 | 21 | 3 |
|
|
Female | 16 | 11 | 5 |
|
| Histology |
|
|
| 0.1076 |
|
SCC | 31 | 27 | 4 |
|
|
Others | 9 | 5 | 4 |
|
| Masaoka stage |
|
|
| 0.9367 |
| IVA,
IVB | 22 | 18 | 4 |
|
|
I–III | 18 | 14 | 4 |
|
| ERCC1
expression |
|
|
| 0.3738 |
|
Positive | 8 | 6 | 2 |
|
|
Negative | 32 | 26 | 6 |
|
Factors associated with the overall
survival of 40 thymic carcinoma patients
In all 40 cases, the 3-year overall survival rate
was 67.3% (Fig. 2A). Only complete
resection was associated with a better prognosis) than incomplete
resection (P=0.0341; Fig. 2B). Other
clinicopathological factors, including the expression of ERCC1 and
TUBB3 proteins, had no effect on overall survival (Table III). The expression of ERCC1 or
TUBB3 proteins did not affect overall survival (Fig. 2C and D).
 | Table III.Factors associated with overall
survival of 40 thymic carcinoma patients. |
Table III.
Factors associated with overall
survival of 40 thymic carcinoma patients.
|
| 3-year
survival |
|---|
|
|
|
|---|
| Characteristic | No. | % |
P-valuea |
|---|
| Age (years) |
|
| 0.106 |
|
>60 | 22 | 77.2 |
|
|
≤60 | 18 | 56.9 |
|
| Sex |
|
| 0.707 |
|
Male | 24 | 67.8 |
|
|
Female | 16 | 68.2 |
|
| Histology |
|
| 0.362 |
|
SCC | 31 | 65.7 |
|
|
Others | 9 | 72.9 |
|
| Masaoka stage |
|
| 0.767 |
| IVA,
IVB | 22 | 73.2 |
|
|
I–III | 18 | 60.0 |
|
| Complete
resection |
|
| 0.0341 |
| R0 | 15 | 91.7 |
|
| R1,
R2 | 25 | 58.4 |
|
| ERCC1
expression |
|
| 0.823 |
|
Positive | 8 | 60.0 |
|
|
Negative | 32 | 68.0 |
|
| Class III β-tubulin
expression |
|
| 0.125 |
|
Positive | 8 | 45.0 |
|
|
Negative | 32 | 73.3 |
|
Clinicopathological features of 19
patients with thymic carcinoma who received platinum-based
chemotherapy (first treatment)
The response rate of platinum-based chemotherapy was
52.6% (10 out of 19 cases). Among 16 cases who received
platinum-based plus paclitaxel chemotherapy, the response rate was
56.3% (9 out of 16 cases). Among the 11 patients who were negative
for both ERCC1 and TUBB3 expression, the response rate was 63.6% (7
out of 11 cases) (Table IV).
 | Table IV.Features of 19 patients with thymic
carcinoma who received platinum-based chemotherapy (first
treatment). |
Table IV.
Features of 19 patients with thymic
carcinoma who received platinum-based chemotherapy (first
treatment).
| No. | Age (years) | Sex | Histology | Masaoka
classification | Regimen | Response rate | ERCC1
expression | TUBB3
expression | OS (months) | Status |
|---|
| 1 | 51 | F | SCC | III | ADOC (X2) | PD | + | + | 23.0 | Dead |
| 2 | 33 | M | Adenocarcinoma | IVb | PVB (X1), PE
(X3) | PR | − | − | 74.4 | Dead |
| 3 | 49 | M | SCC | III | CP (X2) | PR | − | − | 8.9 | Alive |
| 4 | 62 | M | NET | IVb |
CDDP+CPT-11(X2) | SD | + | + | 21.0 | Alive |
| 5 | 55 | M | SCC | IVb | CP (X2) | SD | − | − | 13.3 | Dead |
| 6 | 56 | M | SCC | III | TC (X1) | SD | − | − | 32.1 | Dead |
| 7 | 60 | M | SCC | IVa | CP (X2) | SD | + | − | 92.4 | Alive |
| 8 | 43 | F | SCC | III | CP (X2) | PR | − | − | 6.9 | Dead |
| 9 | 70 | M | SCC | IVb | CP (X3) | PR | − | − | 59.1 | Dead |
| 10 | 58 | M | SCC | IVa | CP (X7) | SD | − | − | 66.2 | Alive |
| 11 | 70 | F | SCC | IVb | CP (X4) | PR | − | + | 62.4 | Alive |
| 12 | 30 | F | NET | IVa | CP (X7) | SD | − | + | 30.1 | Dead |
| 13 | 78 | F | SCC | IVb | CP (X6) | PR | − | − | 46.3 | Alive |
| 14 | 48 | F | SCC | IVb | CP (X2) | SD | − | − | 49.3 | Alive |
| 15 | 54 | M | SCC | IVa | CP (X2) | PR | − | + | 8.7 | Dead |
| 16 | 46 | M | SCC | IVb | CP (X6) | PR | − | − | 35.4 | Alive |
| 17 | 64 | F | SCC | IVb | CP (X2) | PR | − | − | 25.4 | Alive |
| 18 | 66 | M | SCC | IVb | CP (X6) | PR | − | − | 18.1 | Alive |
| 19 | 65 | M | SCC | IVb | CP (X3) | SD | + | − | 9.1 | Alive |
Clinicopathological features of 13
patients with ERCC1 and/or TUBB3 IHC-positive expression
In the ERCC1-positive cases, there was neither
partial response (PR) nor complete response for platinum-based
chemotherapy. Tissues of NET were positive for TUBB3 (all 4 cases).
However, in the squamous cell carcinoma cases of TUBB3-positive and
ERCC1-negative expression, CP chemotherapy was effective in 2 out
of 3 patients, thereby achieving PR.
Expression of ERCC1 and TUBB3 proteins
among 50 NSCLC patients
ERCC1 expression was observed to be positive in 21
cases (42%), and TUBB3 expression was positive in 27 cases
(54%).
Discussion
Thymic carcinoma is a rare and invasive mediastinum
malignant tumor. It is urgent to identify an improved treatment for
advanced and relapsed thymic carcinoma patients, since no optimal
treatment has been established so far. Recently, platinum-based
plus taxane-based chemotherapies are considered the standard
regimens for the treatment of lung cancer patients (24,25). CP
chemotherapy is another regimen for thymic malignant tumors, thymic
carcinomas and thymomas. To a certain degree, case reports and
small-size clinical trials reported the effectiveness of CP
regimens for thymic malignant tumors (26–29).
However, one of the remarkable problems in those previous studies
was that they examined thymic epithelial malignant tumors without
separating them from thymic carcinoma and thymoma. Thymic carcinoma
and thymoma should be distinguished, as they exhibit different
biological symptoms (30). The
reaction towards chemotherapy, prognosis and metastatic form are
different in each tumor type. In the present study, the expression
of ERCC1 and TUBB3 proteins was examined in 40 thymic carcinoma
patients in order to clarify whether the expression of these
proteins was associated with prognosis and clinicopathological
factors.
Among the NSCLC patients who received platinum-based
plus taxane-based chemotherapies (mainly CP), the chemotherapy was
effective for all patients, with the exception of those who
exhibited overexpression of ERCC1 and TUBB3 proteins. According to
a previous large-scale study on lung cancer patients, the
overexpression of ERCC1 and TUBB3 proteins appeared to be
associated with resistance to platinum-based and taxane-based
chemotherapies (15–21). The present study aimed to study the
expression of ERCC1 and TUBB3 proteins in thymic carcinoma
patients. Collecting a large number of patients' tissues (including
those who received platinum-based and taxane-based chemotherapies
in order to examine the effect of therapy) was difficult, as well
as interpreting the results of IHC, due to the limited number of
thymic carcinoma cases, since this tumor is rare. The present study
also examined the expression of ERCC1 and TUBB3 proteins among 50
NSCLC patients in order to compare the protein expression ratio.
The expression of ERCC1 and TUBB3 proteins determined by IHC was
compared between thymic carcinoma and lung cancer patients by using
the absolute scoring assessment and the same immunostaining method
as previously reported (21). The
expression of ERCC1 and TUBB3 proteins in the thymic carcinoma
cases were lower than those in the NSCLC cases. In addition, CP
chemotherapy was also observed to be effective for thymic
carcinoma. The IHC results for ERCC1 and TUBB3 may help to select
the best chemotherapy regimen for thymic carcinoma patients.
In the current study, the expression of ERCC1 and
TUBB3 proteins in the thymic carcinoma cases were lower than those
in the NSCLC cases. As a result of examining NSCLC patients who
received cisplatin-based chemotherapy (mainly CP), it was observed
that chemotherapy was effective for all patients, with the
exception of those overexpressing ERCC1 and TUBB3. The
overexpression of ERCC1 and TUBB3 appeared to be associated with
resistance to platinum- and taxane-based chemotherapies. These
results indicated that platinum-based plus taxane-based regimens,
which are two of the standard treatments for NSCLC, may be
effective for the treatment of thymic carcinoma patients.
Therefore, platinum-based plus taxane-based regimens should be
considered as standard regimens for thymic cancer patients.
The present study also investigated the effect of
platinum-based chemotherapy and the CP regimen in 19 thymic
carcinoma patients (including those receiving advanced and
neo-adjuvant chemotherapy). The response rate to the platinum-based
chemotherapy was 52.6%. Limited to the patients who were negative
for both ERCC1 and TUBB3 and received the CP regimen, the response
rate was 63.6%. For the ERCC1-positive cases, more effective
regimens were identified without using the platinum-based drug.
Since the TUBB3-positive cases, including NET cases, exhibited
resistance to taxane-based chemotherapy, chemotherapy regimens used
in small cell lung cancer may be effective for those cases.
Kaira et al (31) reported the analysis of ERCC1 and TUBB3
expression in thymic epithelial tumors, including 39 thymomas and
17 thymic carcinomas. The authors concluded that ERCC1 and TUBB3
expression may be associated with resistance to CP regimen, in
agreement with the present findings.
Due to its rare frequency, it is difficult to plan
clinical trials for thymic carcinoma patients. Yet, a prospective,
worldwide, randomized, controlled trial of chemotherapy for
advanced and relapsed thymic carcinoma is required.
The present study examined the expression levels of
ERCC1 and TUBB3 proteins, and identified that CP chemotherapy is
effective for thymic carcinoma patients. The results of the present
study also indicated that ERCC1 and TUBB3 expression could serve as
biomarkers that may help to identify the subgroup of thymic
carcinoma patients likely to benefit from platinum-based and
taxane-based chemotherapies.
References
|
1
|
Shimosato Y, Kameya T, Ngai K and Suemasu
K: Squamous cell carcinoma of the thymus. An analysis of eight
cases. Am J Surg Pathol. 1:109–121. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Weksler B, Dhupar R, Parikh V, Nason KS,
Pennathur A and Ferson PF: Thymic carcinoma: A multivariate
analysis of factors predictive of survival in 290 patients. Ann
Thorac Surg. 95:299–303. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhao Y, Zhao H, Hu D, Fan L, Shi J and
Fang W: Surgical treatment and prognosis of thymic squamous cell
carcinoma: A retrospective analysis of 105 cases. Ann Thorac Surg.
96:1019–1024. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Suster S and Rosai J: Thymic carcinoma. A
clinicopathologic study of 60 cases. Cancer. 67:1025–1032. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hsu CP, Chen CY, Chen CL, Lin CT, Hsu NY,
Wang JH and Wang PY: Thymic carcinoma. Ten year' experience in
twenty patients. J Thorac Cardiovasc Surg. 107:615–620.
1994.PubMed/NCBI
|
|
6
|
Takeda S, Sawabata N, Inoue M, Koma M,
Maeda H and Hirano H: Thymic carcinoma. Clinical institutional
experience with 15 patients. Eur J Cardiothorac Surg. 26:401–406.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Agatsuma T, Koizumi T, Kanda S, Ito M,
Urushihata K, Yamamoto H, Hanaoka M and Kubo K: Combination
chemotherapy with doxorubicin, vincristine, cyclophosphamide, and
platinum compounds for advanced thymic carcinoma. J Thorac Oncol.
6:2130–2134. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yoh K, Goto K, Ishii G, Niho S, Ohmatsu H,
Kubota K, Kakinuma R, Nagai K, Suga M and Nishiwaki Y: Weekly
chemotherapy with cisplatin, vincristine, doxorubicin, and
etoposide is an effective treatment for advanced thymic carcinoma.
Cancer. 98:926–931. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Okuma Y, Hosomi Y, Takagi Y, Iguchi M,
Okamura T and Shibuya M: Cisplatin and irinotecan combination
chemotherapy for advanced thymic carcinoma: Evaluation of efficacy
and toxicity. Lung Cancer. 74:492–496. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Magois E, Guigay J, Blancard PS, Margery
J, Milleron B, Lher P and Jounieaux V: Multimodal treatment of
thymic carcinoma: Report of nine cases. Lung Cancer. 59:126–132.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Oguri T, Achiwa H, Kato D, Maeda H, Niimi
T, Sato S and Ueda R: Efficacy of docetaxel as a second-line
chemotherapy for thymic carcinoma. Chemotherapy. 50:279–282. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liang Y, Padda SK, Piess JW, West RB, Neal
JW and Wakelee HA: Pemetrexed in patients with thymic malignancies
previously treated with chemotherapy. Lung Cancer. 87:34–38. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Okuma Y, Shimokawa T, Takagi Y, Hosomi Y,
Iguchi M, Okamura T and Shibuya M: S-1 is an acrive anticancer
agent for advanced thymic carcinoma. Lung Cancer. 70:357–363. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tagawa T, Ohta M, Kuwata T, Awaya H and
Ishida T: S-1 plus cisplatin chemotherapy with concurrent radiation
for thymic basaloid carcinoma. J Thorac Oncol. 5:572–573. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Altaha R, Liang X, Yu JJ and Reed E:
Excision repair cross complementing-group 1: Gene expression and
platinum resistance. Int J Mol Med. 14:959–970. 2004.PubMed/NCBI
|
|
16
|
Lord RV, Brabender J, Gandara D, Alberola
V, Camps C, Domine M, Cardenal F, Sánchez JM, Gumerlock PH, Tarón
M, et al: Low ERCC1 expression correlates with prolonged survival
after cisplatin plus gemcitabine chemotherapy in non-small cell
lung cancer. Clin Cancer Res. 8:2286–2291. 2002.PubMed/NCBI
|
|
17
|
Metzger R, Leichman CG, Danenberg KD,
Danenberg PV, Lenz HJ, Hayashi K, Groshen S, Salonga D, Cohen H,
Laine L, et al: ERCC1 mRNA levels complement thymidylate synthase
mRNA levels in predicting response and survival for gastric cancer
patients receiving combination cisplatin and fluorouracil
chemotherapy. J Clin Oncol. 16:309–316. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Katsetos CD, Legido A, Perentes E and Mörk
SJ: Class III beta-bululin isotype: A key cytoskeletal protein at
the crossroads of developmental neurobiology and tumor
neuropathology. J Child Neurol. 18:851–866; discussion 867. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lu Q and Luduena RF: Removal of beta III
isotype enhances taxol induced microtubule assembly. Cell Struct
Funct. 18:173–182. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kamath K, Wilson L, Cabral F and Jordan
MA: BetaIII-tubulin induces paclitaxel resistance in association
with reduced effects on microtubule dynamic instability. J Biol
Chem. 280:12902–12907. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Okuda K, Sasaki H, Dumontet C, Kawano O,
Yukiue H, Yokoyama T, Yano M and Fujii Y: Expression of excision
repair cross-complementation group 1 and class III beta-tubulin
predict survival after chemotherapy for completely resected
non-small cell lung cancer. Lung Cancer. 62:105–112. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Masaoka A, Monden Y, Nakahara K and
Tanioka T: Follow-up study of thymomas with special reference to
their chlinical stages. Cancer. 48:2485–2492. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kanda Y: Investigation of the freely
available easy-to-use software ‘EZR’ for medical statistics. Bone
Marrow Transplant. 48:452–458. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Schiller JH, Harrington D, Belani CP,
Langer C, Sandler A, Krook J, Zhu J and Johnson DH: Eastern
Cooperative Oncology Group: Comparison of four chemotherapy
regimens for advanced non-small-cell lung cancer. N Engl J Med.
346:92–98. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lilenbaum RC, Herndon JE II, List MA,
Desch C, Watson DM, Miller AA, Graziano SL, Perry MC, Saville W,
Chahinian P, et al: Single-agent versus combination chemotherapy in
advanced non-small-cell lung cancer: The cancer and leukemia group
B (study 9730). J Clin Oncol. 23:190–196. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lemma GL, Lee JW, Aisner SC, Langer CJ,
Tester WJ, Johnson DH and Loehrer PJ Sr: Phase II study of
carboplatin and paclitaxel in advanced thymoma and thymic
carcinoma. J Clin Oncol. 29:2060–2065. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Igawa S, Murakami H, Takahashi T, Nakamura
Y, Tsuya A, Naito T, Kaira K, Ono A, Shukuya T, Tamiya A, et al:
Efficacy of chemotheraoy with carboplatin and paclitaxel for
unresectable thymic carcinoma. Lung Cancer. 67:194–197. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Furugen M, Sekine I, Tsuta K, Horinouchi
H, Nokihara H, Yamamoto N, Kubota K and Tamura T: Combination
chemotherapy with carboplatin and paclitaxel for advanced thymic
cancer. Jpn J Clin Oncol. 41:1013–1016. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hirai F, Yamanaka T, Taguchi K, Daga H,
Ono A, Tanaka K, Kogure Y, Shimizu J, Kimura T, Fukuoka J, et al: A
multicenter phase II study of carboplatin and paclitaxel for
advanced thymic carcinoma: WJOG4207L. Ann Oncol. 26:363–368. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Weiss GJ: Thymic carcinoma: Current and
future therapeutic interventions. Expert Opin Investig Drugs.
19:1007–1016. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kaira K, Serizawa M, Koh Y, Miura S, Kaira
R, Abe M, Nakagawa K, Ohde Y, Okumura T, Naito T, Murakami H,
Takahashi T, Kondo H, Nakajima T, Endo M and Yamamoto N: Expression
of excision repair cross-complementation group 1, breast cancer
susceptibility 1, and β III-tubulin in thymic epithelial tumors. J
Thorac Oncol. 6:606–613. 2011. View Article : Google Scholar : PubMed/NCBI
|