Introduction
The role of the host immune system in the control of
cancer progression has been assessed for several years; at present,
it is vastly accepted that antitumor immunity occurs and that
numerous tumors have developed mechanisms to escape immune control,
leading to malignant progression (1).
The mechanisms responsible for antitumor immunity failure in
individuals with cancer include a wide diversity of soluble
immunosuppressive factors, including transforming growth factor β
(TGFβ), interleukin 10 (IL-10), reactive oxygen species (ROS),
enzymes and inhibitory ligands such as Fas ligand (FasL) or
TNF-related apoptosis-inducing ligand, released by tumor cells or
by other regulatory cells in the tumor microenvironment (2). The majority of immunotherapies against
cancer aim to counteract the action of regulatory cells in order to
achieve tumor remission or prevent recurrences; of these, T
regulatory cells (Tregs) have been the major focus of efforts to
therapeutically modulate their inhibitory activity (3,4).
B cells have been classically associated with
antigen presentation, antibody secretion and T cell activation
during immune responses (5–8). However, in autoimmune diseases, B cells
were demonstrated to exert immune-regulatory roles suppressing
cluster of differentiation (CD)4+ T cell responses,
mainly through IL-10 secretion (9,10).
Therefore, novel studies focusing on the role of B cells as
negative modulators of the antitumor response are currently
underway. Evidence that accounts for the role of B cells in
tumor-induced immunosuppression includes the existence of human B
cells with a regulatory phenotype in solid tumors (11), the B cell-mediated induction of Tregs
expansion (12–14) and the increase of tumor growth
(12,15–17).
Methylcolanthrene-induced murine fibrosarcoma (MCC)
is a highly immunogenic tumor that elicits an early specific
antitumor immune reaction, which is not strong enough to impede
tumor growth (18–21). The antitumor response declines at a
certain tumor volume (~500 mm3) and disappears
comprising a state of tolerance (18–21).
Immunological characteristics of MCC and its rapid growth in
vivo make it a suitable model to study mechanisms underlying
tumor immunity and tumor-induced immunosuppression. Using this
model, previous studies have demonstrated that small tumor-bearing
mice (TBM) are able to reject a secondary distant implant of the
same tumor through a T cell mediated-reaction, phenomenon known as
concomitant immunity (CI) (18).
Later, in the tolerogenic stage, CI is no longer detected and a
second tumor implant grows without being rejected (18). MCC growth was also found to induce a
series of alterations in the cell composition of tumor-draining
lymph nodes (TDLN), including the progressive increase in B cells
with the emergence of an IL-10-producing subpopulation (20,22). These
results suggest that B cells may be implicated in the
downregulation of the antitumor immunity and the establishment of
tumor tolerance in the MCC model. The present study examines the
role of B cells in the immunological control of the MCC tumor
growth.
Materials and methods
Mice
A total of 255 BALB/c and AKR mice (age, 2–3
months), inbred at the animal facilities of The Institute of
Experimental Medicine, National Scientific and Technical Research
Council, National Academy of Science (Buenos Aires, Argentina) were
used in the experiments. Mice were housed at 23°C and exposed to 12
h light/dark cycles, with free access to food and water and handled
according to the policies of the National Institutes of Health
Guide for the Care and Use of Laboratory Animals.
Tumor
The MCC was induced in male BALB/c mice by the
subcutaneous implantation of a methylcholanthrene pellet and was
maintained by syngeneic transplantation. MCC is an immunogenic
murine tumor characterized by an initial immunogenic state (tumor
volume, 100–400 mm3) followed by a tolerance state
(tumor volume, >500 mm3). Tumor size and volume were
assessed every 2 days according to the Attia and Weiss formula:
Tumor volume = 0.4 × (a × b2), where a and b represent
the larger and smaller diameters, respectively.
B-cell depletion
The B cell-depleting monoclonal mouse anti-CD20
antibody (clone, 18B12), kindly provided by Biogen Idec (Cambridge,
MA, USA), or rat non B cell-depleting immunoglobulin G (IgG)
antibody (catalog. no., 10700; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) was injected intraperitoneally (10 mg/kg) 3 days
prior to or 9 days post tumor inoculation (p.i.). Blood samples
were collected on day 0, 12, 16 and 24 and CD19+ cell
presence was analyzed using a FACS flow cytometer (BD Biosciences
San Jose, CA, USA) and WinMDI 2.8 software (developed by Joe
Trotter). Experiments were performed at various times following
antibody administration.
CI assay
For CI experiments, TBM received a second tumor
inoculation of 7×105 tumor cells in the contralateral
flank. The inoculation was performed 12 days p.i. in the group with
B cell-depletion prior to the tumor implant, or 18 days p.i. in the
group with B cell-depletion following the tumor implant. Tumor
rejection was evaluated when the control group (mice with the
second tumor implant only) developed tumors. The physical
appearance, movement and weight of the mice were evaluated daily
and the tumors were assessed for evidence of ulceration and
necrosis.
Cells and culture conditions
Inguinal and axillary TDLN from TBM, equivalent
lymph nodes (LN) from non TBM and tumor tissues were aseptically
excised and mechanically disaggregated in PBS. Tumor-infiltrating
lymphocytes (TILs) were obtained after sedimentation with
Ficoll-Triyoson (1.099 g/cm3), as previously described
(23). For in vitro assays,
single cell suspensions were cultured at 37°C and 5% CO2
atmosphere in complete medium (RPMI-1640; Gibco; Thermo Fisher
Scientific, Inc.) containing 10% fetal bovine serum (Natocor,
Córdoba, Argentina) L-glutamine,
penicillin/streptomycin/amphotericin B and 2-mercaptoethanol
(Gibco; Thermo Fisher Scientific, Inc.).
CD19+ and CD3+
cell purification
For functional assays, CD19+ B cells or
CD3+ T cells were isolated from LN or TILs cell
suspension, respectively, via cell sorting (FACSAria II; BD
Biosciences).
Flow cytometry
Cell suspensions (1×106) were incubated
with appropriate concentrations of the following monoclonal
antibodies (1:100; BD Pharmingen, San Diego, CA, USA): Fluorescein
isothiocyanate (FITC) monoclonal rat anti-mouse CD8α (clone,
53–6.7; catalog no., 553031); FITC monoclonal rat anti-mouse CD4
(clone, H129.19; catalog no., 553651); phycoerythrin (PE)
monoclonal rat anti-mouse CD19 (clone, 1D3; catalog no., 553786);
PE monoclonal rat anti-mouse interferon-γ (IFN-γ; catalog no.,
562020); PE monoclonal rat anti-mouse IL-10 (catalog no., 561060);
PE-Cy5.5 monoclonal rat anti-mouse CD45R/B220 (clone, RA3-6B2;
catalog no., 552771); and PE-Cy5.5 monoclonal rat anti-mouse CD25
(clone, PC61; catalog no., 551071). Cells were analyzed using a
FACS flow cytometer (BD Biosciences) and WinMDI 2.8 software.
Irrelevant isotype-matched antibodies were used as controls. The
intracellular presence of forkhead box P3 (Foxp3) was detected with
PE-rat anti-mouse Foxp3 (clone, FJK-16s; catalog no., 12-5773;
eBioscience, Inc., San Diego, CA, USA) and the Foxp3 staining
buffer set (eBioscience, Inc.). Following the stimulation of LN
cells (2×106 cells/ml) with ionomycin (1 µg/ml;
Sigma-Aldrich; St. Louis, MO, USA) and phorbol 12-myristate
13-acetate (50 ng/ml; Sigma-Aldrich) during 6 h incubation with
brefeldin A (eBioscience, Inc.), intracellular staining of IFN-γ
was detected with the monoclonal rat anti-mouse CD8α and PE
monoclonal rat anti-mouse interferon-γ antibodies using the
Cytofix/Cytoperm and Perm/Wash buffers (BD Pharmingen), according
to the manufacturer's protocol.
B cell inhibitory activity in
vitro
Balb/c mouse LN cells (2×105) were
incubated with 2×105 AKR spleen cells treated with
mitomycin C (Sigma-Aldrich) for 96 h. The final 18 h-pulse was 1
µCurie/well of [3H]-thymidine (DuPont NEN Research
Products, Boston, MA, USA) for a standard allogeneic proliferation.
Balb/c mouse B cells (7.5×104) isolated by cell-sorting
from TBM or non TBM LN were added in co-culture or through a
Transwell chamber, in the presence or absence of IL-10 neutralizing
antibody (5 µg/ml; BD Pharmingen) or granzyme B inhibitor (ZAAD
CMK; 10 µM; Enzo Life Sciences, Inc., Farmingdale, NY, USA), from
the beginning of the incubation.
Cytotoxicity assay
In vitro cytotoxicity was evaluated through
the JAM test (20,24) Briefly, tumor CD3+ T cells
isolated by cell sorting were incubated with
[3H]-thymidine-labeled MCC cells (2×104) for
8 h. Specific killing was calculated as: Specific killing (%) = 100
× (S-E)/S, where S is the spontaneous killing and E is the
experimental killing. Normal [3H]-thymidine-labeled
fibroblasts were used as controls.
Statistical analysis
All statistical analysis was performed using
GraphPad Prism 3.0 software (GraphPad Software, Inc., La Jolla, CA,
USA) and Microsoft Excel 2010 (Microsoft, Redmond, WA, USA).
Differences in tumor growth were evaluated by two-way analysis of
variance and Bonferroni post-tests (Fig.
1). Differences obtained in flow cytometry phenotyping
(Figs. 2, 3 and 4A),
cytotoxicity (Fig. 4B) and B cell
inhibitory activity through Transwell (Fig. 5A) experiments were all evaluated for
significance using the Student's t-test. Differences in B cell
inhibitory activity in the presence or absence of anti-IL-10 or
ZAAD CMK (Fig. 5B and C) were
evaluated by one-way ANOVA and Tukey's Multiple Comparison test.
P<0.05 was considered to indicate a statistically significant
difference (*P<0.05, **P<0.01, ***P<0.001).
Results
Systemic B cell depletion exerts
opposite effects during tumor implantation or tumor growth
Previous studies have described that a progressive
increase in IL-10-expressing B cells occurs at the TDLN, as a tumor
grows and evolves from an immunogenic to a tolerogenic state
(20,22). In order to analyze a possible role for
B cells in the establishment of tolerance and consequent tumor
progression, TBM were depleted of B cells using an anti-CD20
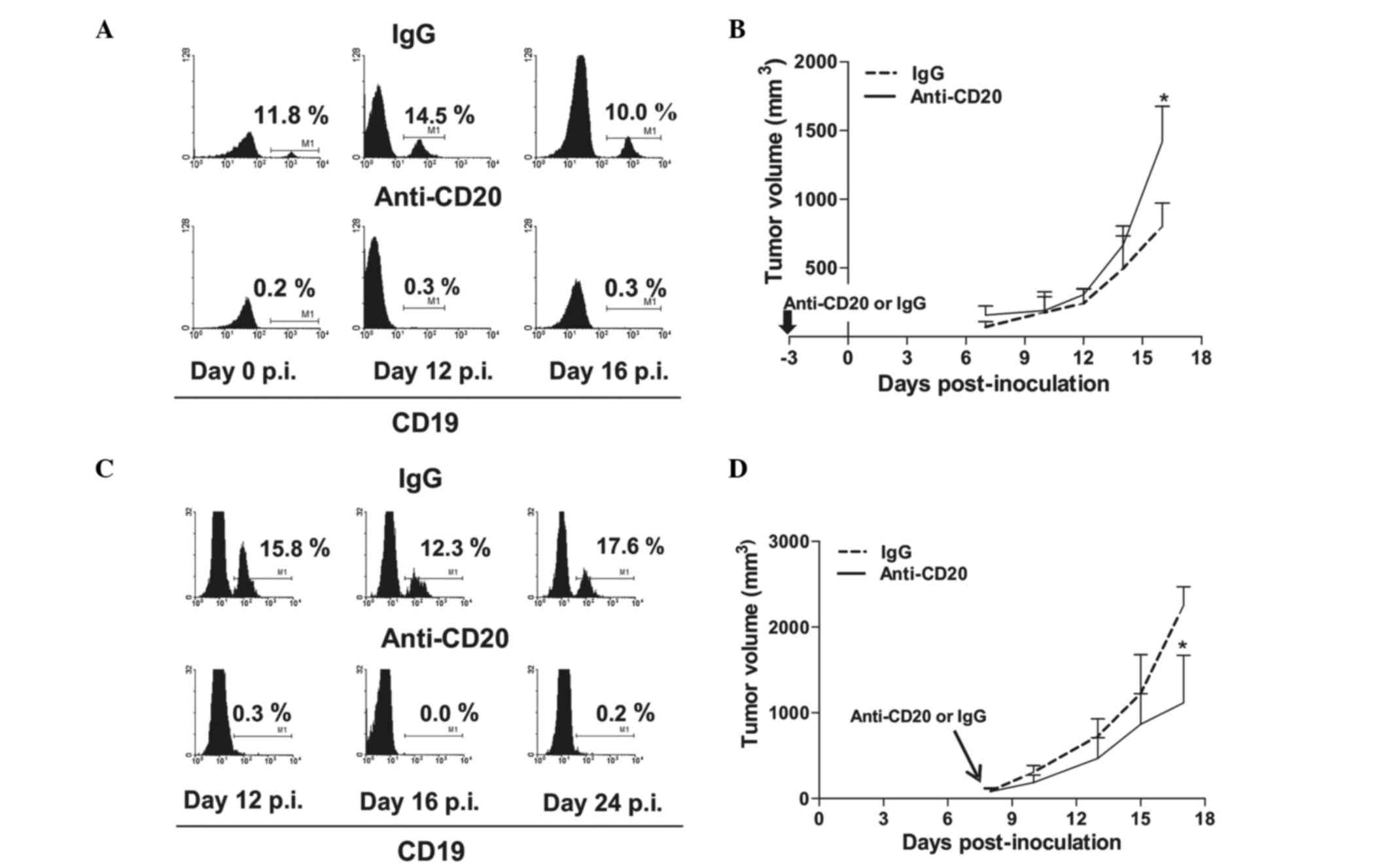
monoclonal antibody, 3 days prior to or 9 days p.i. (Fig. 1). In the TBM, a single administration
of anti-CD20 induced a sustained depletion of peripheral blood B
cells (Fig. 1A and C). Depletion
prior to tumor implantation resulted in increased tumor growth,
which became significant at ~day 15 p.i. (P<0.05; Fig. 1B). By contrast, depletion performed
after tumor implantation induced tumor growth retardation (Fig. 1D). The inhibition of tumor growth
began immediately after antibody inoculum and reached significance
~10 days later (P<0.05). These results may indicate that B cells
are initially necessary for the occurrence of the antitumor
reaction, and are also required for subsequent tumor
progression.
Systemic B cell depletion prior to
tumor inoculation does not modify the early antitumor response at
the TDLN, but enhances Treg number
Lymph node draining the tumor site is the first
place where the immune response against tumor development takes
place. A previous study showed that the initial growth of MCC could
induce the activation of TDLN dendritic and T cells (immunogenic
phase) (20). As the tumor increased
in size, the signs of immune activation at TDLN disappeared as the
mice became tolerant (tolerogenic phase) (20). On the supposition that B cell
depletion prior to tumor implantation could impair the proper onset
of the antitumor immune response and thus enhance tumor growth, the
TDLN from B cell-depleted TMB were analyzed at various times p.i..
Mice were injected with anti-CD20 or IgG antibodies 3 days prior to
tumor inoculum, and the TDLN were excised at days 5 and 20 p.i. and
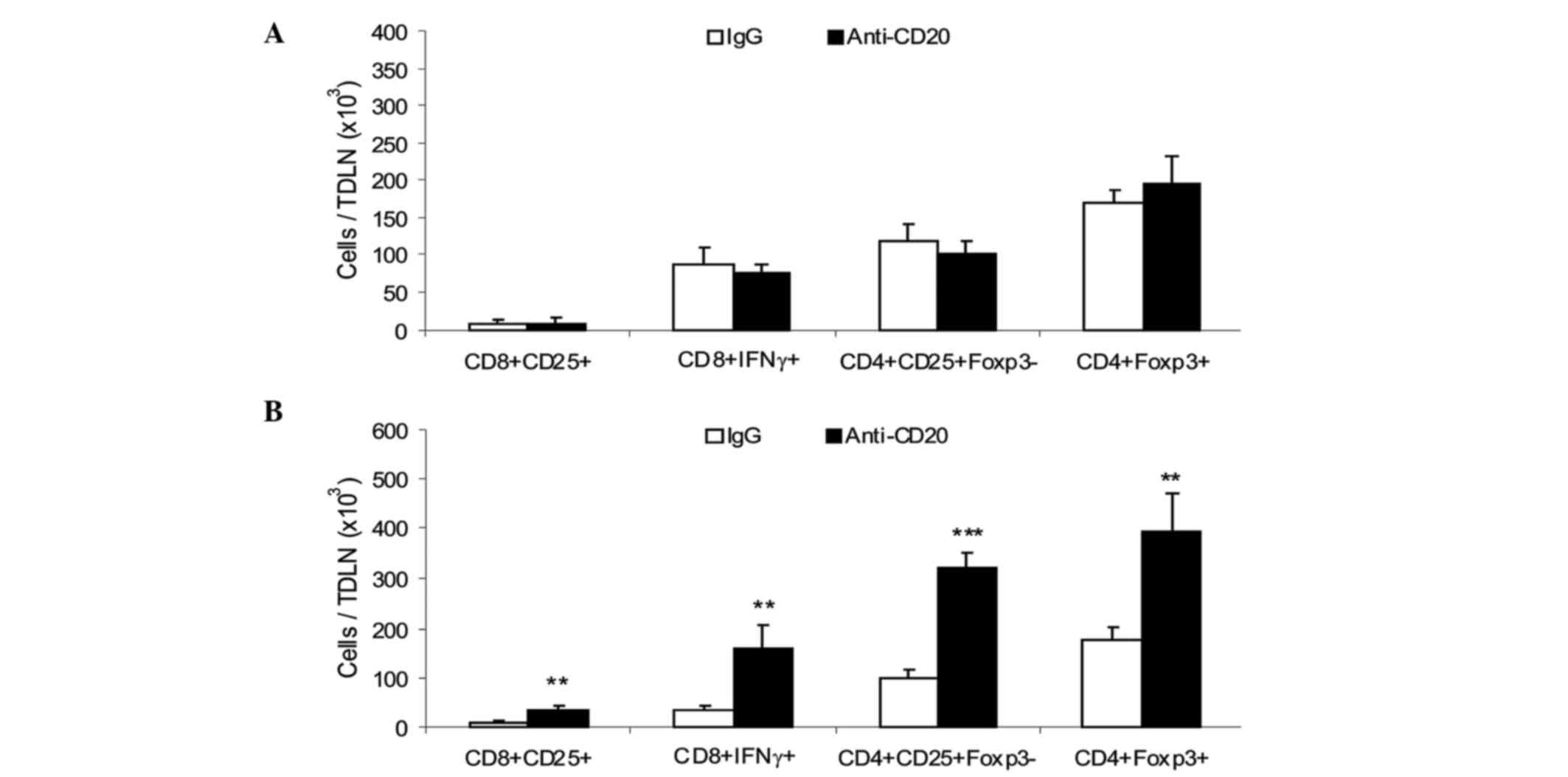
analyzed for cell composition. On day 5, a similar number of total
and activated CD4+ and CD8+ T cells and
CD4+Foxp3+ Tregs were observed in TDLN from
the two groups (Fig. 2A).
Furthermore, the groups were inoculated with a secondary tumor
implant to evaluate the CI, which evaluates the capacity of T cells
to reject a secondary implant (18).
The two groups showed the same proportion of tumor rejection (75%),
suggesting that B cell depletion did not prevent the early
activation stage that occurs in the TDLN following tumor implant.
TDLN from depleted and control mice were analyzed on day 20 p.i.,
when the tumor growth curves of the two groups were clearly
separated. An increased number of activated CD8+ and
CD4+ T cells and increased Tregs were observed in B
cell-depleted mice (Fig. 2B).
Systemic B cell depletion once the
tumor is established prevents the inhibition of an antitumor
response, increases activated T cells and decreases Tregs in TDLN
and tumor tissue
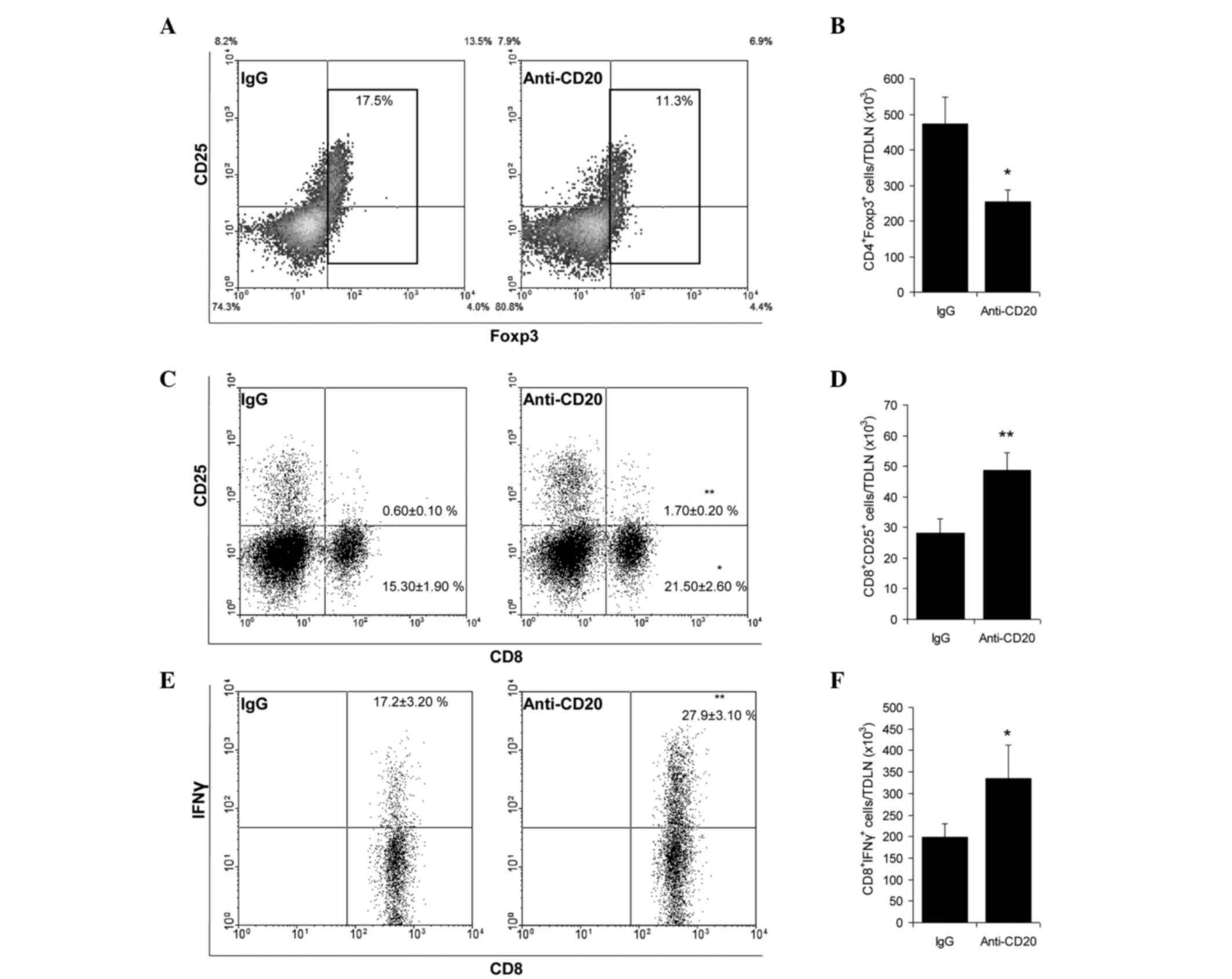
Mice were injected with anti-CD20 or IgG antibodies
9 days p.i. and TDLN were excised and analyzed 8 days later. While
B cell depletion did not affect the absolute number of
CD4+ and CD8+ T cells, an increase in the
number and proportion of activated CD25+- and
IFN-γ-expressing CD8+ T cells and a decrease in the
number and proportion of Tregs was induced (Fig. 3A-F). The subpopulation with putative
inhibitory function, B cells expressing IL-10, also decreased
(28,733±6,294 vs. 11,533±2,463; n=3; P=0.0116). At tumor tissue
level, B cell depletion induced decreased Treg number and increased
CD25+CD4+ activated T cell proportion among
CD4+ TILs (Fig. 4A). In
addition, the functional evaluation of CD3+ T cells
isolated from tumors of depleted mice exhibited an increased
cytotoxic capacity against MCC cells in culture (Fig. 4B). When CI was evaluated in B
cell-depleted mice bearing large tumors (>500 mm3),
an increased rate of tumor rejection (80%) was observed in depleted
mice, whereas no rejection was observed in the control IgG-treated
group (Chi-square test; P=0.00026). This result reinforces the
possibility that B cells may be interfering with T cell activity.
Overall, the results suggest that the absence of B cells could
prevent immune suppression, unveiling the antitumor response.
B cells impair T cell activity,
partially through IL-10 secretion
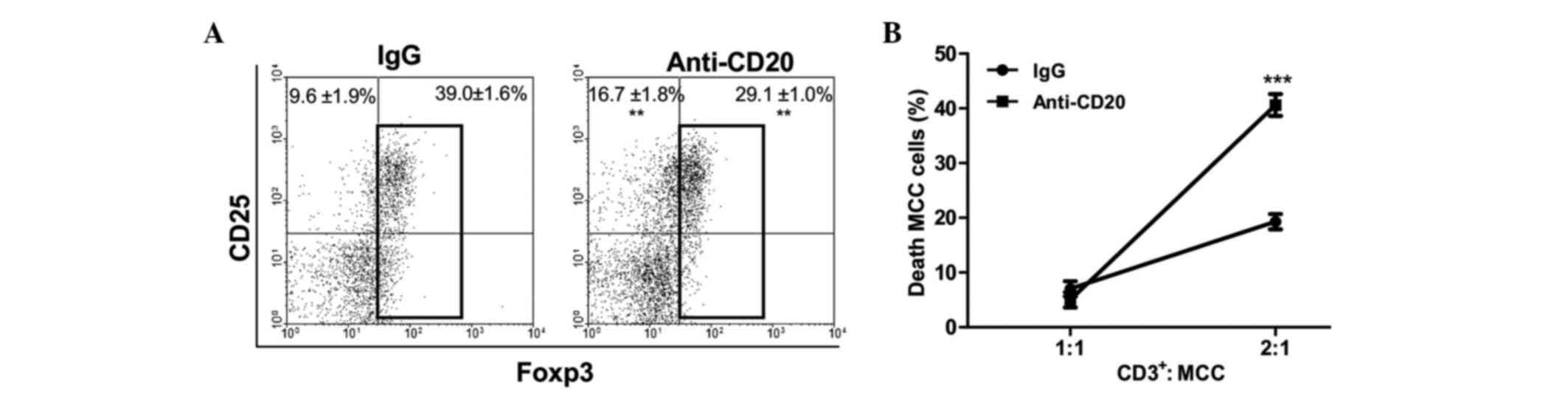
A possible inhibitory role of B cells on T cell
function was further analyzed in vitro. Allogeneic T cell
proliferation was assayed in the presence of B cells isolated from
control- or TBM-LN, either with or without allowing cellular
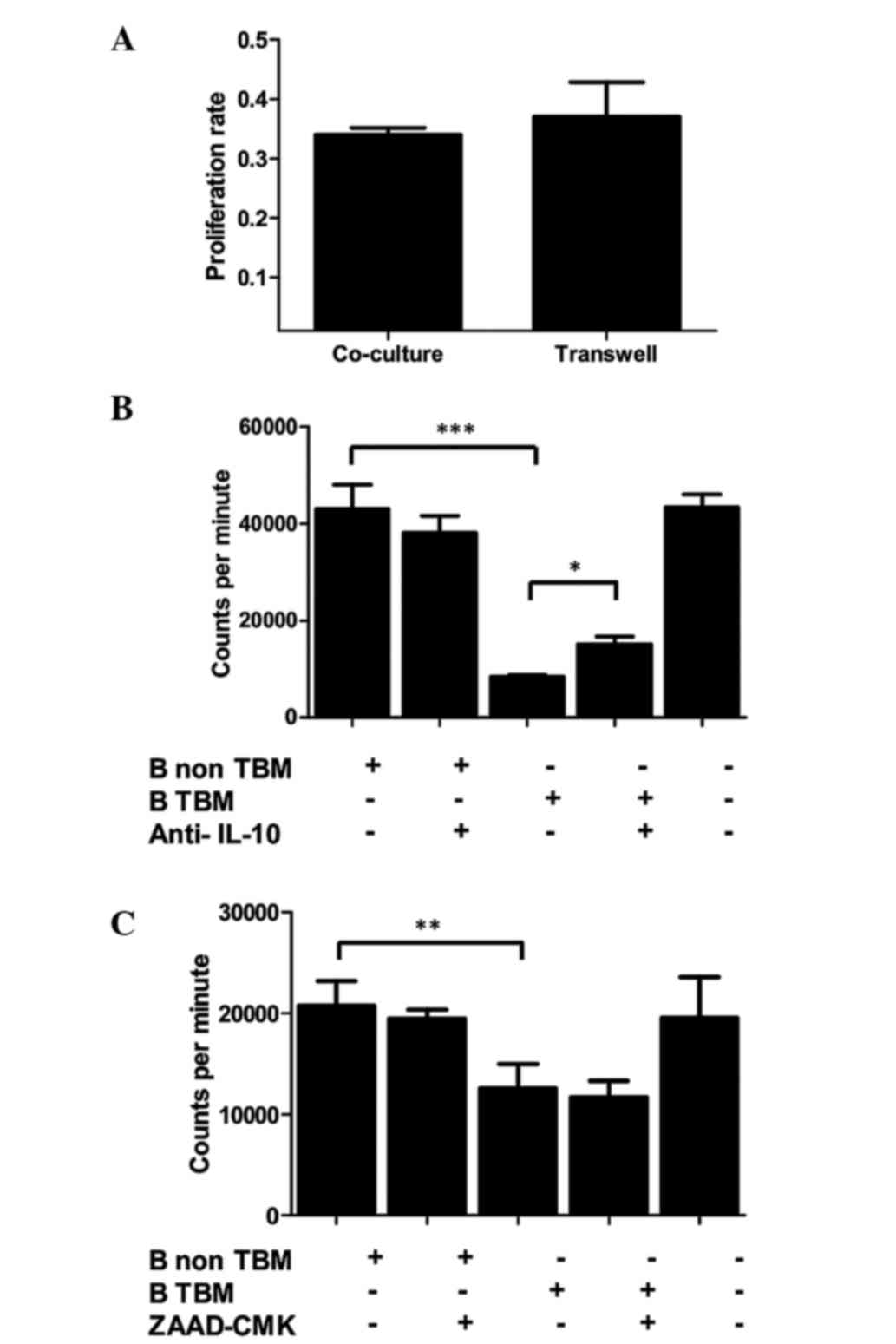
contact (Transwell assay). The results indicated that, unlike
normal B cells, TBM-derived B cells were able to inhibit lymphocyte
proliferation, and that this inhibition did not depend upon cell
contact (Fig. 5A). To analyze the
possible molecules involved in the inhibition, the same experiment
was performed in presence of an IL-10 blocking antibody or a
granzyme B inhibitor (ZAAD-CMK). The B cell-mediated inhibition of
T cell proliferation was observed to be partially prevented by the
blockade of IL-10 (Fig. 5B), while
the inhibition of granzyme B exerted no effect (Fig. 5C). Overall, these results indicate
that B cells from TBM have a direct inhibitory effect upon T
lymphocyte function that is, at least partially, mediated by
IL-10.
Discussion
Tumor presence generally leads to the appearance of
immunosuppressive cell populations that not only affect endogenous
antitumor response but weaken the efficacy of immunotherapies
(25,26). Although Tregs have been classically
assessed as one of the main mediators of tumor-induced
immunosuppression (3,4), recent papers described a subset of B
cells able to negatively regulate cellular immune responses, mainly
through the production of IL-10, in several pathologies including
cancer (27–30). The MCC is a highly immunogenic tumor
that provokes a strong antitumor immune reaction at the early
stages of development. At a certain volume (~400 mm3)
all systemic humoral and cellular antitumor evidences disappear,
and tumor immunogenicity decreases and disappears leading the
immune system to a state of tolerance (20). In the MCC model, the immune status of
the host is reflected at various levels. During the immunogenic
phase, subsequent secondary distant implants of the same tumor are
rejected in a T cell-dependent fashion through CI, whereas this
capacity is lost in the tolerogenic phase (18). Also, at the TDLN level, the cellular
composition of the MCC switches from an activated antitumor profile
to a predominantly immunosuppressive environment with immature
dendritic cells, Tregs and a marked increase in B cells and
IL-10-secreting B cells (20), the
latter suggesting B cell involvement in the loss of tumor immunity.
The present study aimed to elucidate the role of B cells in the
immune response against MCC and in tumor-induced tolerance, by
means of systemic B cell depletion. To get a close insight in B
cell-mediated temporal events, depletion was performed prior to
tumor inoculum or subsequent to tumor establishment, when the
immune status of the host had clearly changed (20). Notably, opposite effects on tumor
growth were obtained. B cell depletion prior to tumor inoculum
enhanced tumor growth, suggesting that B cells are initially
necessary for the onset of the antitumor response. On the contrary,
B cell depletion performed once the immune system had been affected
by the growing tumor resulted in decreased tumor growth, suggesting
that B cells are also involved in tumor-induced tolerance.
The supposition that B cell absence would impair the
initial antitumor response was analyzed through the TDLN cell
composition and the CI response. Contrarily to previous
expectations, the immune response within TDLN was unchanged in B
cell-depleted animals. At day 5 p.i., no differences in activated
and regulatory T cells were observed compared with non depleted
TBM. In addition, the two groups displayed comparable rates of CI.
Later, on day 20 p.i., when the growth curves in the two groups had
clearly separated, an increase in number activated CD8+
and CD4+ T cells, along with an increase in
CD4+Foxp3+ Tregs were observed in the TDLN of
B cell-depleted mice. Speculatively, in the absence of B cells,
tumor-induced mechanisms to evade the immune system may involve a
compensatory exacerbation of the Tregs population, which could
explain the separation of the tumor growth curves at that time.
When depletion was performed following the implant
of the tumor, TDLN from B cell-depleted animals exhibited an
increased number and proportion of IFN-γ-expressing CD8+
T cells and a decreased number and proportion of Tregs compared
with control animals. Also in the tumor tissue, depletion decreased
the proportion of Tregs, increased activated CD4+ T
cells and increased the cytotoxic capacity of CD3+ T
cells. It is feasible that B cell elimination would prevent or
delay the negative impact on the immune system and the induction of
regulatory cells induced by the growing tumor. In addition, a
remarkable 80% of T cell-mediated secondary tumor rejection (CI)
was obtained in B cell-depleted mice, whereas no tumor rejection
was evidenced in control mice. Overall, these findings suggested
that the absence of B cells would extend the immunogenic period,
probably by allowing proper T cell action.
A regulatory role for B cells in cancer immunity
that favors tumor progression has previously been assessed in other
tumor models. Qin et al (31)
demonstrated that low immunogenicity exhibited by a weakly
immunogenic breast tumor is associated with B cell presence, while
Tadmor et al (12)
demonstrated the inhibition of a murine breast tumor and decrease
in Tregs in B cell deficient mice. In addition, Shah et al
(16) determined that B cells may
inhibit an antitumor T cell-mediated response by antigen
nonspecific mechanisms, and Inoue et al (17) showed that robust antitumor
cytotoxicity can be developed only in B cell-knockout mice. While
these studies have been performed in B cell deficient mice, to the
best of our knowledge, only few studies have analyzed a role of B
cells in cancer in B cell-depleted animal models. DiLillo et
al (8) demonstrated that B cell
depletion induced tumor growth exacerbation when performed prior to
melanoma implantation; no effect was evidenced when depletion was
performed following tumor implantation. These results led the
authors to postulate that B cells were required for the onset of T
cell activation in the melanoma model, though a promoting action of
B cells on tumor growth was not observed (8). In another system Kim et al
(15) found that B cell depletion
following the establishment of the tumor not only retarded tumor
growth but augmented the immunotherapeutic efficacy of a vaccine
approach. The results of the present study partially agree with
these two studies (8,15). An exacerbation of tumor growth was
induced by B cell depletion prior to tumor implant; however, no
effects of B cell depletion were detected in two of the recognized
markers (activated cells within TDLN and CI) of an immune antitumor
reaction in MCC. By contrast, depletion following tumor
implantation impaired tumor growth, increased activated T cells and
decreased Tregs. The different time frames in which B cell
depletion is performed, the extent of depletion and even the
subsets of B cells in various situations may account for these
differences.
Multiple regulatory pathways were recently described
for B cells in other pathologies, and certain pathways, such as
FasL (32) and programmed cell death
1 ligand 2 (33), require
cell-to-cell contact to be effective, while others, including TGFβ
(34), granzyme B (11) and IL-10 (13,27,28,35,36),
are mediated by soluble molecules secreted by B cells. Accordingly,
a previous study reported that B cells expressing IL-10, granzyme B
and FasL are present during MCC growth (22). The present study demonstrates that B
cells from MCC bearing mice are able to inhibit T cell
proliferation in a contact-independent manner, with IL-10 being
partially involved in this inhibition. Our previous results, which
indicated a lack of TGFβ secretion by TDLN cells (20) and the results of the present study
which demonstrated no effect of Granzyme B in T cell proliferation
inhibition enable the involvement of these mediators to be ruled
out. Future studies are required in order to elucidate whether
another nontraditional soluble molecule could be implicated in the
inhibition of T cell proliferation induced by B cells.
In conclusion, the results of the present study
suggest that B cells from MCC bearing mice contribute to
tumor-induced immunosuppression and tolerance, favoring tumor
progression, by affecting T lymphocyte function, increasing Treg
number at TDLN and preventing T cell activation and cytotoxicity at
the tumor site. Thus, B cell absence creates an imbalance in tumor
immunity, which delays the establishment of tolerance and extends
the period during which the immune system is able to react against
the tumor. The present authors postulate that modulating B cells in
a determined time frame could improve immunotherapeutic approaches
against cancer.
Acknowledgments
The present study was supported by grants from the
National Institute of Cancer (grant no. 6, ministerial resolution
1006/2016, Argentina), the National Scientific and Technical
Research Council (grant no. 11220120100628, Argentina), the
National Agency of Scientific Promotion and Technology (grant no
2014-1590, Argentina) and the Alberto J Roemmers Foundation,
Argentina. The authors are grateful to Dr Christiane Dosne
Pasqualini (National Academy of Medicine, Buenos Aires, Argentina)
for a critical discussion of this article.
Glossary
Abbreviations
Abbreviations:
|
MCC
|
methylcolanthrene-induced murine
fibrosarcoma
|
|
Tregs
|
T regulatory cells
|
|
CI
|
concomitant immunity
|
|
LN
|
lymph node
|
|
TDLN
|
tumor-draining lymph node
|
|
TBM
|
tumor bearing mice
|
|
p.i
|
post tumor inoculation
|
References
|
1
|
Cavallo F, De Giovanni C, Nanni P, Forni G
and Lollini PL: 201: The immune hallmarks of cancer. Cancer Immunol
Immunother. 60:319–326. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rabinovich GA, Gabrilovich D and Sotomayor
EM: Immunosuppressive strategies that are mediated by tumor cells.
Annu Rev Immunol. 25:267–296. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Petrausch U, Poehlein CH, Jensen SM,
Twitty C, Thompson JA, Assmann I, Puri S, LaCelle MG, Moudgil T,
Maston L, et al: Cancer immunotherapy: The role regulatory T cells
play and what can be done to overcome their inhibitory effects.
Curr Mol Med. 9:673–682. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rech AJ, Mick R, Martin S, Recio A, Aqui
NA, Powell DJ Jr, Colligon TA, Trosko JA, Leinbach LI, Pletcher CH,
et al: CD25 blockade depletes and selectively reprograms regulatory
T cells in concert with immunotherapy in cancer patients. Sci
Transl Med. 4:134ra622012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
LeBien TW and Tedder TF: B lymphocytes:
How they develop and function. Blood. 112:1570–1580. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Crawford A, Macleod M, Schumacher T,
Corlett L and Gray D: Primary T cell expansion and differentiation
in vivo requires antigen presentation by B cells. J Immunol.
176:349–3506. 2006. View Article : Google Scholar
|
|
7
|
Coughlin CM, Vance BA, Grupp SA and
Vonderheide RH: RNA-transfected CD40-activated B cells induce
functional T-cell responses against viral and tumor antigen
targets: Implications for pediatric immunotherapy. Blood.
103:2046–2054. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
DiLillo DJ, Yanaba K and Tedder TF: B
cells are required for optimal CD4+ and CD8+ T cell tumor immunity:
Therapeutic B cell depletion enhances B16 melanoma growth in mice.
J Immunol. 184:4006–4016. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fillatreau S, Gray D and Anderton SM: Not
always the bad guys: B cells as regulators of autoimmune pathology.
Nat Rev Immunol. 8:391–397. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bouaziz JD, Yanaba K and Tedder TF:
Regulatory B cells as inhibitors of immune responses and
inflammation. Immunol Rev. 224:201–214. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lindner S, Dahlke K, Sontheimer K, Hagn M,
Kaltenmeier C, Barth TF, Beyer T, Reister F, Fabricius D, Lotfi R,
et al: Interleukin 21-induced granzyme B-expressing B cells
infiltrate tumors and regulate T cells. Cancer Res. 73:2468–2479.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tadmor T, Zhang Y, Cho HM, Podack ER and
Rosenblatt JD: The absence of B lymphocytes reduces the number and
function of T-regulatory cells and enhances the antitumor response
in a murine tumor model. Cancer Immunol Immunother. 60:609–619.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Olkhanud PB, Damdinsuren B, Bodogai M,
Gress RE, Sen R, Wejksza K, Malchinkhuu E, Wersto RP and Biragyn A:
Tumor-evoked regulatory B cells promote breast cancer metastasis by
converting resting CD4+ T cells to T-regulatory cells. Cancer Res.
71:3505–3515. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang Y, Eliav Y, Shin SU, Schreiber TH,
Podack ER, Tadmor T and Rosenblatt JD: B lymphocyte inhibition of
antitumor response depends on expansion of Treg but is independent
of B-cell IL-10 secretion. Cancer Immunol Immunother. 62:87–99.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kim S, Fridlender ZG, Dunn R, Kehry MR,
Kapoor V, Blouin A, Kaiser LR and Albelda SM: B-cell depletion
using an anti-CD20 antibody augments antitumor immune responses and
immunotherapy in nonhematopoetic murine tumor models. J Immunother.
31:446–457. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shah S, Divekar AA, Hilchey SP, Cho HM,
Newman CL, Shin SU, Nechustan H, Challita-Eid PM, Segal BM, Yi KH,
et al: Increased rejection of primary tumors in mice lacking B
cells: inhibition of antitumor CTL and TH1 cytokine responses by B
cells. Int J Cancer. 117:574–586. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Inoue S, Leitner WW, Golding B and Scott
D: Inhibitory effects of B cells on antitumor immunity. Cancer Res.
66:7741–7747. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Franco M, Bustuoabad OD, Di Gianni PD,
Goldman A, Pasqualini CD and Ruggiero R: A serum- mediated
mechanism for concomitant resistance shared by immunogenic and
non-immunogenic murine tumors. Br J Cancer. 74:178–186. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bustuoabad OD, Ruggiero RA, Di Gianni PD,
Lombardi G, Beli C, Camerano GV, Dran GI, Schere-Levy C, Costa H,
Isturiz MA, et al: Tumor transition zone: A new putative
morphological and functional hallmark of tumor aggressiveness.
Oncol Res. 15:169–182. 2005.PubMed/NCBI
|
|
20
|
Maglioco A, Machuca D, Mundiñano J,
Cabrera G, Camicia G, Bruzzo J, Camerano G, Costa H, Ruggiero RA
and Dran GI: Lymphadenectomy exacerbates tumor growth while
lymphadenectomy plus the adoptive transfer of autologous cytotoxic
cells and low-dose cyclophosphamide induces regression of an
established murine fibrosarcoma. Cancer Immunol Immunother.
60:389–399. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chiarella P, Vulcano M, Bruzzo J,
Vermeulen M, Vanzulli S, Maglioco A, Camerano GV, Palacios V,
Fernández G, Brando RF, et al: Anti-inflammatory pretreatment
enables an efficient dendritic cell-based immunotherapy against
established tumors. Cancer Immunol Immunother. 57:701–718. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Maglioco A, Machuca D, Camerano G, Costa
H, Ruggiero R and Dran GI: Regulatory B cells presence in lymph
nodes draining a murine tumor. Medicina (B Aires). 74:185–188.
2014.PubMed/NCBI
|
|
23
|
Rosenberg SA, Spiess P and Lafreniere R: A
new approach to the adoptive immunotherapy of cancer with
tumor-infiltrating lymphocytes. Science. 233:1318–1321. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Matzinger P: The JAM test. A simple assay
for DNA fragmentation and cell death. J Immunol Methods.
145:185–192. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zou W: Regulatory T cells, tumour immunity
and imunotherapy. Nat Rev Immunol. 6:295–307. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Whiteside T: Inhibiting the inhibitors:
Evaluating agents targeting cancer immunosuppression. Expert Opin
Biol Ther. 10:1019–1035. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mauri C, Gray D, Mushtaq N and Londei M:
Prevention of arthritis by interleukin 10-producing B cells. J Exp
Med. 197:489–501. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Watanabe R, Ishiura N, Nakashima H, Kuwano
Y, Okochi H, Tamaki K, Sato S, Tedder TF and Fujimoto M: Regulatory
B cells (B10 cells) have a suppressive role in murine lupus: CD19
and B10 cell deficiency exacerbates systemic autoimmunity. J
Immunol. 184:4801–4809. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Matsushita T, Yanaba K, Bouaziz JD,
Fujimoto M and Tedder TF: Regulatory B cells inhibit EAE initiation
in mice while other B cells promote disease progression. J Clin
Invest. 118:3420–3430. 2008.PubMed/NCBI
|
|
30
|
DiLillo DJ, Matsushita T and Tedder TF:
B10 cells and regulatory B cells balance immune responses during
inflammation, autoimmunity and cancer. Ann N Y Acad Sci.
1183:38–57. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Qin Z, Richter G, Schüler T, Ibe S, Cao X
and Blankenstein T: B cells inhibit induction of T cell-dependent
tumor immunity. Nat Med. 4:627–630. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lundy S: Killer B lymphocytes: The
evidence and the potential. Inflamm Res. 58:345–357. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhong X, Tumang JR, Gao W, Bai C and
Rothstein TL: PD-L2 expression extends beyond dendritic
cells/macrophages to B1 cells enriched for V(H)11/V(H)12 and
phosphatidylcholine binding. Eur J Immunol. 37:2405–2410. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Parekh VV, Prasad DV, Banerjee PP, Joshi
BN, Kumar A and Mishra GC: B cells activated by lipopolysaccharide,
but not by anti-Ig and anti-CD40 antibody, induce anergy in CD8+ T
cells: Role of TGF-beta 1. J Immunol. 170:5897–5911. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Moore KW, De Waal Malefyt R, Coffman RL
and O'Garra A: Interleukin-10 and the interleukin-10 receptor. Annu
Rev Immunol. 19:683–765. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zheng SG, Wang JH, Gray JD, Soucier H and
Horwitz DA: Natural and induced CD4+CD25+ cells educate CD4+CD25-
cells to develop suppressive activity: The role of IL-2, TGF-beta,
and IL-10. J Immunol. 172:5213–5221. 2004. View Article : Google Scholar : PubMed/NCBI
|