Introduction
Liver cancer is one of the most common human
malignancies. A number of signaling pathways, including the
transforming growth factor β (TGF-β)/mothers against
decapentaplegic homolog (1),
proto-oncogene Wnt/β-catenin (2), rat
sarcoma GTPase/mitogen-activated protein kinase (MAPK) (3), phosphoinositide 3-kinase (PI3K)/protein
kinase B (Akt) (4), c-Jun N-terminal
kinase (JNK)/signal transducer and activator of transcription
(STAT) (5,6), hedgehog and tumor protein 53
transduction pathways, serve key roles in the pathogenesis of liver
cancer. Among these signaling pathways, the TGF-β signaling pathway
is one of the most important (1).
TGF-β1 is as a member of the TGF-β family able to
produce tumor-inhibiting and promoting effects (7–9). TGF-β1
has been associated with immunosuppression, tumor angiogenesis,
tumor cell migration, proliferation, differentiation, development,
apoptosis and invasion, as well as other processes, in numerous
types of cancer (10,11). For example, TGF-β1 expression is
increased in liver cancer (12),
intrahepatic cholangiocarcinoma (13), prostate cancer (14,15) and in
head and neck squamous cell carcinoma (16), and leads to increased tumor growth.
Conversely, TGF-β1 expression levels in patients with leukemia are
significantly decreased compared with healthy subjects (17).
Cell growth, the cell cycle and apoptosis are
closely associated with the genesis and development of liver
cancer, and multiple factors are involved in their regulation,
including proliferating cell nuclear antigen (PCNA), gankyrin,
general vesicular transport factor p115 (p115), X-linked inhibitor
of apoptosis protein (XIAP), survivin and caspase-3 (18–21). PCNA
is a highly conserved protein; in addition to DNA replication, the
functions of PCNA are associated with other vital cellular
processes, including chromatin remodeling, DNA repair,
sister-chromatid cohesion and cell cycle control (22). Recent studies have reported that tumor
cells express increased levels of PCNA, identifying it as a
potential target for cancer therapy (23,24).
Gankyrin is a chaperone of the ubiquitin-proteasome and a novel
oncogene, and has been demonstrated to be overexpressed in numerous
types of cancer (25–27), including liver cancer (28–30),
breast cancer (31,32), colorectal cancer (33), estrogen-driven endometrial carcinoma
(26) and oral cancer (34). Gankyrin serves an essential role in
tumor occurrence and development (25–34). p115
is a tether protein that has an important role in a number of
signaling pathways required for cell proliferation, and has been
extensively studied (35,36). A previous study demonstrated that p115
is a potential tumor biomarker and therapeutic target that is
overexpressed in human gastric cancer cells (36). The inhibitor of apoptosis protein
(IAP) family comprises internal apoptosis suppressors, and its
members are able to bind to caspase and inhibit cell apoptosis.
XIAP, an important member of the IAP family, possesses inhibitory
activity and serves an important role in cell apoptosis (19,20).
Survivin, a novel member of the IAP family with the lowest relative
molecular weight, is the most potent suppressor of apoptosis that
has been identified so far (21).
However, there are currently few studies concerning the association
between TGF-β1 and PCNA, gankyrin, p115, XIAP and survivin in liver
cancer.
Following previous observations, TGF-β1 expression
was knocked down in the present study using small interfering RNA
(siRNA). Subsequently, cell growth, cell cycle distribution and
apoptosis, as well as PCNA, gankyrin, p115, XIAP and survivin
protein expression, was observed in HepG2 liver cancer cells. The
present study aimed to further elucidate the association between
TGF-β1 and the aforementioned factors, and the role of TGF-β1
during the genesis and development of liver cancer.
Materials and methods
Cell culture
HepG2 liver cancer cells were obtained from the Type
Culture Collection of the Chinese Academy of Sciences (Shanghai,
China) and plated in culture flasks. Cells were cultured in
Dulbecco's modified Eagle's medium (DMEM; HyClone; GE Healthcare
Life Sciences, Logan, UT, USA) supplemented with 10% v/v fresh
fetal calf serum (FCS; TBD Biotechnology Corporation, Tianjin,
China), at 37°C in a humidified atmosphere containing 5%
CO2. To maintain the cell line, cells were replated
following digestion for 1 min in 0.25% trypsin when they reached
confluency.
Transient transfection
siRNA directed against TGF-β1 was designed and
synthesized by Shanghai GenePharma Co., Ltd. (Shanghai, China). The
TGF-β1 siRNA sequences were as follows: Sense, 5′-GAC ACC AAC UAU
UGC UUC ATT-3′ and antisense, 5′-UGA AGC AAU AGU UGG UGU CTT-3′.
The scrambled negative control siRNA sequences were as follows:
Sense 5′-UUC UCC GAA CGU GUC ACG UTT-3′ and antisense, 5′-ACG UGA
CAC GUU CGG AGA ATT-3′.
A total of 3×105 cells were plated in
6-well plates in triplicate and grown to 30–50% confluency. For the
transfection, 10 µl X-tremeGENE siRNA Transfection Reagent (Roche
Diagnostics, Basel, Switzerland) and 2 µg siRNA were mixed in 200
µl DMEM for 15–20 min. The 210-µl mixture was added to the cells
alongside 2 ml DMEM supplemented with 10% v/v FCS, and the plates
were subsequently agitated. The cells were cultured at 37°C in a
humidified atmosphere containing 5% CO2. Cells were
harvested at 72 and 96 h, and the proteins were isolated for
further analysis. In addition, a negative control was created using
the control siRNA and subsequently analyzed. Untreated cells were
also analyzed. All experiments were performed in triplicate.
Detection of TGF-β1 protein expression
using western blotting
Transfected cells were lysed in
radioimmunoprecipitation assay buffer (Beyotime Institute of
Biotechnology, Haimen, China) supplemented with
phenylmethylsulfonyl fluoride (1:200) and phosphatase inhibitors
(1:200) on ice for 15 min, at 72 and 96 h following transfection,
prior to protein isolation. The total protein concentration was
determined using a bicinchoninic acid kit (Beyotime Institute of
Biotechnology). Protein samples (60 µg) were separated using 12%
SDS-PAGE and then transferred onto polyvinylidene difluoride
membranes (EMD Millipore, Billerica, MA, USA). The membranes were
blocked by incubation in PBS with 5% skimmed milk at room
temperature for 1 h. The membranes were subsequently incubated with
primary antibodies against TGF-β1 (1:200; cat. no. sc-146; Santa
Cruz Biotechnology, Inc., Dallas, TX, USA) and β-actin (1:200; cat.
no. sc-47778; Santa Cruz Biotechnology, Inc.) at 4°C overnight. The
membranes were washed three times using PBS, followed by incubation
with a horseradish peroxidase-conjugated secondary antibody
(1:5,000; cat. no. sc-2005; Santa Cruz Biotechnology, Inc.) at 37°C
for 1 h. The membranes were washed three times using PBS and
visualized using a BeyoECL Plus kit (cat. no. BYT-P0018; Beyotime
Institute of Biotechnology). Images were captured using a
fluorescence imager (Champgel 5500; Beijing Sage Creation Science
and Technology Ltd., Beijing, China) and analyzed using Quantity
One® software (version 4.5.2; Bio-Rad Laboratories,
Inc., Hercules, CA, USA). The quantitative results of grayscale
analysis were used for the statistical analysis.
Detection of cell growth using the
Cell Counting Kit-8 (CCK-8) assay
The HepG2 groups analyzed were as follows: i) the
control group; ii) the negative siRNA control group; iii) the
TGF-β1 siRNA transfected group. The following doses of exogenous
TGF-β1 were added to HepG2 cells (the exogenous TGF-β1 group), 0,
5, 10, 20, 30, 40 and 50 µg/l.
A total of 1.5×104 transfected cells were
plated in 96-well plates in triplicate and grown to 30–50%
confluency, prior to transfection. A total of 0.8 µl X-tremeGENE
siRNA Transfection Reagent and 0.15 µg siRNA were mixed in 30 µl
DMEM for 15–20 min. The 30 µl mixture, or increasing doses of
exogenous TGF-β1, were administered to the cells, in addition to
150 µl DMEM supplemented with 10% v/v FCS, and the plates were
subsequently agitated. Cells were cultured at 37°C in a humidified
atmosphere containing 5% CO2. A total of 20 µl CCK-8
reagent (Beyotime Institute of Biotechnology) was added to the cell
medium 24, 48, 72 and 96 h following transfection or treatment with
exogenous TGF-β1. Following a 2-h incubation at 37°C, the
absorbance was measured using a microplate reader (MK3; Thermo
Labsystems, Inc., Beverly, MA, USA) at 450 nm in order to determine
the number of viable cells. The control and negative control groups
were also analyzed. All experiments were performed in triplicate.
The data were normalized to their respective controls. The cell
growth inhibition rates were calculated as follows: (the absorbance
ratio of the control group-the absorbance ratio of the transfection
group)/the absorbance ratio of the control group ×100. Following
analysis, the optimum duration and dose of treatment was used in
subsequent experiments.
Detection of cell cycle distribution
using flow cytometry
The following groups were assessed: i) the control
group; ii) the 24 h exogenous TGF-β1 group; iii) the 48 h exogenous
TGF-β1 group; iv) the 72 h exogenous TGF-β1 group; v) the 72 h
TGF-β1-knockdown group; vi) the 96 h TGF-β1-knockdown group. Cells
in the exogenous TGF-β1 groups were treated with 25 µg/l
TGF-β1.
At the respective time points the culture medium was
removed, and the cells were washed in PBS, trypsinized, harvested,
washed in PBS, centrifuged three times at 4°C and 1,000 × g
for 5 min, added to 2 ml ice-cold 70% ethanol and preserved at 4°C.
The cells were washed three times, and RNases and proteins were
removed using a Cell Cycle Detection kit (BD Biosciences, Franklin
Lakes, NJ, USA). The cells were subsequently incubated in 10 g/ml
propidium iodide (PI) at 4°C for 10 min in the dark and the cell
cycle distribution was analyzed using a FACScan flow cytometer
(Sysmex Partec GmbH, Görlitz, Germany) and CyViewTM software
version 6.0 (Sysmex Partec GmbH) within 2 h. All experiments were
performed in triplicate.
Detection of apoptosis using flow
cytometry
The cell groups described in the cell cycle
distribution section were used. Cell apoptosis was detected using
an Annexin V-fluorescein isothiocyanate (FITC)/PI Apoptosis
Detection kit (BD Biosciences). At the respective time points, the
cells were collected, centrifuged three times at 4°C and 1,000 × g
for 5 min, and resuspended in 500 µl 1X binding buffer. The cell
density was adjusted to 1×106 cells/ml. A total of 100
µl cells were incubated with 5 µl Annexin V-FITC and 5 µl PI for 15
min in the dark at room temperature. Cell apoptosis was evaluated
using a FACScan flow cytometer. For each determination, a minimum
of 50,000 cells was analyzed. All experiments were performed in
triplicate.
Viable cells stained negative for PI and annexin
V-FITC, early apoptotic cells stained positive for annexin V-FITC
and negative for PI, and late apoptotic cells stained positive for
annexin V-FITC and PI. Nonviable cells, which underwent necrosis,
stained positive for PI but negative for annexin V-FITC.
Evaluation of PCNA, gankyrin, p115,
XIAP and survivin expression using western blotting
The cell groups described in the cell cycle
distribution section were used. At the respective time points,
total protein was extracted. The protein levels were evaluated
using western blotting, following the aforementioned protocol used
for the TGF-β1 protein. The following primary antibodies were used
at 4°C overnight: Gankyrin (1:500; cat. no. GTX48519; GeneTex,
Inc., Irvine, CA, USA), p115 (1:1,000; cat. no. GTX115115; GeneTex,
Inc.), PCNA, XIAP (each 1:500; cat. no. BS1289 and BS1609,
respectively; Bioworld Technology, Inc., St. Louis Park, MN, USA),
and survivin (1:200; cat. no. sc-10811; Santa Cruz Biotechnology,
Inc.). The horseradish peroxidase-conjugated secondary antibody was
obtained from Santa Cruz Biotechnology, Inc. (1:5,000; cat. no.
sc-2004) and incubated with the membrane for 1 h at 37°C.
Statistical analysis
Values are expressed as the mean ± standard
deviation of triplicate data, and were compared using the Student's
t-test and a one-way analysis of variance. Statistical analyses
were conducted using GraphPad Prism (version 6; GraphPad Software,
Inc., La Jolla, CA, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
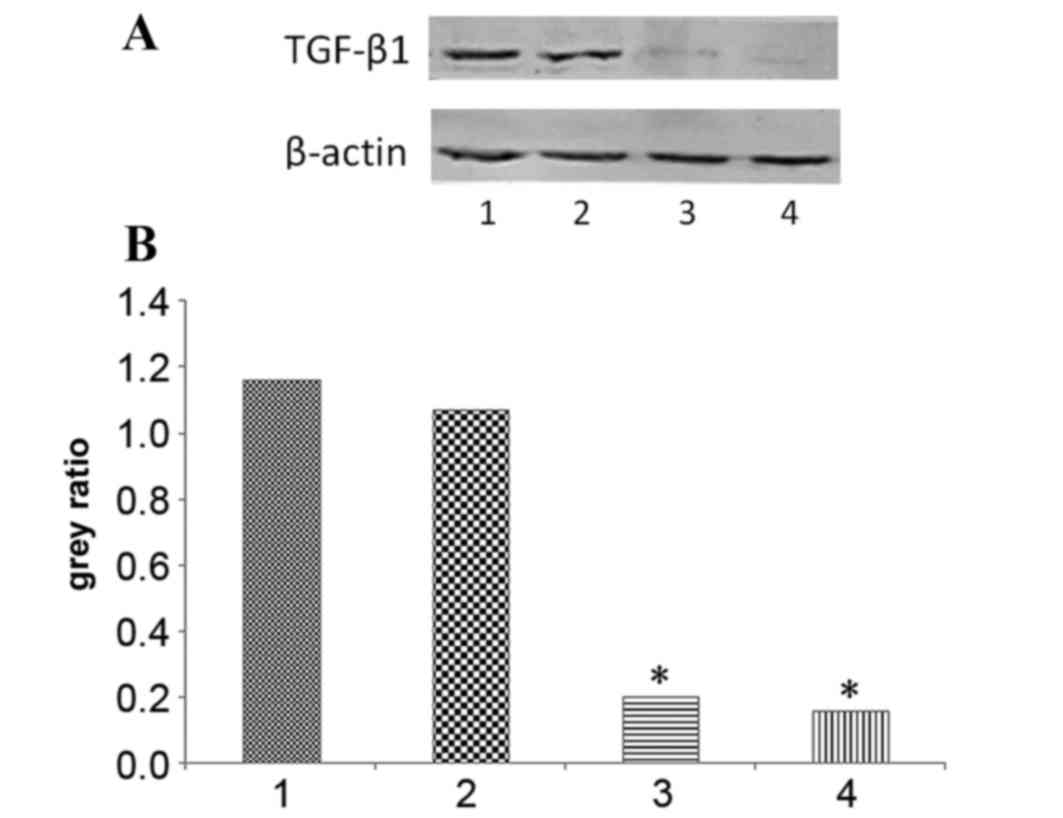
TGF-β1 protein expression
Following siRNA transfection, the TGF-β1 protein
expression levels at 72 and 96 h were evaluated using western
blotting, and were observed to be significantly decreased (P=0.0016
and P=0.0055, respectively; Fig. 1).
No significant difference was observed in the TGF-β1 protein levels
of the negative siRNA control group, as compared with the control
group. These results indicate that the siRNA against TGF-β1 was
effective.
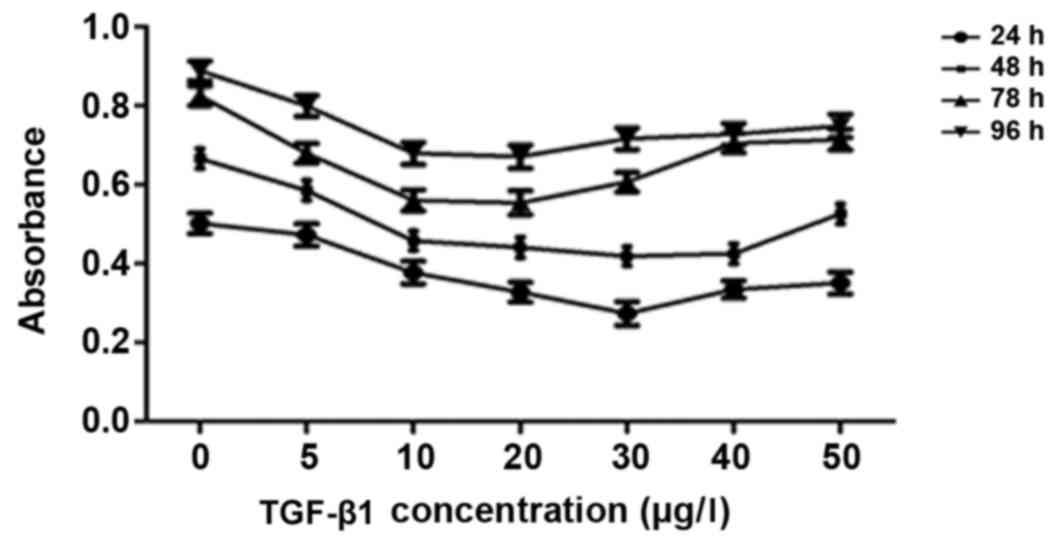
Effect of TGF-β1 on HepG2 cell
growth
Treatment with exogenous TGF-β1 inhibits HepG2
cell growth
The results of the CCK-8 assays are presented in
Fig. 2. Treatment with 0–20 µg/l
TGF-β1 inhibited HepG2 cell growth in a dose-dependent manner;
however, at doses >30 µg/l, the inhibitory effect of treatment
with TGF-β1 on HepG2 cell growth was decreased. Therefore, the
optimum dose of TGF-β1 to inhibit HepG2 cell growth was between 20
and 30 µg/l (Fig. 2). Therefore, 25
µg/l TGF-β1 was used in subsequent experiments.
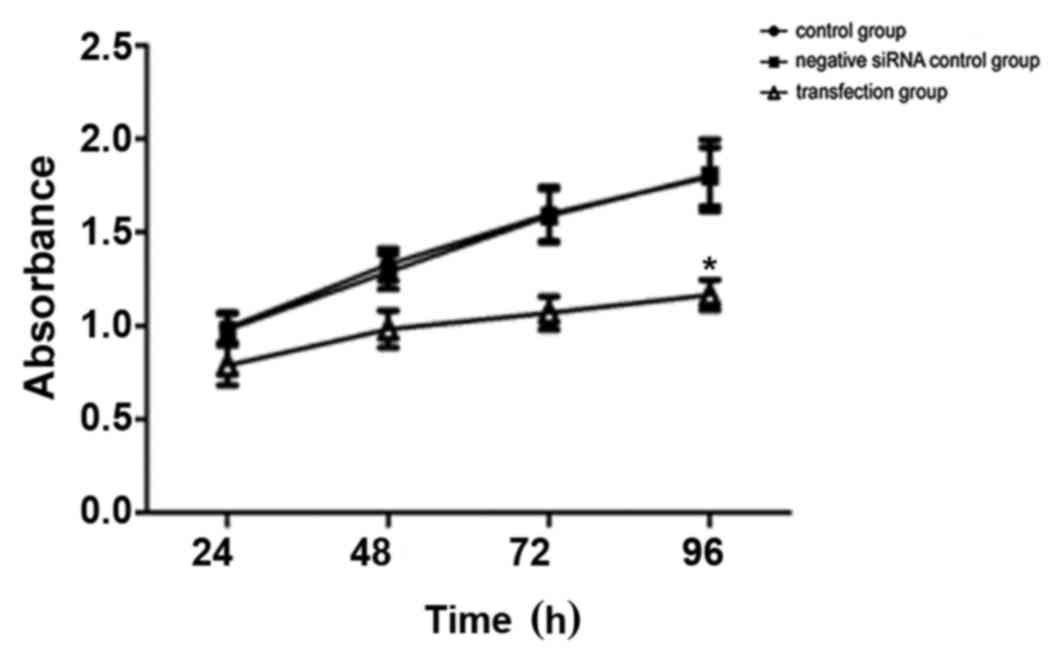
The effect of silencing TGF-β1 on HepG2 cell
growth
The results of the CCK-8 assays are presented in
Fig. 3. No significant difference in
cell viability was observed in the control group compared with the
negative siRNA control group, whereas a significant decrease in the
number of viable cells was observed in the TGF-β1 siRNA-transfected
group (P=0.042; Fig. 3). The cell
growth inhibition rates induced by the TGF-β1 siRNA were 12.9% at
24 h, 21.0% at 48 h, 34.3% at 72 h and 35.0% at 96 h. These results
indicated that HepG2 cell growth was inhibited, or that cell death
was increased, between 24 and 96 h following transfection, and that
the optimum inhibition times were 72 and 96 h (Fig. 3). These results suggest that
short-term inhibition of cell growth or promotion of cell death
occurs following transient siRNA transfection.
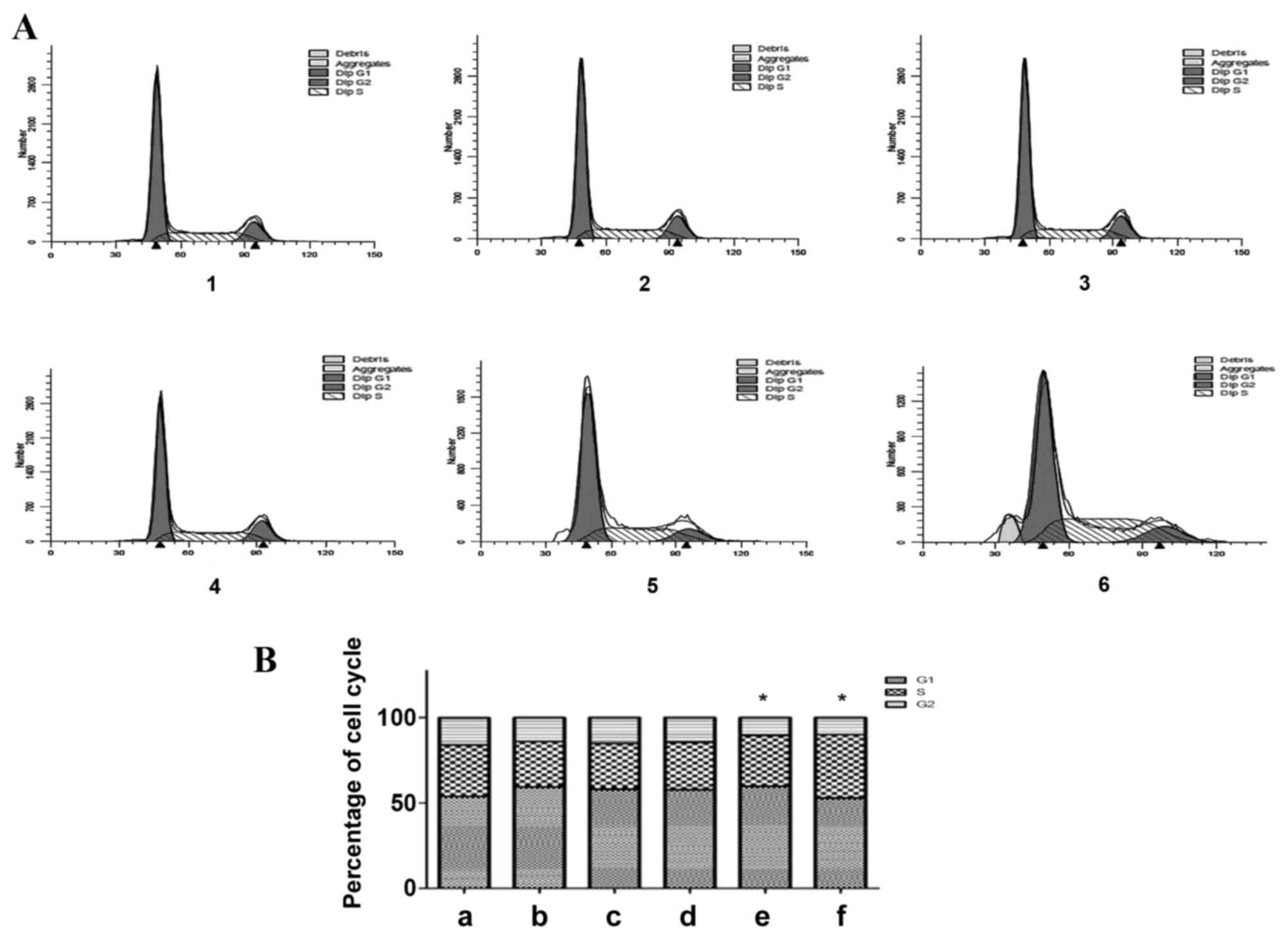
Effect of altered TGF-β1 expression on HepG2 cell
cycle distribution
The flow cytometric results demonstrated that the
percentage of G1-phase cells increased and the
percentage of S-phase and G2-phase cells decreased, 24,
48 and 72 h following treatment with exogenous TGF-β1, as compared
with the control group (Fig. 4). A
total of 72 h following TGF-β1 knockdown, the percentage of
G1-phase cells increased, the percentage of S-phase
cells exhibited no significant alteration and the percentage of
G2-phase cells decreased, as compared with the control
group (P=0.0425). A total of 96 h following TGF-β1 knockdown, the
percentage of G1-phase cells decreased, the percentage
of S-phase cells increased and the percentage of
G2-phase cells decreased, as compared with the control
group (P=0.0326). In the exogenous TGF-β1 group, cells were
arrested in the G1 phase, and the number of cells in the
S and G2 phases decreased. In addition, cells in the
TGF-β1-knockdown group were also arrested in the G1
phase, and the number of cells in the S phase remained unchanged
and decreased in the G2 phase 72 h following
transfection. By contrast, 96 h following knockdown, the cells were
arrested in the S phase, the number of cells in the G2
phase decreased and the apoptosis peak was visible. The effect of
TGF-β1 knockdown on cell cycle distribution was considered to be
statistically significant. These results indicate that TGF-β1
knockdown inhibits cell cycle progression, therefore inhibiting
cell growth.
Effect of altered TGF-β1 expression on HepG2 cell
apoptosis
The flow cytometric results demonstrated that in the
exogenous TGF-β1 group, the percentage of early apoptotic cells
increased compared with the control group, particularly at 48 h
(Fig. 5). However, the percentage of
late apoptotic cells did not change significantly following
treatment with exogenous TGF-β1 compared with the control group
(Fig. 5). In the TGF-β1-knockdown
group, early apoptosis significantly increased at 72 h, and early
and late apoptosis significantly increased at 96 h, as compared
with the control group (P=0.0461 and P=0.0433, respectively;
Fig. 5). The results indicate that
TGF-β1 knockdown increases cell apoptosis, therefore inhibiting
cell growth.
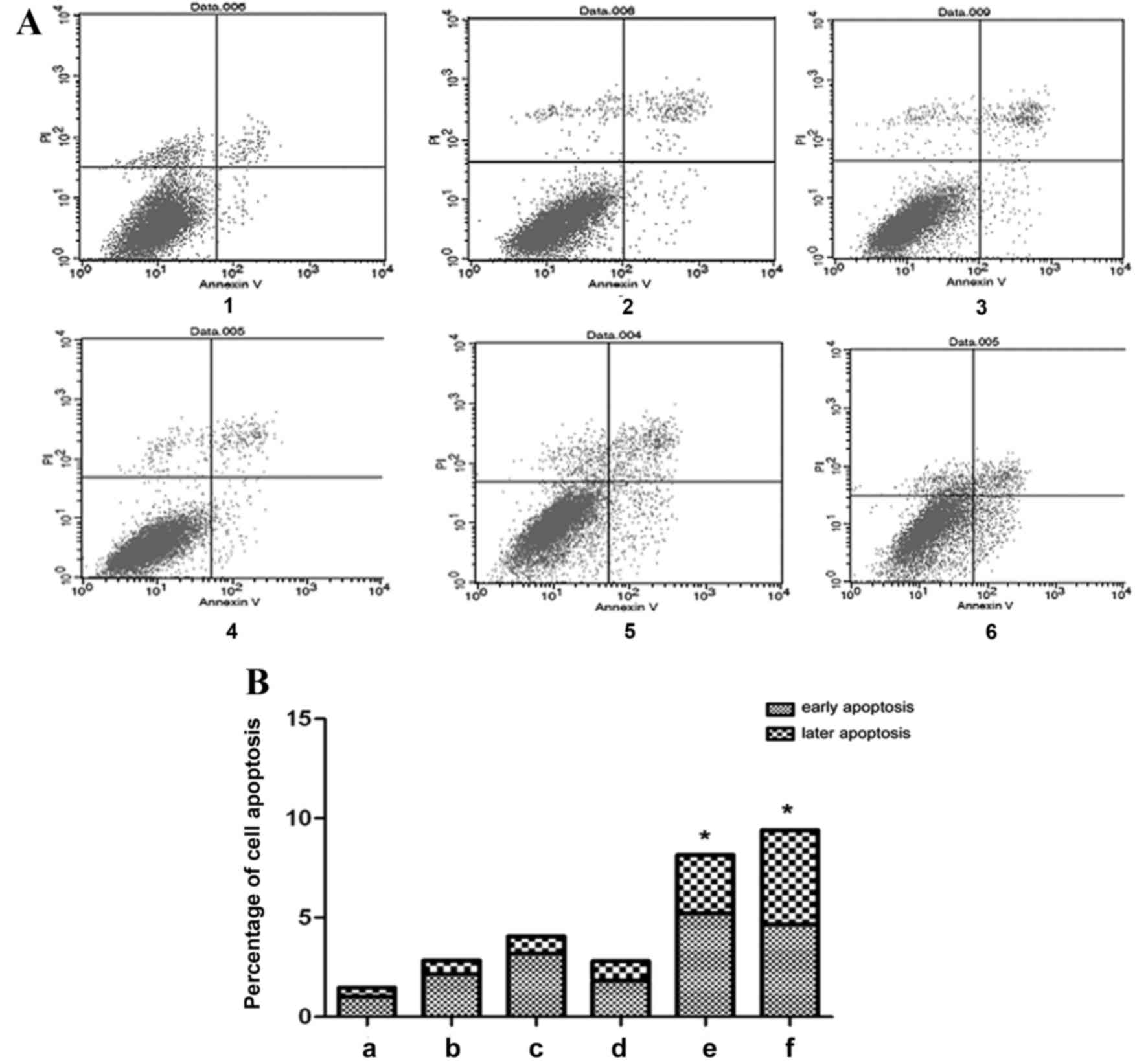 | Figure 5.Effect of altered TGF-β1 on HepG2
cell apoptosis. (A) Flow cytometric analysis of HepG2 cell
apoptosis and (B) quantification of the flow cytometric data. 1,
control group; 2, 24 h exogenous TGF-β1 group; 3, 48 h exogenous
TGF-β1 group; 4, 72 h exogenous TGF-β1 group; 5, 72 h
TGF-β1-knockdown group; 6, 96 h TGF-β1-knockdown group. In the
scatter diagram, the upper left quadrant represents mechanically
damaged cells, the upper right quadrant represents late apoptotic
cells, the lower left quadrant represents normal cells and the
lower right quadrant represents early apoptotic cells. *P<0.05,
vs. the control group. TGF-β1, transforming growth factor-β-1; PI,
propidium iodide. |
Effect of altered TGF-β1 expression on PCNA,
gankyrin, p115, XIAP and survivin protein expression
HepG2 cells were treated with exogenous TGF-β1 or
TGF-β1 siRNA, and protein expression was evaluated using western
blotting. In the exogenous TGF-β1 group, PCNA protein expression
was significantly decreased at 24, 48 and 72 h following treatment
compared with the control group (P=0.0016, P=0.0051 and P=0.0109,
respectively); however, the most significant decrease was observed
at 24 h (Fig. 6). Gankyrin expression
was significantly decreased at 24 h (P=0.039), significantly
increased at 72 h (P=0.028) and unchanged at 48 h compared with the
control group (Fig. 6). p115 protein
expression was significantly increased at 24 h (P=0.0382), but
significantly decreased at 48 h (P=0.0289) and 72 h (P=0.0026)
compared with the control group (Fig.
6). XIAP protein expression was significantly decreased
compared with the control group (P24 h=0.0015, P48
h=0.0289, P72 h=0.0025; Fig. 7). Survivin protein expression at 24 h
was significantly decreased compared with the control group
(P=0.0041); however, no significant difference was observed at 48
and 72 h (P=0.1177 and P=0.5671, respectively; Fig. 7). In the TGF-β1-knockdown group, PCNA,
gankyrin, XIAP and survivin protein expression was significantly
decreased compared with the control group (PPCNA72
h=0.0004, PPCNA96 h=0.0106,
Pgankyrin72 h=0.028, Pgankyrin96
h=0.023, PXIAP72 h=0.0026,
PXIAP96 h=0.0227, Psurvivin72
h=0.0135, Psurvivin96 h=0.0203; Figs. 6 and 7).
p115 expression was significantly increased at 96 h compared with
the control group (P=0.0292; Fig. 6).
PCNA, XIAP and survivin protein expression increased following
TGF-β1 knockdown in a time-dependent manner compared with 72 h and
96 h following transfection, whereas gankyrin exhibited the
opposite pattern (Figs. 6 and
7).
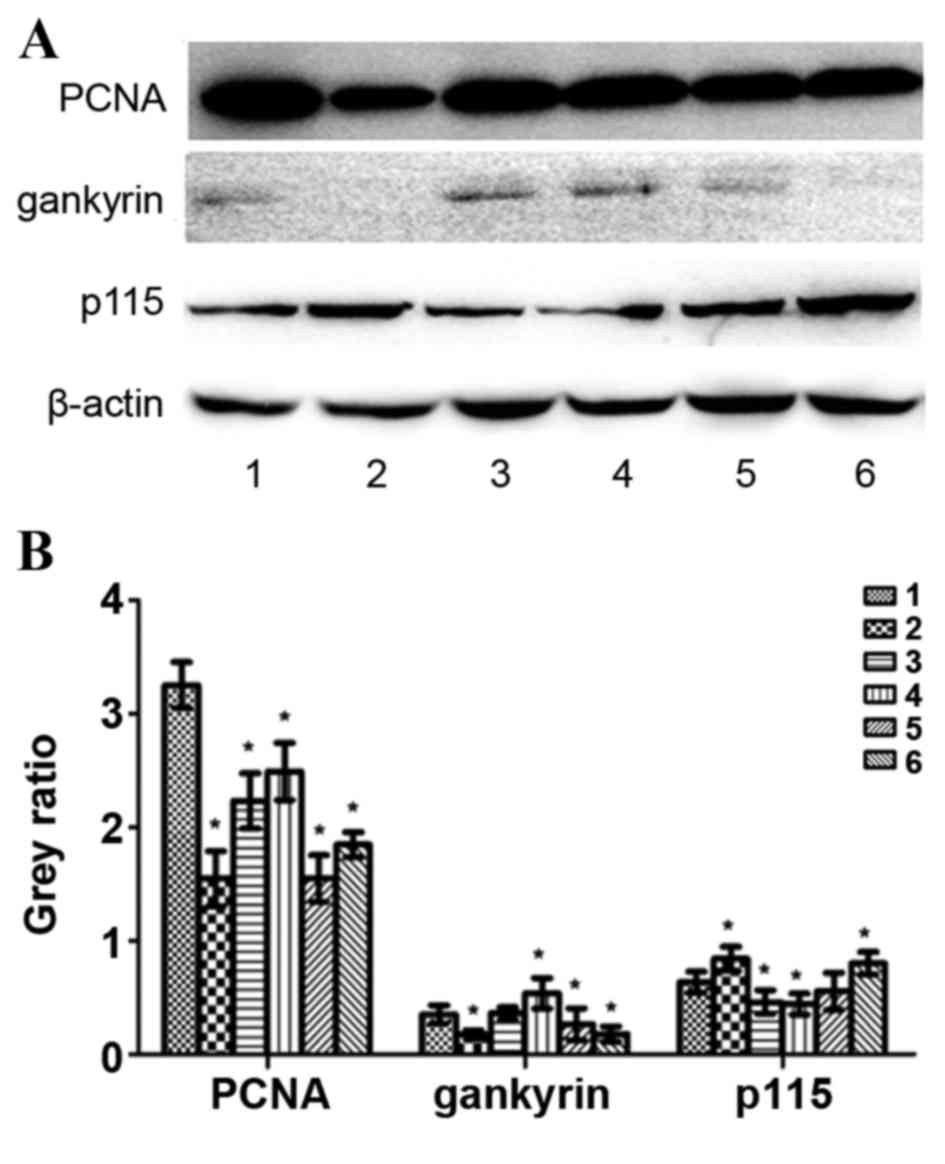 | Figure 6.Protein expression levels of PCNA,
gankyrin, p115 and β-actin following treatment with exogenous
TGF-β1 or TGF-β1 siRNA in HepG2 cells. (A) Representative protein
bands from the western blotting. (B) Protein band quantification.
1, control group; 2, 24 h exogenous TGF-β1 group; 3, 48 h exogenous
TGF-β1 group; 4, 72 h exogenous TGF-β1 group; 5, 72 h
TGF-β1-knockdown group; 6, 96 h TGF-β1-knockdown group. *P<0.05,
vs. the control group. PCNA, proliferating cell nuclear antigen;
p115, general vesicular transport factor p115; TGF-β1, transforming
growth factor-β-1; siRNA, small interfering RNA. |
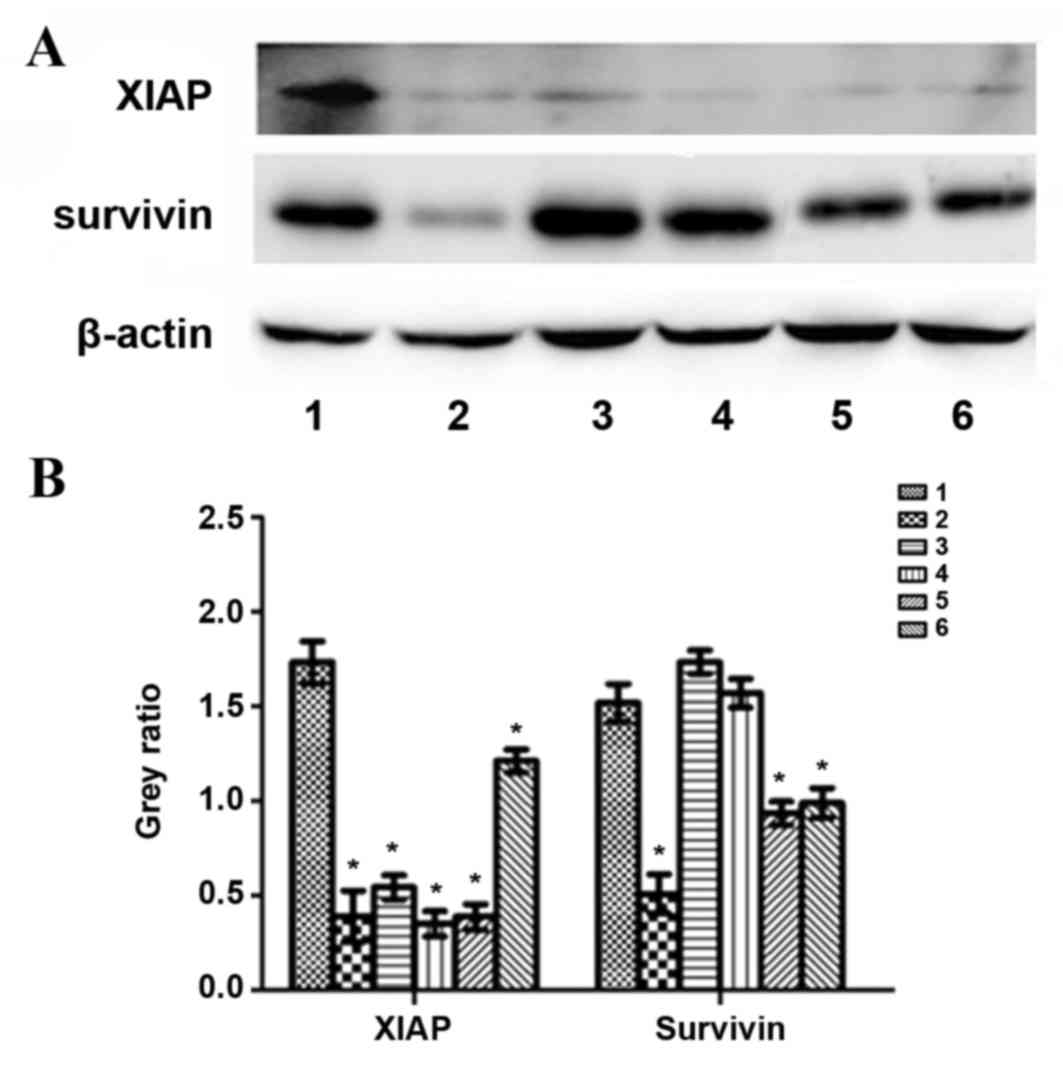 | Figure 7.Protein levels of XIAP, survivin and
β-actin following treatment with exogenous TGF-β1 or TGF-β1 siRNA
in HepG2 cells. (A) Representative protein bands from the western
blotting. (B) Protein band quantification. 1, control group; 2, 24
h exogenous TGF-β1 group; 3, 48 h exogenous TGF-β1 group; 4, 72 h
exogenous TGF-β1 group; 5, 72 h TGF-β1-knockdown group; 6, 96 h
TGF-β1-knockdown group. *P<0.05 vs. the control group. XIAP,
X-linked inhibitor of apoptosis protein; TGF-β1, transforming
growth factor-β-1; siRNA, small interfering RNA. |
Discussion
RNA interference has been successfully used to study
gene function, and has assisted in determining associations between
upstream and downstream factors in various signaling pathways
(37). In the present study, TGF-β1
protein expression was observed to be decreased 72 and 96 h
following siRNA transfection, indicating that the siRNA against
TGF-β1 was effective.
In the present study, treatment with exogenous
TGF-β1 inhibited HepG2 cell growth; the degree of inhibition
following treatment with concentrations between 20 and 30 µg/l was
the most significant. The effect of treatment with exogenous TGF-β1
on cell growth may be due to TGF-β1 having a role as a tumor
suppressor in the early stage of tumor development, and is involved
in two-way regulation during the genesis and development of liver
cancer (7–9). In addition, an siRNA directed against
TGF-β1 inhibited HepG2 cell growth, potentially as TGF-β1 is
overexpressed in liver cancer (12).
However, it remains unclear why treatment with exogenous TGF-β1 and
siRNA against TGF-β1 inhibited HepG2 cell growth, and the
underlying molecular mechanisms require further study.
Previously, TGF-β1 has been revealed to induce
G1-phase cell cycle arrest or prolong the time of the
G1-S phase transition in mesothelioma and breast cancer
(38), which is consistent with the
results of the present study. In the exogenous TGF-β1 group, cells
were arrested in the G1 phase, and the percentage of
cells in the S and G2 phases decreased. The
TGF-β1-knockdown cells were also arrested in the G1 and
S phases 72 and 96 h following transfection, respectively. These
results are consistent with the results of cell growth.
In the present study, treatment with exogenous
TGF-β1 or siRNA against TGF-β1 inhibited HepG2 cell growth, cell
cycle progression and apoptosis; however, the effect of TGF-β1
knockdown was more significant. This is potentially as exogenous
TGF-β1 has inconsistent effects on the expression of related
factors, including PCNA, gankyrin, p115, XIAP and survivin. As the
effect was more significant in the TGF-β1-knockdown group, the
changes due to TGF-β1 knockdown will be discussed. The effect of
treatment with exogenous TGF-β1 requires further study.
PCNA serves an important role in the priming of cell
proliferation and is therefore an indicator of cell proliferation.
(18) For example, antisense TGF-β1
oligonucleotides may lead to significantly decreased expression
levels of PCNA and inhibit cell growth in oral squamous cell
carcinoma (39). Gankyrin, a novel
oncogene, regulates the cell cycle and apoptosis (28). LBH589 inhibits the proliferation and
metastasis of hepatocellular carcinoma through the inhibition of
the gankyrin/STAT3/Akt signaling pathway (28). However, there are few studies on
gankyrin, and the association between TGF-β1 and gankyrin remains
unclear. In the present study, treatment with exogenous TGF-β1
resulted in decreased protein expression levels of PCNA and
gankyrin compared with the control group; therefore, treatment with
exogenous TGF-β1 may inhibit cell growth and enhance apoptosis.
p115 is a potential tumor biomarker and therapeutic target in human
gastric cancer (36). However, in the
present study, TGF-β1 knockdown resulted in increased p115 protein
expression levels as compared with the control group. The
underlying molecular mechanism remains to be completely
elucidated.
XIAP and survivin are considered to be IAPs and
their decreased expression causes caspase-3 to become
phosphorylated, therefore increasing cellular apoptosis. TGF-β may
upregulate certain anti-apoptotic genes, including B-cell
lymphoma-2 like 2 and XIAP (10), and
XIAP knockdown abolishes the TGF-β1-induced proliferation of
malignant meningioma cells (11).
TGF-β signaling pathway antagonists similarly activate the survivin
promoter, rendering cells refractory to further promoter activation
by insulin-like growth factor-1 (40). Similarly, in the present study, TGF-β1
knockdown resulted in decreased XIAP and survivin protein
expression levels as compared with the control group, therefore
enhancing cellular apoptosis.
In conclusion, the TGF-β signaling pathway affects
cell growth, the cell cycle and apoptosis by regulating the protein
expression of PCNA, gankyrin, p115, XIAP and survivin. The results
of the present study may improve current understanding of liver
cancer pathogenesis and the respective role of the TGF-β signaling
pathway. By understanding these processes in detail, it may be
possible to treat tumors by modulating TGF-β signal transduction
cascades within cells, and to specifically control cell growth,
differentiation and apoptosis.
Glossary
Abbreviations
Abbreviations:
|
TGF-β
|
transforming growth factor-β
|
|
MAPK
|
mitogen-activated protein kinase
|
|
PI3K
|
phosphoinositide 3-kinase
|
|
JNK
|
c-Jun N-terminal kinase
|
|
STAT
|
signal transducer and activator of
transcription
|
|
PCNA
|
proliferating cell nuclear antigen
|
|
p115
|
general vesicular transport factor
p115
|
|
XIAP
|
X-linked inhibitor of apoptosis
protein
|
|
siRNA
|
small interfering RNA
|
|
DMEM
|
Dulbecco's modified Eagle's medium
|
|
FCS
|
fetal calf serum
|
|
CCK-8
|
Cell Counting kit-8
|
|
PI
|
propidium iodide
|
|
FITC
|
fluorescein isothiocyanate
|
References
|
1
|
Balzarini P, Benetti A, Invernici G,
Cristini S, Zicari S, Caruso A, Gatta LB, Berenzi A, Imberti L,
Zanotti C, et al: Transforming growth factor-beta1 induces
microvascular abnormalities through a down-modulation of neural
cell adhesion molecule in human hepatocellular carcinoma. Lab
Invest. 92:1297–1309. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Monga SP: β-catenin signaling and roles in
liver homeostasis, injury, and tumorigenesis. Gastroenterology.
148:1294–1310. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Huang JL, Ren TY, Cao SW, Zheng SH, Hu XM,
Hu YW, Lin L, Chen J, Zheng L and Wang Q: HBx-related long
non-coding RNA DBH-AS1 promotes cell proliferation and survival by
activating MAPK signaling in hepatocellular carcinoma. Oncotarget.
6:33791–33804. 2015.PubMed/NCBI
|
|
4
|
Tang H, Li RP, Liang P, Zhou YL and Wang
GW: miR-125a inhibits the migration and invasion of liver cancer
cells via suppression of the PI3K/AKT/mTOR signaling pathway. Oncol
Lett. 10:681–686. 2015.PubMed/NCBI
|
|
5
|
Zhao H, Guo Y, Li S, Han R, Ying J, Zhu H,
Wang Y, Yin L, Han Y, Sun L, et al: A novel anti-cancer agent
Icaritin suppresses hepatocellular carcinoma initiation and
malignant growth through the IL-6/Jak2/Stat3 pathway. Oncotarget.
6:31927–31943. 2015.PubMed/NCBI
|
|
6
|
Wang XH, Liu BR, Qu B, Xing H, Gao SL, Yin
JM, Wang XF and Cheng YQ: Silencing STAT3 may inhibit cell growth
through regulating signaling pathway, telomerase, cell, cycle,
apoptosis and angiogenesis in hepatocellular carcinoma: Potential
uses for gene therapy. Neoplasma. 58:158–171. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang S, Guo L, Dong L, Guo L, Li S, Zhang
J and Sun M: TGF-beta1 signal pathway may contribute to
rhabdomyosarcoma development by inhibiting differentiation. Cancer
Sci. 101:1108–1116. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sjölund J, Boström AK, Lindgren D, Manna
S, Moustakas A, Ljungberg B, Johansson M, Fredlund E and Axelson H:
The notch and TGF-β signaling pathways contribute to the
aggressiveness of clear cell renal cell carcinoma. PLoS One.
6:e230572011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gore AJ, Deitz SL, Palam LR, Craven KE and
Korc M: Pancreatic cancer-associated retinoblastoma 1 dysfunction
enables TGF-β to promote proliferation. J Clin Invest. 124:338–352.
2014. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Caja L, Bertran E, Campbell J, Fausto N
and Fabregat I: The transforming growth factor-beta (TGF-β)
mediates acquisition of a mesenchymal stem cell-like phenotype in
human liver cells. J Cell Physiol. 226:1214–1223. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gogineni VR, Gupta R, Nalla AK, Velpula KK
and Rao JS: uPAR and cathepsin B shRNA impedes TGF-β1-driven
proliferation and invasion of meningioma cells in a XIAP-dependent
pathway. Cell Death Dis. 3:e4392012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lee D, Chung YH, Kim JA and Lee YS, Lee D,
Jang MK, Kim KM, Lim YS, Lee HC and Lee YS: Transforming growth
factor beta 1 overexpression is closely related to invasiveness of
hepatocellular carcinoma. Oncology. 82:11–18. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu W, Chen JR, Hsu CH, Li YH, Chen YM,
Lin CY, Huang SJ, Chang ZK, Chen YC, Lin CH, et al: A zebrafish
model of intrahepatic cholangiocarcinoma by dual expression of
hepatitis B virus X and hepatitis C virus core protein in liver.
Hepatology. 56:2268–2276. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Darrington E, Zhong M, Vo BH and Khan SA:
Vascular endothelial growth factor A, secreted in response to
transforming growth factor-β1 under hypoxic conditions, induces
autocrine effects on migration of prostate cancer cells. Asian J
Androl. 14:745–751. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fuzio P, Ditonno P, Rutigliano M,
Battaglia M, Bettocchi C, Loverre A, Grandaliano G and Perlino E:
Regulation of TGF-β1 expression by androgen deprivation therapy of
prostate cancer. Cancer Lett. 318:135–144. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Freudlsperger C, Bian Y, Wise Contag S,
Burnett J, Coupar J, Yang X, Chen Z and Van Waes C: TGF-β and NF-κB
signal pathway cross-talk is mediated through TAK1 and SMAD7 in a
subset of head and neck cancers. Oncogene. 32:1549–1559. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wu Y, Chen P, Huang HF, Huang MJ and Chen
YZ: Reduction of transforming growth factor-β1 expression in
leukemia and its possible role in leukemia development. Leuk
Lymphoma. 53:145–151. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yuan SX, Tao QF, Wang J, Yang F, Liu L,
Wang LL, Zhang J, Yang Y, Liu H, Wang F, et al: Antisense long
non-coding RNA PCNA-AS1 promotes tumor growth by regulating
proliferating cell nuclear antigen in hepatocellular carcinoma.
Cancer Lett. 349:87–94. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bao W, Zhu F, Duan Y, Yang Y and Cai H:
HtrA1 resensitizes multidrug-resistant hepatocellular carcinoma
cells by targeting XIAP. Biomed Pharmacother. 70:97–102. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wu WY, Kim H, Zhang CL, Meng XL and Wu ZS:
Clinical significance of autophagic protein LC3 levels and its
correlation with XIAP expression in hepatocellular carcinoma. Med
Oncol. 31:1082014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Vogl TJ, Oppermann E, Qian J, Imlau U,
Tran A, Hamidavi Y, Korkusuz H, Bechstein WO, Nour-Eldin NE,
Gruber-Rouh T, et al: Transarterial chemoembolization of
hepatocellular carcinoma in a rat model: The effect of additional
injection of survivin siRNA to the treatment protocol. BMC Cancer.
16:3252016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Strzalka W and Ziemienowicz A:
Proliferating cell nuclear antigen (PCNA): A key factor in DNA
replication and cell cycle regulation. Ann Bot. 107:1127–1140.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dillehay KL, Lu S and Dong Z: Antitumor
effects of a novel small molecule targeting PCNA chromatin
association in prostate cancer. Mol Cancer Ther. 13:2817–2826.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dillehay KL, Seibel WL, Zhao D, Lu S and
Dong Z: Target validation and structure-activity analysis of a
series of novel PCNA inhibitors. Pharmacol Res Perspect.
3:e001152015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zheng T, Hong X, Wang J, Pei T, Liang Y,
Yin D, Song R, Song X, Lu Z, Qi S, et al: Gankyrin promotes tumor
growth and metastasis through activation of IL-6/STAT3 signaling in
human cholangiocarcinoma. Hepatology. 59:935–946. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bai Z, Tai Y, Li W, Zhen C, Gu W, Jian Z,
Wang Q, Lin JE, Zhao Q, Gong W, et al: Gankyrin activates IL-8 to
promote hepatic metastasis of colorectal cancer. Cancer Res.
73:4548–4558. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang J, Yang Y, Zhang Z, He Y, Liu Z, Yu
Y, Wu S, Cai B and Feng Y: Gankyrin plays an essential role in
estrogen-driven and GPR30-mediated endometrial carcinoma cell
proliferation via the PTEN/PI3K/AKT signaling pathway. Cancer Lett.
339:279–287. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Song X, Wang J, Zheng T, Song R, Liang Y,
Bhatta N, Yin D, Pan S, Liu J, Jiang H and Liu L: LBH589 Inhibits
proliferation and metastasis of hepatocellular carcinoma via
inhibition of gankyrin/STAT3/Akt pathway. Mol Cancer. 12:1142013.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sun W, Ding J, Wu K, Ning BF, Wen W, Sun
HY, Han T, Huang L, Dong LW, Yang W, et al: Gankyrin-mediated
dedifferentiation facilitates the tumorigenicity of rat hepatocytes
and hepatoma cells. Hepatology. 54:1259–1272. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Qian YW, Chen Y, Yang W, Fu J, Cao J, Ren
YB, Zhu JJ, Su B, Luo T, Zhao XF, et al: 28(GANK) prevents
degradation of Oct4 and promotes expansion of tumor-initiating
cells in hepatocarcinogenesis. Gastroenterology. 142:1547–1558.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gao L, Xie H, Dong L, Zou J, Fu J, Gao X,
Ou L, Xiang S and Song H: Gankyrin is essential for hypoxia
enhanced metastatic potential in breast cancer cells. Mol Med Rep.
9:1032–1036. 2014.PubMed/NCBI
|
|
32
|
Zhen C, Chen L, Zhao Q, Liang B, Gu YX,
Bai ZF, Wang K, Xu X, Han QY, Fang DF, et al: Gankyrin promotes
breast cancer cell metastasis by regulating Rac1 activity.
Oncogene. 32:3452–3460. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mine H, Sakurai T, Kashida H, Matsui S,
Nishida N, Nagai T, Hagiwara S, Watanabe T and Kudo M: Association
of gankyrin and stemness factor expression in human colorectal
cancer. Dig Dis Sci. 58:2337–2344. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li J, Knobloch TJ, Kresty LA, Zhang Z,
Lang JC, Schuller DE and Weghorst CM: Gankyrin, a biomarker for
epithelial carcinogenesis, is overexpressed in human oral cancer.
Anticancer Res. 31:2683–2692. 2011.PubMed/NCBI
|
|
35
|
Millarte V, Boncompain G, Tillmann K,
Perez F, Sztul E and Farhan H: Phospholipase C γ1 regulates early
secretory trafficking and cell migration via interaction with p115.
Mol Biol Cell. 26:2263–2278. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li XJ, Luo Y and Yi YF: P115 promotes
growth of gastric cancer through interaction with macrophage
migration inhibitory factor. World J Gastroenterol. 19:8619–8629.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Liu X, Sun G and Sun X: RNA
interference-mediated silencing of speckle-type POZ protein
promotes apoptosis of renal cell cancer cells. Onco Targets Ther.
9:2393–2402. 2016.PubMed/NCBI
|
|
38
|
Li B, Wen G, Zhao Y, Tong J and Hei TK:
The role of TGFBI in mesothelioma and breast cancer: Association
with tumor suppression. BMC Cancer. 12:2392012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kim SG and Song JY: Therapeutic targeting
of oncogenic transforming growth factor-β1 signaling by antisense
oligonucleotides in oral squamous cell carcinoma. Oncol Rep.
28:539–544. 2012.PubMed/NCBI
|
|
40
|
Song K, Shankar E, Yang J, Bane KL,
Wahdan-Alaswad R and Danielpour D: Critical role of a
survivin/TGF-β/mTORC1 axis in IGF-I-mediated growth of prostate
epithelial cells. PLoS One. 8:e618962013. View Article : Google Scholar : PubMed/NCBI
|