Introduction
Breast cancer is the most common malignancy
occurring in females from Western countries (1). In Brazil, it has been estimated that
57,960 new cases and 14,207 deaths due to the disease will occur in
2016 (2,3). Breast cancer prognosis is directly
associated with tumor staging at the time of diagnosis (4,5). For
almost a century, radical mastectomy plus axillary lymph node
dissection, introduced by Halsted in 1882 until the 1970s of the
20th century (6), was the standard
surgical treatment for all breast tumor stages, resulting in
serious complications in the upper limb ipsilateral to surgery
(7–11).
Breast cancer is currently diagnosed at earlier
stages of the disease. The majority of patients have clinically
negative axillae. Axillary staging methods that do not require an
axillary lymph node dissection have been investigated, as axillary
nodal status is the most important prognostic factor and most
effective indicator of long-term survival (12–15).
Sentinel lymph node biopsy (SNB) is the gold standard for
histopathological staging of early-stage breast carcinoma, as
information concerning axillary lymph node status is able to be
achieved with a lower complication rate (13). However, SNB involves a complex
laboratory technique, increasing surgical costs. In addition,
clinical complications may arise, including anaphylactic reactions,
lower sensitivity and strength of the ipsilateraley upper limb and
even the rare occurrence of lymphedema (16,17).
Alternative methods to replace sentinel lymph node
biopsy have been assessed (15).
Therefore, analysis of ultrasonographic characteristics of axillary
lymph nodes, particularly ultrasound-guided fine-needle aspiration
(US-guided FNA) cytology is required. The main US features of
suspicious lymph nodes are nodal size, cortical thickening, round
morphology, hypoechogenicity, loss of central fatty hilum and
eccentrically bulging cortex (15).
However, thickness of the lymph node cortex is most highly
associated with the presence of metastasis and may contribute to
US-guided FNA (5). Oz et al
(5) revealed that a cortical
thickness >4.0 mm had a sensitivity of 86% and specificity of
87%, whereas at the cut-off point of 3.0 mm, specificity decreased
to 37%. However, results in the literature are heterogenous, as
ultrasonographic examination is operator-dependent and
machine-dependent and there is a great variation in sensitivity and
specificity when only US is used for evaluation of axillary lymph
nodes (18–20). By contrast, using US-guided FNA,
certain authors have demonstrated a sensitivity and specificity
ranging from 42.2–89 and 81–100%, respectively (20–28).
US-guided FNA has a high accuracy rate (5,7).
Therefore, controversy concerning US-guided FNA
cytology of axillary lymph nodes vs. SNB in early-stage breast
cancer, in addition to the paucity of comparative studies between
the two methods in Brazilian women, informed the current study
design.
Patients and methods
Patients
The present study involved 30 female patients, aged
33–73 years, diagnosed with operable early invasive breast
carcinoma, with any histological tumor subtype and with indications
for SNB during surgery. These women had been managed at the Breast
Disorder Clinic of the Getulio Vargas Hospital, Federal University
of Piaui (Piaui, Brazil) from May 2015 to April 2016. Three
patients were excluded from the study due to previous axillary
surgeries, allergic reaction to dye injection or refusal to
participate in the study. The Internal Review Board of the Federal
University of Piaui approved the study and all patients signed an
informed consent form prior to admission.
Methods
US-guided FNA of axillary lymph nodes was performed
at the most common site for sentinel lymph node (SLN) appearance,
representative of benign or suspicious ultrasonographic nodal size
and morphological features. A Logiq E portable ultrasound machine
(GE Medical Systems, Jiangsu, China), with a 12 L linear probe, and
7.5 to 12 MHz imaging frequency was used. Contents of the aspirated
material were expelled onto a glass slide for cellular distension.
The smeared slides were fixed in 99.3% alcohol (29). The sentinel lymph node was obtained
during the scheduled surgical procedure for each patient, following
its location by Blue Patent dye V injection or Tc-99 m phytate
lymphoscintigraphy scan. A γ probe was used intraoperatively to
locate the SLN. The SLN was sent to the laboratory without any
fixative agent for histopathological examination during the
intraoperative period. The analysis of cytology and sentinel lymph
node was performed by the same professional, with SLN considered as
the gold standard to evaluate the performance of FNA.
Data obtained were stored in an electronic database
created in the Excel 2010 program (Windows 7; Microsoft
Corporation, Redmond, WA, USA). Subsequently, statistical analysis
was performed using calculation of sample proportions in Excel
2010.
Results
The patients ranged in age from 33–73 years (mean
age, 51 years). The mean size of the primary breast tumor was 1.7
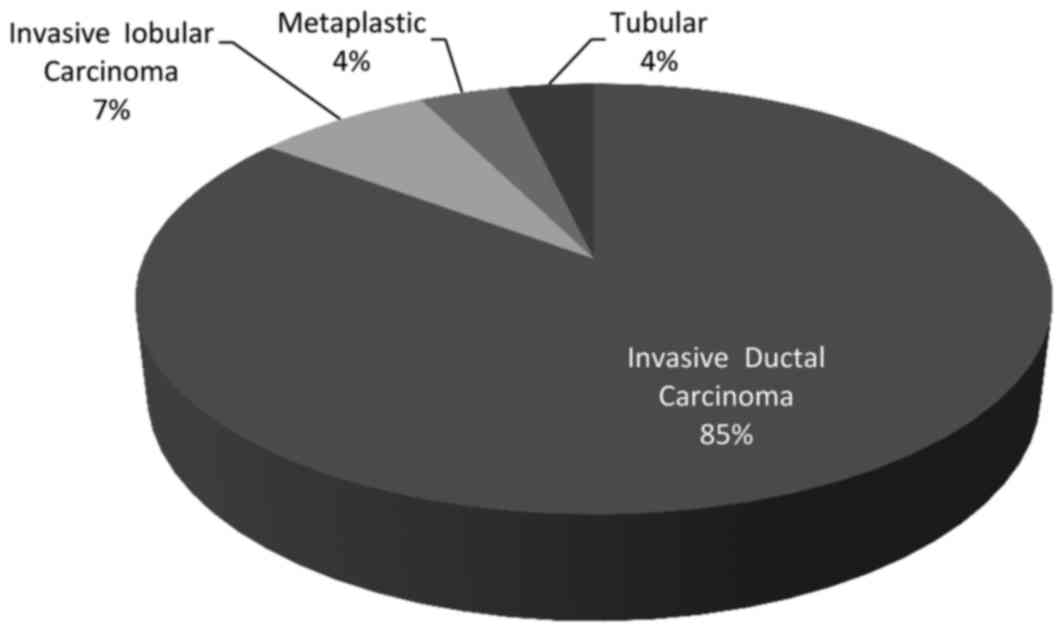
cm (range, 0.7–3.7 cm). There were 23 (85%) invasive ductal tumors,
2 (7%) invasive lobular tumors, 1 (4%) tubular tumor, 1 (4%)
metaplastic carcinoma and an average of 2 SLNs were removed per
patient (range, 1–6) (Table I;
Fig. 1). Of the 27 axillae included
in the study, 11 (41%) were positive for metastatic carcinoma
according to the SNB. Of these 11 positive cases, US-guided FNA
cytology was positive for malignancy in 5 cases (19%). None of the
FNA cases that tested positive for malignancy were identified in
SNBs that were negative for metastatic carcinoma. The sensitivity
of SNB cytology was 45%, specificity was 100%, positive predictive
value was 100% and negative predictive value was 73% in comparison
with SNB histopathology (Table
II).
 | Table I.Characteristics of patients
studied. |
Table I.
Characteristics of patients
studied.
| Characteristics | Mean (range) |
|---|
| Age, years | 51 (33–73) |
| Tumor size, cm | 1.7 (0.7–3.7) |
| Sentinel lymph
nodes | 2 (1–6) |
 | Table II.Comparison between FNA cytology and
histopathology in the evaluation of axillary lymph node metastasis
in patients with breast cancer. |
Table II.
Comparison between FNA cytology and
histopathology in the evaluation of axillary lymph node metastasis
in patients with breast cancer.
|
Histopathology/Cytology | With metastasis | Without
metastasis | Total |
| With metastasis | 5 | – | 5 |
| Without
metastasis | 6 | 16 | 22 |
| Total | 11 | 16 | 27 |
Discussion
Previous studies have revealed certain limitations
of fine needle aspiration cytology (FNAC) compared with
histopathology of sentinel lymph node biopsy. In addition to US
examination, Bonnema et al (30) used US-guided fine needle aspiration in
non-palpable axillary lymph nodes of patients with breast cancer
and made a comparison with sentinel lymph node histopathology. This
previous study obtained a sensitivity of 80% and specificity of
100%. Subsequently, various other studies were conducted to
consolidate this technique, which is simpler and less expensive
than SNB in daily practice (15,16,18,20).
Nevertheless, all these studies have demonstrated a limiting factor
in this evaluation, which is a moderate negative predictive value.
Negative cytology is not always accurate, as a negative result may
not exclude axillary lymph node metastasis since it may be a false
negative result, particularly in cases of very small (<5.0 mm)
tumor implants (7). However, at each
new study the detection rate increases, due to advances in imaging
tests with ultrasonographic devices that reveal more detailed lymph
node structure (20).
Therefore, the present study was developed to
compare the detection rates of lymph node metastasis by US-guided
FNA vs. SNB in women with early-stage breast cancer. The purpose of
the study was to corroborate this diagnostic method as an
alternative to SNB, which remains the gold standard of care. The
number of sentinel lymph nodes detected (a mean of 2 nodes per
patient) is consistent with other studies published; however, it
has been reported that certain surgeons opt to remove para-sentinel
lymph nodes (12).
The moderate negative predictive value is currently
the greatest limitation of using FNA. In the present study, the
negative predictive value was 73% due to false-negative cases. This
value increases when the method is used in patients regardless of
the presence of axillary lymph nodes with altered images suspicious
of metastatic implants (7,18). A reason for these false-negative
results in FNA may be the failure to detect lymph nodes with small
tumor deposits (<5 mm) or micrometastasis. This false-negative
result is similar to that obtained by core-needle biopsy
(thick-needle biopsy of tissue sample). In lymph nodes with small
metastatic tumor deposits, it is challenging to reach this small
area, and the technique is more invasive and expensive (7,15,31). Furthermore, assuring that the removed
lymph node corresponds to the sentinel lymph node was not
possible.
In the present study, axillary lymph node cytology
in patients with early breast cancer in comparison with SNB had 6
false-negative results. Cytology was negative when histological
examinations of sentinel lymph nodes revealed metastasis. By
contrast, there were no false-positive results, as all positive
cytology results were confirmed by sentinel lymph node
histopathology. However, FNA sensitivity was low in association
with that of SNB. Of the 11 metastatic axillae, only 5 were
revealed by FNA, resulting in a sensitivity of only 45%. A probable
reason for this discrepancy may be the small sample included in
this study, in view of such a prevalent disease. In a study by
Oruwari et al (22), the
sample was similar, but included patients with more advanced
disease. As a result, the sensitivity and specificity were higher.
On the other hand, of the 16 patients without axillary lymph node
metastasis, all had a negative FNA, and specificity was 100%.
Similarly, in 5 patients with positive cytology, all had sentinel
lymph node metastasis, and the positive predictive value was
100%.
By contrast, positive fine-needle aspiration may
avoid sentinel lymph node mapping in cases where direct axillary
lymph node dissection is selected. Whether the performance of
axillary lymph node dissection may be avoided in these cases
remains unknown. According to the ACOSOG Z0011 (13) and EORTC AMAROS (12) studies, radiotherapy to the axilla
minimally affected by metastasis may promote survival rates similar
to those of patients undergoing axillary lymph node dissection
(7,32). However, an important study reported
that positive preoperative cytology indicates a greater extension
of axillary metastatic disease. Axillary lymph node dissection may
not be avoided in the majority of patients (94%), which is
consistent with inclusion criteria of the ACOSOG Z011 trial. This
may avoid sentinel lymph node mapping in these patients, who have a
mean number of 4 lymph nodes involved, in addition to extracapsular
disease (16).
This method may decrease the cost of sentinel lymph
node mapping, which is an invasive method with a longer surgical
and anesthetic duration. Furthermore, it requires the use of
nuclear medicine for lymphoscintigraphy scan or dyes that may cause
anaphylactic reactions. Lymph node dissection may be avoided in
cases where the sentinel lymph node is not located, either in obese
patients or those with afferent lymphatic vessel congestion by
tumor cells (18,25). In addition, the adverse effects of
sentinel lymph node mapping, including pain, loss of sensitivity
and lymphedema of the upper limb may be prevented (7,18,19). Nevertheless, despite the results of
the present study revealing specificity of cytology similar to the
SNB, the sample size of this study was small and there is a
requirement for further studies with larger sample sizes to improve
the analysis of results.
In conclusion, the results of the current study
revealed that fine-needle aspiration cytology of breast carcinoma
in comparison with histological examination of SNB has a
sensitivity, specificity, positive and negative predictive value of
45, 100, 100 and 73%, respectively. Therefore, positive FNA
cytology has a specificity similar to SNB in cases of axillary
metastatic disease. However, it is not able to rule out metastatic
implants when the test is negative, due to its low sensitivity.
References
|
1
|
Shah R, Rosso K and Nathanson SD:
Pathogenesis, prevention, diagnosis and treatment of breast cancer.
World J Clin Oncol. 5:283–298. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
National Cancer Institute (INCA) José
Alencar Gomes: Coordination of prevention and surveillance.
Estimate for 2016: Cancer incidence in Brazil/INCA-Rio de Janeiro:
INCA. 2015.http://www.inca.gov.br/estimativa/2016/estimativa-2016-2v11.pdf
|
|
3
|
National Cancer Institute (INCA) José
Alencar Gomes: Coordination of prevention and surveillance.
Estimate for 2014: Cancer incidence in Brazil/INCA-Rio de Janeiro:
INCA. 2014.http://www.inca.gov.br/estimativa/2014/index.asp?ID=2
|
|
4
|
National Cancer Institute (INCA) Jose
Alencar Gomes: Coordination of prevention and surveillance.
Estimate for 2012: Cancer incidence in Brazil/INCA-Rio de Janeiro:
INCA. 2012.http://portal.saude.sp.gov.br/resources/ses/perfil/gestor/homepage/estimate
|
|
5
|
Oz A, Demirkazik FB, Akpinar MG, Soygur I,
Baykal A, Onder SC and Uner A: Efficiency of ultrasound and
ultrasound-guided fine needle aspiration cytology in preoperative
assessment of axillary lymph node metastases in breast cancer. J
Breast Cancer. 15:211–217. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Plesca M, Bordea C, El Houcheimi B, Ichim
E and Blidaru A: Evolution of radical mastectomy for breast cancer.
J Med Life. 9:183–186. 2016.PubMed/NCBI
|
|
7
|
Maniero MB: Regional lymph node staging in
breast cancer: The increasing role of imaging and ultrasound-guided
axillary lymph node fine needle aspiration. Radiol Clin North Am.
48:989–997. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Song SE, Seo BK, Lee SH, Vie A, Lee KY,
Cho KR, Woo OH, Cha SH and Kim BH: Classification of metastatic
versus non-metastatic axillary nodes in breast cancer patients:
Value of cortex-hilum area ratio with ultrasound. J Breast Cancer.
15:65–70. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Abe H, Schmidt RA, Sennett CA, Shimauchi A
and Newstead GM: Us-guided core needle biopsy of axillary lymph
nodes in patients with breast cancer: Why and how to do it.
Radiographics. 27:(Suppl 1). S91–S99. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Krishnamurthy S: Current applications and
future prospects of fine-needle aspiration biopsy of locoregional
lymph nodes in the management of breast cancer. Cancer.
117:451–462. 2009.PubMed/NCBI
|
|
11
|
Ecanow JS, Abe H, Newstead GM, Ecanow DB
and Feske FM: Axillary staging of breast cancer: What the
radiologist should know? Radiographics. 33:1589–1612. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Donker M, Van Tienhoven G, Straver ME,
Meijnen P, Van de Velde CJ, Mansel RE, Cataliotti L, Westenberg AH,
Klinkenbijl JH, Orzalesi L, et al: Radiotherapy or surgery of the
axilla after a positive sentinel node in breast cancer (EORTC
10981–22023 AMAROS): A randomised, multicenter, open-label, phase 3
non-inferiority trial. Lancet Oncol. 15:1303–1310. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Giuliano AE, Hunt KK, Ballman KV, Beitsch
PD, Whitworth PW, Blumeneranz PW, Leitch AM, Saha S, Mccall LM and
Morrow M: Axillary dissection vs no axillary dissection in women
with invasive breast cancer and sentinel node metastase: A
randomized clinical trial. JAMA. 305:569–575. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lyman GH, Temin S, Edge SB, Newman LA,
Turner RR, Weaver DL, Benson AB III, Bosserman LD, Burstein HJ,
Cody H III, et al: Sentinel lymph node biopsy for patients with
early-stage breast cancer: American society of clinical oncology
clinical practice guideline update. J Clin Oncol. 32:1365–1383.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fung AD, Collins JA, Campassi C, Ioffe OB
and Staats PN: Performance characteristics of ultrasound-guided
fine-needle aspiration of axillary lymph nodes for metastatic
breast cancer employing rapid on-site evaluation of adequacy:
analysis of 136 cases and review of the literature. Cancer
Cytopathol. 122:282–291. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Boland MR, Prichard RS, Daskalova I,
Lowery AJ, Evoy D, Geraghty J, Rothwell J, Quinn CM, O'Doherty A
and McDermott EW: Axillary nodal burden in primary breast cancer
patients with positive pre-operative ultrasound guided fine needle
aspiration cytology: Management in the era of ACOSOG Z011. Eur J
Surg Oncol. 41:559–565. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Moorman AM, Bourez RL, de Leeuw DM and
Kouwenhoven EA: Pre-operative ultrasonographic evaluation of
axillary lymph nodes in breast cancer patients: For which group
still of additional value and in which group cause for special
attention? Ultrasound Med Biol. 41:2842–2848. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Boughey JC, Moriarty JP, Degnim AC, Gregg
MS, Egginon JS and Long KH: Cost modeling of preoperative axillary
ultrasound and fine-needle aspiration to guide surgery for invasive
breast cancer. Ann Surg Oncol. 17:953–958. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cho N, Moon WK, Han W, Park IA, Cho J and
Noh DY: Preoperative sonographic classification of axillary lymph
nodes in patients with breast cancer: Node-to-node correlation with
surgical histology and sentinel node biopsy results. AJR Am J
Roentgenol. 193:1731–1737. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pessoa EC, Rodrigues JR, Pessoa CP,
Véspoli HM and Uemura G: Axillary lymph node aspiration biopsy
guided by ultrasound is effective as lymph node involvement
prediction method in patients with breast cancer? Rev Bras Ginecol
Obstet. 36:118–123. 2014.(In Portuguese). View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Deurloo EE, Tanis PJ, Guilhuijs KG, Muller
SH, Kröger R, Peterse JL, Rutgers EJ, Olmos Valdés R and Schultze
Kool LJ: Reduction in the number of sentinel lymph node procedures
by preoperative ultrasonography of the axilla in breast cancer. Eur
J Cancer. 39:1068–1073. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Oruwari JU, Chung MA, Koelliker S,
Steinhoff MM and Cady B: Axillary staging using ultrasound-guided
fine needle aspiration biopsy in locally advanced breast cancer. Am
J Surg. 184:307–309. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lemos S, Dias M, Gonçalo M, Pinto E,
Fernandes G and Oliveira C: Detection of axillary metastases in
breast cancer patients using ultrasound and colour doppler combined
with fine needle aspiration cytology. Eur J Gynaecol Oncol.
26:165–166. 2005.PubMed/NCBI
|
|
24
|
Ciatto S, Brancato B, Risso G, Ambrogetti
D, Bulgaresi P, Maddau C, Turco P and Houssami N: Accuracy of fine
needle aspiration cytology (FNAC) of axillary lymph nodes as a
triage test in breast cancer staging. Breast Cancer Res Treat.
103:85–91. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sapino A, Cassoni P, Zanon E, Fraire F,
Croce S, Coluccia C, Donadio M and Bussolati G:
Ultrasonographically-guided fine-needle aspiration of axillary
lymph nodes: Role in breast câncer management. Br J Cancer.
88:702–706. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
van Rijk MC, Deurloo EE, Nieweg OE,
Gilhuijs KG, Peterse JL, Rutgers EJ, Kröger R and Kroon BB:
Ultrasonography and fine-needle aspiration cytology can spare
breast cancer patients unnecessary sentinel lymph node biopsy. Ann
Surg Oncol. 13:31–35. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Popli MB, Sahoo M, Mehrotra N, Chouhury M,
Kumar A, Pathania OP and Thomas S: Preoperative ultrasound-guided
fine-needle aspiration cytology for axillary staging in breast
carcinoma. Australas Radiol. 50:122–126. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Altomare V, Guerriero G, Carino R,
Battista C, Primavera A, Altomare A, Vaccaro D, Esposito A, Ferri
AM and Rabitti C: Axillary lymph node echo-guided fine-needle
aspiration cytology enables breast cancer patients to avoid a
sentinel lymph node biopsy. Preliminary experience and a review of
the literature. Surg Today. 37:735–739. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Caputo LFG, Mota EM and Gitirana LB:
Concepts and methods for the training of professionals in health
laboratories. EPSJV. 2:189–213. 2010.
|
|
30
|
Bonnema J, Van Geel AN, Van Ooijen B, Mali
SP, Tjiam SL, Henzen-Logmans SC, Schmitz PI and Wiggers T:
Ultrasound guided aspiration biopsy for detection of axillary node
metastases in breast cancer patients: New diagnostic method. World
J Surg. 21:270–274. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Rao R, Lilley L, Andrews V, Radford L and
Ulissey M: Axillary staging by percutaneous biopsy: Sensitivity of
fine-needle aspiration versus core needle biopsy. Ann Surg Oncol.
16:1170–1175. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Galimberti V, Ribeiro-Fontana SK,
Maisonneuve P, Steccanella F, Vento AR, Intra M, Naninato P,
Caldarella P, Iorfida M, Colleoni M, et al: Sentinel node biopsy
after neoadjuvant treatment in breast cancer: Five-year follow-up
of patients with clinically node-negative or node-positive disease
before treatment. Eur J Surg Oncol. 42:361–368. 2016. View Article : Google Scholar : PubMed/NCBI
|