Introduction
Osteosarcoma is the most common type of primary
malignant tumor originating from bone in young adolescents
(1). Although progress has been made
in surgical technology and combined therapeutic strategies, the
total survival rate of osteosarcoma remains poor, particularly in
patients with lung metastasis (2).
Several molecules, including cluster of differentiation 44, tumor
protein p53, PLA2G16, vascular endothelial growth factor and
periostin have been identified for their use in the prognosis of
osteosarcoma (3–7). However, it was reported that p53 only
predicted a decreased short-term survival rate, but not 3- or
5-year survival, and cluster of differentiation 44 expression level
is not associated with overall survival rate and metastasis
(8,9).
Therefore, it is important to identify novel and effective
predictors of tumor progression and survival rate for patients with
osteosarcoma.
MicroRNAs (miRNAs) are transcribed from non-protein
coding genes or introns, and mediate the translational suppression
or cleavage of their target mRNAs by binding to the complementary
sites of their 3′-untranslated region. The aberrant expression of
miRNA, as oncogenes or anti-oncogenes, is observed in various types
of cancer (10,11). In osteosarcoma, certain miRNAs
including miR-1247 and miR-27 have been identified as oncogenes
(12,13), while others, including miR-15a,
miR-646 and miR-218, are regarded as anti-oncogenes (14–16). These
previous findings prove that miRNAs are a key element in the
tumorigenesis of osteosarcoma.
Previously, miR-335 has been identified as a tumor
suppressor in multiple types of cancer, including pancreatic,
ovarian, breast, small cell lung and renal cancer (17–21).
Furthermore, our former study identified that miR-335 could inhibit
the metastasis of osteosarcoma by directly targeting the regulation
of Rho-associated serine-threonine protein kinase 1 (Rock1), in
order to mediate Rho signaling (22).
Until recently, there was little research focusing on the
prognostic values of miR-335 and Rock1 in osteosarcoma. An
increasing number of studies have revealed that combined miRNA and
target gene expression profiling may provide vital information for
the diagnosis and prognosis of numerous types of human cancer
(23,24). However, there have been a limited
number of findings on the clinical significance of the combined
expression of miR-335 and Rock1 in human osteosarcoma. Considering
the aforementioned results, the present study hypothesized that
there would be a connection between the combined abnormal
expression of miR-335 and its target Rock1 with tumor progression
and prognosis in patients with osteosarcoma.
The present study, examined miR-335 and Rock1
expression levels in osteosarcoma tissues and noncancerous bone
tissues using in situ hybridization and
immunohistochemistry, respectively. The clinical significance of
abnormal miR-335 and Rock1 expression in osteosarcomas was
explored.
Materials and methods
Patients and tissue samples
Approval for the present study was obtained from the
Medical Ethics Committee of China Medical University (Liaoning,
China). The need for written informed consents by the patients was
waived due to the retrospective nature of the present study. A
total of 91 osteosarcoma specimens were collected from patients
with osteosarcoma who underwent curative tumor resection at the
First Affiliated Hospital of China Medical University (Liaoning,
China) between January 2003 and January 2008. During surgery, a
total of 47 adjacent non-tumorous bone tissues were collected as
controls. No patients underwent chemotherapy or radiotherapy prior
to surgery. The clinical stage of the patients was classified
according to the 6th edition of the Tumor Node Metastases
Classification of Malignant Tumors, International Union against
Cancer (25). The clinicopathological
information for all patients is presented in Table I. All 91 patients with osteosarcoma
were monitored during follow-up appointments, which lasted between
72 and 132 months. Mortality occurred in 9 patients during the
follow-up period. The median overall survival (OS) and disease-free
survival (DFS) of patients was 68 and 55 months, respectively.
 | Table I.Clinicopathological characteristics of
patients with osteosarcoma. |
Table I.
Clinicopathological characteristics of
patients with osteosarcoma.
| Characteristics | No. of cases | % |
|---|
| Age at diagnosis,
years | 91 |
|
|
<18 | 45 | 49.5 |
|
≥18 | 46 | 50.5 |
| Gender | 91 |
|
|
Female | 50 | 54.9 |
|
Male | 41 | 45.1 |
| Tumor size, cm | 91 |
|
|
<5 | 45 | 49.5 |
| ≥5 | 46 | 50.5 |
| Anatomic
location | 91 |
|
|
Tibia/femur | 56 | 61.5 |
|
Elsewhere | 35 | 38.5 |
| Histological
subtype | 90 |
|
|
Osteoblastic | 30 | 33.3 |
|
Chondroblastic | 30 | 33.3 |
|
Fibroblastic | 12 | 20.0 |
|
Mixed | 18 | 13.4 |
| Clinical stage | 87 |
|
|
I+IIA | 54 | 62.1 |
|
IIB/III | 33 | 37.9 |
| Distant
metastasis | 91 | 45.1 |
|
Absent | 55 | 60.4 |
|
Present | 36 | 39.6 |
In situ hybridization
miR-335 expression and subcellular localization in
osteosarcoma tissues and matched noncancerous bone tissues were
measured using in situ hybridization. Briefly, following the
manufacturer's protocol, the tissue slides were mixed with
5′-digoxigenin LNA-modified-miR-335 (Exiqon A/S, Vedbaek, Denmark)
using the IsHyb in situ Hybridization kit (BioChain
Institute Inc., Eureka Drive, Newark, CA, USA).
Immunohistochemistry analysis
Rock1 protein expression and subcellular
localization in osteosarcoma tissues and matched noncancerous bone
tissues was detected using immunohistochemical staining. Firstly,
tissue sections (4 µm thick) were incubated with the rabbit
monoclonal Rock1 antibody (dilution, 1:100; cat. no. ab134181;
Abcam, Cambridge, UK) at 4°C overnight and subsequently incubated
with biotinylated secondary antibodies (dilution, 1:1,000; cat. no.
E043201; Dako; Agilent Technologies, Inc., Santa Clara, CA, USA) at
37°C for 30 min. Samples were subsequently incubated with
streptavidin horseradish peroxidase for another 30 min (LSAB kit;
Dako; Agilent Technologies, Inc.) and stained with
3,3-diaminobenzidine. Finally, the slides were counterstained with
hematoxylin, dehydrated in a graded ethanol series (absolute ethyl
alcohol for 3 min, 95% ethanol for 3 min and 85% ethanol for 3
min), then mounted. Negative control sections were performed under
the same conditions, except without primary antibodies.
Evaluation of in situ hybridization
and immunostaining
The immunoreactivity intensity was scored according
to four values: 0) Negative staining; i) weak positive staining;
ii) moderate positive staining; and iii) strong positive staining.
The proportion of stained cells for that intensity over the total
number of tumor cells on the slide was recorded in 5% increments
from a range of 0–100. The final immunoreactive score was obtained
by multiplying the intensity score with the percentage of
positively stained cells, and ranged between 0 and 300% and
assigned with 5% increments (0, 5, 10, …300%). These scores were
used to determine the cutoff value in receiver operating
characteristic (ROC) curves to discriminate between tumors of high
miR-335 or Rock1 expression and those of low expression. The
sensitivity and specificity was plotted on ROC curves to
investigate the survival status of patients with osteosarcoma.
Statistical analysis
All data were analyzed using SPSS 17.0 (SPSS, Inc.,
Chicago, IL, USA). The Student's t-test was used to compare
the difference for data with normal distribution, while Wilcoxon
rank-sum tests or Kruskal-Wallis tests were used to compare the
difference for data with unequal variance. The association between
miR-335 and Rock1 expression and clinicopathological
characteristics in patients with osteosarcoma was evaluated using
the Pearson's χ2 test or Fisher's exact probability
test. The association between miR-335 and Rock1 expression was
assessed by Spearman's rank correlation analysis. The Kaplan-Meier
curves were plotted to demonstrate the survival difference, and the
survival probabilities were assessed using a log-rank test.
Univariate and multivariate Cox's proportional hazards regression
models were used to assess the association of potential confounding
variables with the OS or DFS. P≤0.05 was considered to indicate a
statistically significant difference.
Results
Subcellular localizations and
expression patterns of miR-335 and Rock1 in osteosarcoma
tissues
In situ hybridization and
immunohistochemistry analysis were used to confirm the subcellular
localizations and the expression patterns of miR-335 and Rock1
protein in 91 osteosarcoma samples and 47 noncancerous bone samples
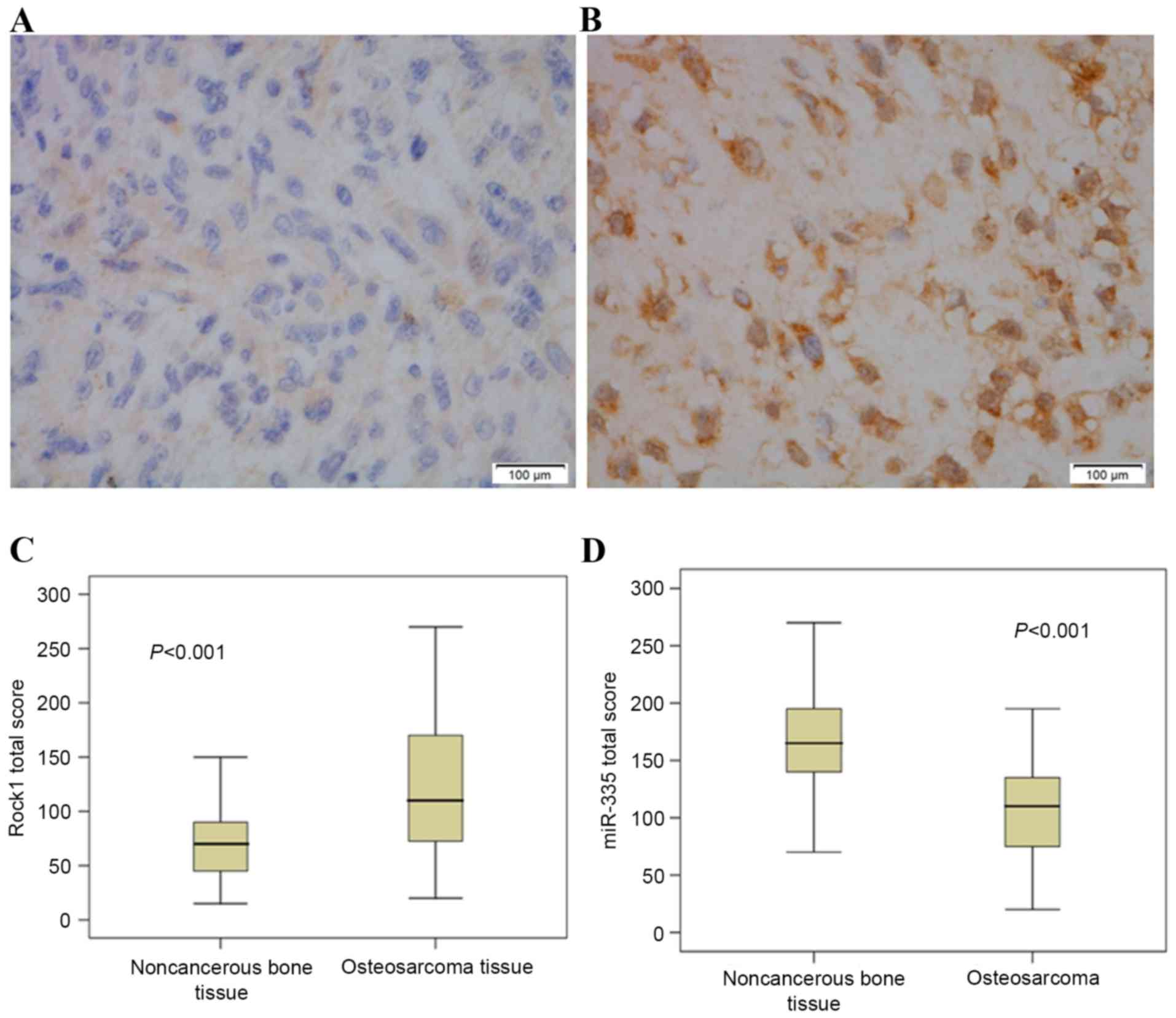
respectively. As demonstrated in Fig.
1, miR-335 and Rock1 immunopositive staining were mainly
localized in the cytoplasm. It was also observed that compared to
noncancerous bone tissues, the miR-335 expression levels were
considerably decreased in osteosarcoma tissues (P<0.001), while
Rock1 expression levels increased significantly in osteosarcoma
tissues (P<0.001).
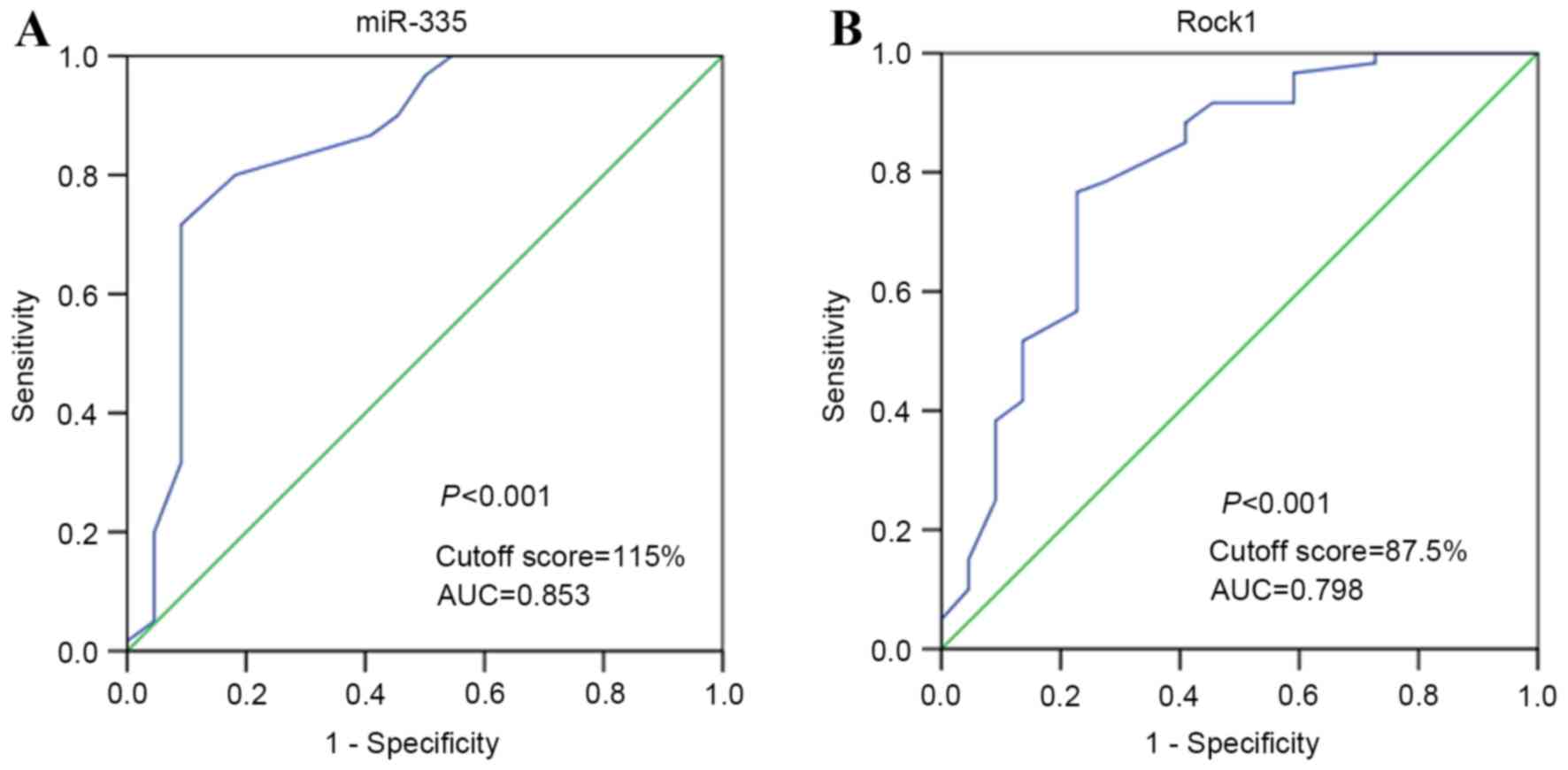
Selection of the cutoff value for
miR-335 and Rock1
ROC curve analysis was applied to determine an
optimal cutoff value for the expression of miR-335 and ROCK1 in
osteosarcoma samples. Considering the OS status, cutoff values of
115 and 87.5% were selected for the expression of miR-335 and
Rock1, respectively (Fig. 2A and B).
Tumors in which the immunohistological scores were ≥115 and
<115% were defined as those of high and low expression of
miR-335, respectively. Tumors with immunohistological scores ≥87.5
and <87.5% were defined as those with high and low expression of
Rock1, respectively.
miR-335 expression associates with
Rock1 expression in osteosarcoma tissues
In Table II, it can
be observed how miR-335 and Rock1 were expressed in 91 osteosarcoma
samples. As shown in the cutoff value used in the present study,
11/91 (12.09%) samples were of high miR-335 and high Rock1
expression, 11/91 (12.09%) were of low miR-335 and low Rock1
expression, 31/91 (34.07%) were of high miR-335 expression, but low
Rock1 expression, and 38/91 (41.76%) were of low miR-335 expression
but high Rock1 expression. There was a significant negative
correlation between miR-335 and Rock1 expression in osteosarcoma
tissues (r=−0.378, P<0.001, Table
II), according to Spearman's correlation analysis.
 | Table II.Expression of miR-335 and Rock1
proteins in 91 patients with osteosarcoma. |
Table II.
Expression of miR-335 and Rock1
proteins in 91 patients with osteosarcoma.
|
| Rock1
expression |
|
|---|
|
|
|
|
|---|
| miR-335 | High (n=55) | Low (n=36) | P-value |
|---|
| High (n=42) | 11 | 31 | <0.001 |
| Low (n=49) | 38 | 11 |
|
Association between combined
low-expression of miR-335 and high-expression of Rock1 and the
aggressive clinicopathological features of patients with
osteosarcoma
As demonstrated in Table
III, the low expression of miR-335 was significantly associated
with distant metastasis (P=0.016) and grade IIB/III osteosarcoma
(P=0.004). The high expression of Rock1 was also significantly
associated with distant metastasis (P=0.022), grade IIB/III
osteosarcoma (P=0.027) and higher tumor size (P=0.013). Table IV presents the association of
combined miR-335 and Rock1 expression with distant metastasis and
clinical stage. Out of all comparisons investigated in the present
study, tumors with a high expression of Rock1 but low expression of
miR-335 were the most strongly associated with distant metastasis
(P=0.010) and a higher clinical stage (P=0.010).
 | Table III.Association of miR-335 and Rock1
expression with the clinicopathological features of patients with
osteosarcoma. |
Table III.
Association of miR-335 and Rock1
expression with the clinicopathological features of patients with
osteosarcoma.
|
Characteristics | No. of cases | miR-335 low, n
(%) | P-value | Rock1 high, n
(%) | P-value |
|---|
| Age at diagnosis,
years |
|
| 0.746 |
| 0.346 |
|
<18 | 45 | 25 (55.6) |
| 25 (55.6) |
|
|
≥18 | 46 | 24 (52.2) |
| 30 (65.2) |
|
| Gender |
|
| 0.845 |
| 0.722 |
|
Female | 40 | 22 (55.0) |
| 25 (62.5) |
|
|
Male | 51 | 27 (52.9) |
| 30 (58.8) |
|
| Tumor size, cm |
|
| 0.113 |
| 0.013 |
|
<5 | 46 | 21 (45.7) |
| 22 (47.8) |
|
| ≥5 | 45 | 28 (62.2) |
| 33 (73.3) |
|
| Anatomic
location |
|
| 0.618 |
| 0.090 |
|
Tibia/femur | 56 | 29 (51.8) |
| 30 (53.6) |
|
|
Elsewhere | 35 | 20 (57.1) |
| 25 (71.4) |
|
| Histological
subtype |
|
| 0.067 |
| 0.510 |
|
Osteoblastic | 30 | 11 (36.7) |
| 16 (53.3) |
|
|
Chondroblastic | 30 | 19 (63.3) |
| 19 (63.3) |
|
|
Fibroblastic | 12 | 6 (50.0) |
| 6 (50.0) |
|
|
Mixed | 18 | 13 (72.2) |
| 5 (27.8) |
|
| Clinical stage |
|
| 0.004 |
| 0.027 |
|
I+IIA | 54 | 22 (40.7) |
| 28 (51.9) |
|
|
IIB/III | 33 | 24 (72.7) |
| 25 (75.8) |
|
| Distant
metastasis |
|
| 0.016 |
| 0.022 |
|
Absent | 55 | 24 (43.6) |
| 28 (50.9) |
|
|
Present | 36 | 25 (69.4) |
| 27 (75.0) |
|
 | Table IV.Association of the combination of
miR-335 and Rock1 expression with clinical stage and distant
metastasis of osteosarcoma. |
Table IV.
Association of the combination of
miR-335 and Rock1 expression with clinical stage and distant
metastasis of osteosarcoma.
|
|
| miR-335 and Rock1
expression |
|
|---|
|
|
|
|
|
|---|
| Variables | No. of cases |
miR-335-low/Rock1-high, n (%) | Both high or
low |
miR-335-high/Rock1-low, n (%) | P-value |
|---|
| Clinical stage |
|
|
|
| 0.010 |
|
I+IIA | 54 | 17 (31.5) | 16 (29.6) | 21 (38.9) |
|
|
IIB/III | 33 | 20 (60.6) | 9 (27.3) | 4 (12.1) |
|
| Distant
metastasis |
|
|
|
| 0.010 |
|
Absent | 55 | 16 (29.1) | 20 (36.4) | 19 (34.5) |
|
|
Present | 33 | 22 (61.1) | 8 (22.2) | 6 (16.7) |
|
Prediction of poor prognosis of
osteosarcoma patients using combined downregulated miR-335 and
upregulated Rock1
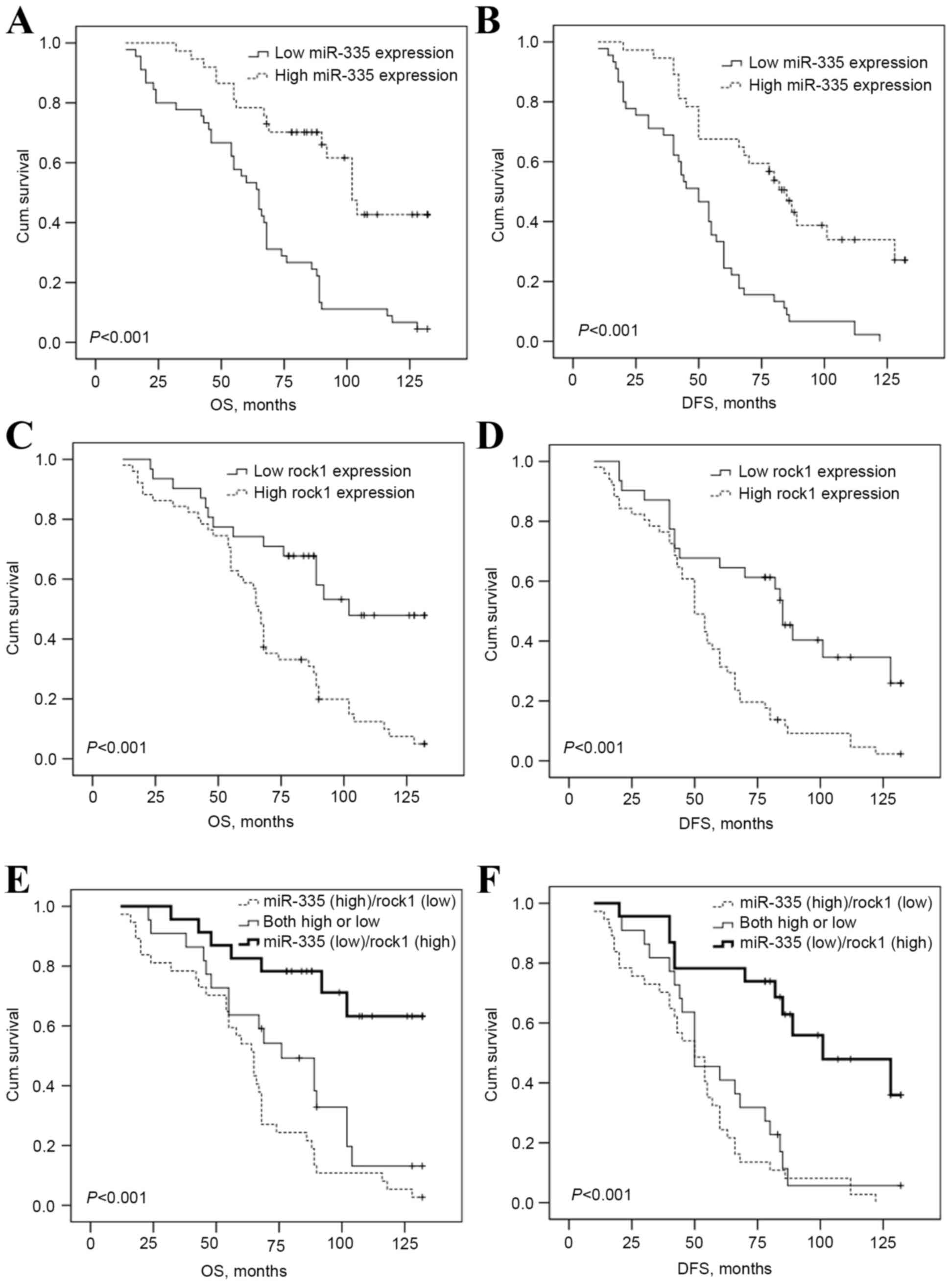
Kaplan-Meier analysis and log-rank test were used to
evaluate the association of different miR-335 and Rock1 expression
level with the OS or DFS in osteosarcoma patients. The data
demonstrates that a high expression of Rock1 was significantly
associated with shorter OS (P<0.001, Fig. 3A) and DFS (P<0.001, Fig. 3B), and the low expression of miR-335
was also evidently associated with shorter OS (P<0.001, Fig. 3C) and DFS (P<0.001, Fig. 3D). Furthermore, univariate and
multivariate Cox's proportional hazards regression models were used
to evaluate the association of potential confounding variables with
the OS or DFS.
Univariate results identified that gender (P=0.020
for OS), higher tumor size (P=0.008 for DFS; P=0.001 for OS),
higher clinical stage (P<0.001 for DFS; P<0.001 for OS),
distant metastasis (P=0.006 for DFS; P<0.001 for OS), anatomic
location (P<0.001 for DFS), miR-335 low expression (P<0.001
for DFS; P<0.001 for OS) and high Rock1 expression (P<0.001
for DFS; P=0.001 for OS), were associated with the OS and DFS in
patients with osteosarcoma (Table V).
In addition, multivariate Cox regression analysis identified that
higher clinical stage (P=0.002 for DFS; P=0.015 for OS), distant
metastasis (P=0.024 for DFS; P=0.002 for OS) and low expression of
miR-335 (P<0.001 for DFS; P=0.002 for OS) remained independent
prognostic factors for OS in patients with osteosarcoma.
Subsequently, the present study combined miR-335 and Rock1
expression into three groups: High expression of miR-335 and Rock1,
either high expression of miR-335 or Rock1, and low expression
miR-335 and Rock1 to investigate whether the combined expression of
miR-335 and Rock1 exhibited an effect on the progression and
prognosis of osteosarcoma.
 | Table V.Univariate Cox regression analysis of
OS and DFS in patients with osteosarcoma. |
Table V.
Univariate Cox regression analysis of
OS and DFS in patients with osteosarcoma.
|
| Disease-free
survival | Overall
survival |
|---|
|
|
|
|
|---|
| Category | RR (95% CI) | P-value | RR (95% CI) | P-value |
|---|
| Age, years
(≥18/<18) | 1.151
(0.714–1.714) | 0.564 | 1.172
(0.705–1.705) | 0.541 |
| Gender
(male/female) | 1.105
(0.681–1.681) | 0.686 | 0.951
(0.570–1.570) | 0.020 |
| Tumor size, cm
(≥5.0/<5.0) | 1.912
(1.184–3.184) | 0.008 | 2.326
(1.388–3.388) | 0.001 |
| Clinical stage
(IB/III/I+IIA) | 4.031
(2.377–6.377) | <0.001 | 4.098
(2.396–7.396) | <0.001 |
| Histological
subtype (mixed/fibroblastic/ | 1.052
(0.870–1.870) | 0.603 | 1.051
(0.858–1.858) | 0.630 |
|
(Mixed/fibroblastic/chondroblastic/osteoblastic) |
|
|
|
|
| Distant metastasis
(present/absent) | 1.991
(1.216–3.216) | 0.006 | 3.450
(1.999–5.999) | <0.001 |
| Anatomic location
(elsewhere/tibia/femur) | 2.513
(1.63–3.63) | <0.001 | 1.539
(0.919–2.919) | 0.101 |
| miR-335 expression
(negative/positive) | 0.310
(0.184–0.184) | <0.001 | 0.287
(0.163–0.163) | <0.001 |
| Rock1 expression
(positive/negative) | 2.633
(1.528–4.528) | <0.001 | 2.887
(1.579–5.579) | 0.001 |
| miR-335/Rock1
expression | 2.542
(1.553–4.553) | <0.001 | 2.931
(1.735–4.735) | <0.001 |
| (miR-335-low and
Rock1-high/others) |
|
|
|
|
Kaplan-Meier analysis and log-rank tests (Fig. 3E and F) revealed that the OS and DFS
of patients with high expression of Rock1 and low expression of
miR-335 was considerably shorter compared with those with high or
low expression of miR-335 and Rock1, and those with low Rock1
expression and high miR-335 expression (P<0.001 for the two).
Results of univariate and multivariate Cox proportional hazards
regression analysis are presented in Table VI. The findings demonstrate that
combined high Rock1 expression and low miR-335 expression was
considered as an independent prognostic factor for shorter OS
(P=0.050) and DFS (P=0.021) in patients with osteosarcoma.
 | Table VI.Multivariate cox regression analysis
of OS and DFS in osteosarcoma patients. |
Table VI.
Multivariate cox regression analysis
of OS and DFS in osteosarcoma patients.
|
| Disease-free
survival | Overall
survival |
|---|
|
|
|
|
|---|
| Category | RR (95% CI) | P-value | RR (95% CI) | P-value |
|---|
| Gender
(male/female) | – | – | 0.748
(0.428–1.428) | 0.307 |
| Tumor size, cm
(≥5.0/<5.0) | 1.324
(0.776–2.776) | 0.314 | 1.810
(0.964–3.964) | 0.065 |
| Clinical stage
(IB/III/I+IIA) | 2.607
(1.427–4.427) | 0.002 | 2.243
(1.169–4.169) | 0.015 |
| Distant metastasis
(present/absent) | 1.956
(1.091–3.091) | 0.024 | 3.056
(1.514–6.514) | 0.002 |
| Anatomic location
(elsewhere/tibia/femur) | 1.443
(0.855–2.855) | 0.169 | 1.119
(0.637–1.637) | 0.696 |
| miR-335 expression
(negative/positive) | 0.173
(0.065–0.065) | <0.001 | 0.170
(0.056–0.056) | 0.002 |
| Rock1 expression
(positive/negative) | 2.182
(0.917–5.917) | 0.078 | 2.469
(0.903–6.903) | 0.078 |
| miR-335/Rock1
expression | 0.243
(0.073–0.073) | 0.021 | 0.253
(0.064–0.064) | 0.050 |
| (miR-335-low and
Rock1-high/others) |
|
|
|
|
Discussion
The present study used a number of clinical samples
in order to attempt to detect the combined expression of miR-335
and its target gene Rock1 in osteosarcoma tissues, to analyze its
association with osteosarcoma clinicopathological characteristic
and to assess the correlation of combined miR-335 and Rock1
expression with the prognosis of patients with osteosarcoma. The
present findings provide evidence that the combined downregulation
of miR-335 and upregulation of Rock1 may be associated with the
progression and undesirable prognosis in osteosarcoma.
Recently, numerous studies have reported that
miRNAs, which target various oncogenes or anti-oncogenes, may
perform a diverse role in tumorigenesis (26–28).
Therefore, miRNAs are suggested as a type of prognostic biomarker
and potential therapeutic site for tumor treatment. As a transcript
of genomic region chromosome 7q32.2, the function of miR-335 has
been widely reported (29). It was
revealed that miR-335 functions as a tumor suppressor in malignant
tumors and that the loss of miR-335 expression may lead to the
progression of aggressive tumor phenotypes. Wang et al
(30) reported that miR-335 could
directly suppress B-cell lymphoma 2 and lead to inhibition of the
proliferation and invasion in renal cell carcinoma cells. Gao et
al (18) reported that miR-335
could target the regulatory oncoprotein c-Met and suppress the
migration of breast cancer cells. However, miR-335 also functions
as a tumor oncogene in certain types of cancer. Shu et al
(31) identified that targeting
oncogenic miR-335 suppressed the growth and invasion of malignant
astrocytoma cells. Shi et al (32) reported that miR-335 directly targeted
retinoblastoma 1 and promoted meningiomas cellular proliferation.
In a previous study, we revealed that miR-335 could target Rock1
and cause the suppression of migration and invasion in osteosarcoma
cells (22). Our data proved that
there is a marked decline in the expression levels of miR-335 in
human osteosarcoma tissues compared with noncancerous bone tissues,
and that there is also a close correlation of downregulated miR-335
expression with osteosarcoma progression, and even adverse outcomes
in patients with osteosarcoma, which increases evidence that
miR-335 may be a tumor suppressor in osteosarcoma.
Rock consists of two isoforms, Rock1 and Rock2
(23), and is the key kinase of Rho
signaling (22). Activated Rocks
promote the reassembly of focal contacts and the formation of
stress-fibers while interacting with the actin cytoskeleton
(33). Subsequently, this regulates
various cellular processes, including cellular proliferation,
assembly of focal adhesion, fomation of invadopodium and actomyosin
contractility (34). An increasing
number of evidence demonstrates the key role Rocks perform in
oncogenesis, particularly regarding Rock1. Oellers et al
(35) identified that Rock1 was
essential for glioma cell migration on myelinated axons. Kamai
et al (36) revealed a
prominent association of the Rho/Rock pathway with the
invasion/migration of bladder cancer. Akagi et al (37) demonstrated that Rock1 could be a novel
prognostic marker in vulvar cancer. In osteosarcoma, Liu et
al (38) revealed that the
downregulation of Rock1 could suppress proliferation but promote
apoptosis in osteosarcoma cells. Cai et al (23) identified that the high expression of
Rock1 was significantly associated with the metastasis of
osteosarcoma and the response to preoperative chemotherapy. The
present study identified a significant elevation of Rock1 but
decreased expression of miR-335 in human osteosarcoma tissues.
Evidence also demonstrated that there was a close correlation of
the upregulated Rock1 expression with osteosarcoma progression and
adverse outcomes in patients with osteosarcoma, which additionally
suggests that Rock1 may be an oncogene in osteosarcoma.
However, whether the combined expression of miR-335
and Rock1 is applicable for use in the clinical setting remains
unknown in osteosarcoma. The findings of the present study
demonstrate that the combined high expression of Rock1 and low
expression of miR-335 was positively associated with distant
metastasis, higher clinical stage and worse OS and DFS. Therefore,
the prediction of advanced tumor progression and unfavorable
prognosis can be determined by the downregulation of miR-335 and
the upregulation of its target gene, Rock1, for patients with
osteosarcoma.
In summary, miR-335 was identified to be
downregulated, while Rock1 was upregulated in patients with
osteosarcoma. Additionally, the combined high expression of Rock1
and low expression of miR-335 was positively correlated with
distant metastasis, higher clinical stage, and poor OS and DFS. In
addition, multivariate survival analysis demonstrated that the
combined low expression of miR-335 and high expression of Rock1
were independent prognostic factors for OS and DFS. Finally, data
in the present study provided evidence that the combined
downregulation of miR-335 and upregulation of Rock1 may be
associated with tumor progression and poor prognosis in patients
with osteosarcoma.
Acknowledgements
The present study was supported by grants from the
National Natural Science Foundation of China (grant no. 81502333),
the China Postdoctoral Science Foundation (grant no., 2016M591437),
the PhD Start-up Research Foundation of Liaoning Province (grant
no. 201601225), the Education Fund Item of Liaoning Province (grant
no., L2014418) and the SMC General Science Foundation (grant no.
20151002).
References
|
1
|
Gill J, Ahluwalia MK, Geller D and Gorlick
R: New targets and approaches in osteosarcoma. Pharmacol Ther.
137:89–99. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ta HT, Dass CR, Choong PF and Dunstan DE:
Osteosarcoma treatment: State of the art. Cancer Metastasis Rev.
28:247–263. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen D, Zhang YJ, Zhu KW and Wang WC: A
systematic review of vascular endothelial growth factor expression
as a biomarker of prognosis in patients with osteosarcoma. Tumour
Biol. 34:1895–1899. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fu HL, Shao L, Wang Q, Jia T, Li M and
Yang DP: A systematic review of p53 as a biomarker of survival in
patients with osteosarcoma. Tumour Biol. 34:3817–3821. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hu F, Shang XF, Wang W, Jiang W, Fang C,
Tan D and Zhou HC: High-level expression of periostin is
significantly correlated with tumour angiogenesis and poor
prognosis in osteosarcoma. Int J Exp Pathol. 97:86–92. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liang S, Ren Z, Han X, Yang J, Shan L, Li
L, Wang B, Zhang Q, Mu T, Chen K, et al: PLA2G16 expression in
human osteosarcoma is associated with pulmonary metastasis and poor
prognosis. PloS One. 10:e01272362015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu Y, Wu Y, Gu S, Sun Z, Rui Y, Wang J,
Lu Y, Li H, Xu K and Sheng P: Prognostic role of CD44 expression in
osteosarcoma: Evidence from six studies. Diagn Pathol. 9:1402014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yao D, Cai GH, Chen J, Ling R, Wu SX and
Li YP: Prognostic value of p53 alterations in human osteosarcoma: A
meta analysis. Int J Clin Exp Pathol. 7:6725–6733. 2014.PubMed/NCBI
|
|
9
|
Liu Y, Wu Y, Gu S, Sun Z, Rui Y, Wang J,
Lu Y, Li H, Xu K and Sheng P: Prognostic role of CD44 expression in
osteosarcoma: Evidence from six studies. Diagn Pathol. 9:1402014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Calin GA and Croce CM: MicroRNA signatures
in human cancers. Nat Rev Cancer. 6:857–866. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kumar MS, Lu J, Mercer KL, Golub TR and
Jacks T: Impaired microRNA processing enhances cellular
transformation and tumorigenesis. Nat Genet. 39:673–677. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pan W, Wang H, Jianwei R and Ye Z:
MicroRNA-27a promotes proliferation, migration and invasion by
targeting MAP2K4 in human osteosarcoma cells. Cell Physiol Biochem.
33:402–412. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhao F, Lv J, Gan H, Li Y, Wang R, Zhang
H, Wu Q and Chen Y: MiRNA profile of osteosarcoma with CD117 and
stro-1 expression: miR-1247 functions as an onco-miRNA by targeting
MAP3K9. Int J Clin Exp Pathol. 8:1451–1458. 2015.PubMed/NCBI
|
|
14
|
Jin J, Cai L, Liu ZM and Zhou XS:
miRNA-218 inhibits osteosarcoma cell migration and invasion by
down-regulating of TIAM1, MMP2 and MMP9. Asian Pac J Cancer Prev.
14:3681–3684. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sun XH, Geng XL, Zhang J and Zhang C:
miRNA-646 suppresses osteosarcoma cell metastasis by downregulating
fibroblast growth factor 2 (FGF2). Tumour Biol. 36:2127–2134. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tian X, Zhang J, Yan L, Dong JM and Guo Q:
MiRNA-15a inhibits proliferation, migration and invasion by
targeting TNFAIP1 in human osteosarcoma cells. Int J Clin Exp
Pathol. 8:6442–6449. 2015.PubMed/NCBI
|
|
17
|
Cao J, Cai J, Huang D, Han Q, Yang Q, Li
T, Ding H and Wang Z: miR-335 represents an invasion suppressor
gene in ovarian cancer by targeting Bcl-w. Oncol Rep. 30:701–706.
2013.PubMed/NCBI
|
|
18
|
Gao L, Yang Y, Xu H, Liu R, Li D, Hong H,
Qin M and Wang Y: MiR-335 functions as a tumor suppressor in
pancreatic cancer by targeting OCT4. Tumour Biol. 35:8309–8318.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gao Y, Zeng F, Wu JY, Li HY, Fan JJ, Mai
L, Zhang J, Ma DM, Li Y and Song FZ: MiR-335 inhibits migration of
breast cancer cells through targeting oncoprotein c-Met. Tumour
Biol. 36:2875–2883. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gong M, Ma J, Guillemette R, Zhou M, Yang
Y, Yang Y, Hock JM and Yu X: miR-335 inhibits small cell lung
cancer bone metastases via IGF-IR and RANKL pathways. Mol Cancer
Res. 12:101–110. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang K, Chen X, Zhan Y, Jiang W, Liu X,
Wang X and Wu B: miR-335 inhibits the proliferation and invasion of
clear cell renal cell carcinoma cells through direct suppression of
BCL-W. Tumour Biol. 36:6875–6882. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang Y, Zhao W and Fu Q: miR-335
suppresses migration and invasion by targeting ROCK1 in
osteosarcoma cells. Mol Cell Biochem. 384:105–111. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cai H, Lin L, Tang M and Wang Z: Combined
microRNA-340 and ROCK1 mRNA profiling predicts tumor progression
and prognosis in pediatric osteosarcoma. Int J Mol Sci. 15:560–573.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yu L, Zhang J, Guo X, Li Z and Zhang P:
MicroRNA-224 upregulation and AKT activation synergistically
predict poor prognosis in patients with hepatocellular carcinoma.
Cancer Epidemiol. 38:408–413. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Spiessl B, Scheibe O and Wagner G: Soft
tissue sarcomas. In: International Union Against Cancer (UICC)
TNM-AtlasIllustrated Guide to the Classification of Malignant
Tumours. Springer-Verlag; Berlin: pp. 170–172
|
|
26
|
Liu P, Zhang H, Liang X, Ma H, Luan F,
Wang B, Bai F, Gao L and Ma C: HBV preS2 promotes the expression of
TAZ via miRNA-338-3p to enhance the tumorigenesis of hepatocellular
carcinoma. Oncotarget. 6:29048–29059. 2015.PubMed/NCBI
|
|
27
|
Santhi WS, Prathibha R, Charles S, Anurup
KG, Reshmi G, Ramachandran S, Jissa VT, Sebastian P and Pillai
Radhakrishna M: Oncogenic microRNAs as biomarkers of oral
tumorigenesis and minimal residual disease. Oral Oncol. 49:567–575.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xu C, Zeng Q, Xu W, Jiao L, Chen Y, Zhang
Z, Wu C, Jin T, Pan A, Wei R, et al: miRNA-100 inhibits human
bladder urothelial carcinogenesis by directly targeting mTOR. Mol
Cancer Ther. 12:207–219. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shu M, Zhou Y, Zhu W, Zhang H, Wu S, Chen
J and Yan G: MicroRNA 335 is required for differentiation of
malignant glioma cells induced by activation of cAMP/protein kinase
A pathway. Mol Pharmacol. 81:292–298. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang K, Chen X, Zhan Y, Jiang W, Liu X,
Wang X and Wu B: miR-335 inhibits the proliferation and invasion of
clear cell renal cell carcinoma cells through direct suppression of
BCL-W. Tumour Biol. 36:6875–6882. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shu M, Zheng X, Wu S, Lu H, Leng T, Zhu W,
Zhou Y, Ou Y, Lin X, Lin Y, et al: Targeting oncogenic miR-335
inhibits growth and invasion of malignant astrocytoma cells. Mol
Cancer. 10:592011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shi L, Jiang D, Sun G, Wan Y, Zhang S,
Zeng Y, Pan T and Wang Z: miR-335 promotes cell proliferation by
directly targeting Rb1 in meningiomas. J Neurooncol. 110:155–162.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Schofield AV and Bernard O: Rho-associated
coiled-coil kinase (ROCK) signaling and disease. Crit Rev Biochem
Mol Biol. 48:301–316. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Patel RA, Liu Y, Wang B, Li R and Sebti
SM: Identification of novel ROCK inhibitors with anti-migratory and
anti-invasive activities. Oncogene. 33:550–555. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Oellers P, Schröer U, Senner V, Paulus W
and Thanos S: ROCKs are expressed in brain tumors and are required
for glioma-cell migration on myelinated axons. Glia. 57:499–509.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kamai T, Tsujii T, Arai K, Takagi K, Asami
H, Ito Y and Oshima H: Significant association of Rho/ROCK pathway
with invasion and metastasis of bladder cancer. Clin Cancer Res.
9:2632–2641. 2003.PubMed/NCBI
|
|
37
|
Akagi EM, Lavorato-Rocha AM, Bde Maia M,
Rodrigues IS, Carvalho KC, Stiepcich MM, Baiocchi G, Sato-Kuwabara
Y, Rogatto SR, Soares FA and Rocha RM: ROCK1 as a novel prognostic
marker in vulvar cancer. BMC cancer. 14:8222014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Liu X, Choy E, Hornicek FJ, Yang S, Yang
C, Harmon D, Mankin H and Duan Z: ROCK1 as a potential therapeutic
target in osteosarcoma. J Orthop Res. 29:1259–1266. 2011.
View Article : Google Scholar : PubMed/NCBI
|