Introduction
Prostate cancer is a primary public health problem
in Western countries, according to the 2014 cancer statistics, and
represents the most commonly diagnosed type of cancer and second
leading cause of cancer-associated male death in the USA (1). It is well-known that primary prostate
tumors respond poorly to high dosages of chemotherapeutic agents or
radiotherapy (2). Testosterone is the
major circulating androgen, therefore patients with advanced
prostate cancer often undergo androgen-deprivation therapy (ADT)
that directly decreases the endogenous testosterone level through a
radical prostatectomy and/or inhibition of the androgen receptor
activity using pharmacological inhibitors (3). However, therapeutic benefits from ADT
are frequently short-lived because castration-resistant and
incurable tumors may emerge in patients with prostate cancer
(4).
Numerous chemical agents or nutritional supplements
have been reported to exhibit significant prostate
chemopreventative activities in experimental animal models
(5). Therefore, a number of
population-based studies were performed to examine the potential
prostate chemopreventative effects of selected natural or synthetic
compounds (6). In contrast with
initial expectations, however, none of the compounds produced
meaningful chemopreventative effects that were able to be
translated into the clinical setting (7), suggesting that the identification of
novel medications that were able to inhibit the growth of prostate
cancer cells remains of clinical importance. The aim of the present
study was to identify novel medicinal plant extracts that may exert
inhibitory effects on the growth of prostate cancer, and an
acetonitrile extract of Salvia miltiorrhiza Radix was
demonstrated to exhibit marked suppressive effects on the growth of
prostate cancer cells.
Materials and methods
Cell culture, chemicals and
reagents
All traditional medicinal plant extracts used in the
present study were purchased from the Korean Plant Extract Bank
(Ochang, South Korea). RPMI-1640 medium, heat-inactivated fetal
bovine serum (FBS), penicillin/streptomycin and PBS were purchased
from Welgene (Gyeongsan, South Korea). PC-3, LNCaP and DU-145
prostate cancer cells were purchased from the Korean Cell Line Bank
(Seoul, South Korea) and cultured in RPMI-1640 medium, containing
10% heat-inactivated FBS and 1X penicillin and streptomycin at 37°C
in a humidified 5% CO2 incubator. Dimethyl sulfoxide
(DMSO), N-acetylcysteine (NAC), bromodeoxyuridine (BrdU) and
horseradish peroxidase (HRP)-conjugated secondary antibodies
(catalog nos. sc-2031 and sc-2054) were purchased from Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA). Paraformaldehyde, the
bicinchoninic acid (BCA) protein assay kit and polyvinylidene
fluoride (PVDF) membranes were purchased from EMD Millipore
(Billerica, MA, USA). The DeadEnd™ fluorimetric terminal
deoxynucleotidyltransferase-mediated dUTP nick end labeling (TUNEL)
system kit was purchased from Promega Corporation (Madison, WI,
USA). Propidium iodide (PI), trypan blue solution,
2′,7′-dichlorofluorescin diacetate (DCF-DA) and antibodies against
BrdU (catalog no. sc-32323), 8′-hydroxyguanosine (8-OH-G) and
β-actin were purchased from Sigma-Aldrich; Merck Millipore
(Darmstadt, Germany). All other primary antibodies related to cell
cycle arrest and apoptosis analysis [Cell Cycle Regulation Sampler
kit (catalog no. 9932) or Apoptosis Antibody Sampler kit (catalog
no. 9915)] were purchased from Cell Signaling Technology, Inc.
(Danvers, MA, USA). Fluorescein isothiocyanate (FITC)-conjugated
secondary antibody was purchased from Jackson ImmunoResearch
Laboratories, Inc. (West Grove, PA, USA). Matrigel™ basement
membrane matrix was purchased from BD Biosciences (Franklin Lakes,
NJ, USA).
MTT assay
PC-3 cells (3×104 cells/100 µl/well) were
plated in 96-well culture plates. Following incubation at 37°C in a
humidified 5% CO2 incubator with separate acetonitrile
and water extracts (data not shown) of 400 traditional medicinal
plants at a concentration of 20 µg/ml for 24 h, cells were mixed
with 50 µl MTT stock solution (2 mg/ml) for 4 h at 37°C. PC-3 cells
were washed with 1X PBS and lysed with 50 µl DMSO, followed by
spectrophotometric measurement at 540 nm. The percentage of viable
cells was determined in comparison with the control group. Cells
were also pretreated with 10 µM NAC for 24 h.
Trypan blue exclusion assay
LNCaP, DU-145 and PC-3 prostate cancer cells
(3×105 cells/1 ml/well in quadruplicate) were plated in
6-well culture plates and exposed to various concentrations (5, 20
and 100 µg/ml) of the acetonitrile extract of S.
miltiorrhiza Radix for 24 or 48 h. Following washing with 1X
PBS, cells were harvested by centrifugation at 800 × g for 5 min at
room temperature, and stained with 0.4% trypan blue solution for 3
min at room temperature. The number of viable cells was determined
using a light microscope and a hemocytometer.
BrdU incorporation assay
PC-3 cells (7.5×104 cells on a cover
glass) were exposed to BrdU (10 µM) alone or in combination with
the acetonitrile extract of S. miltiorrhiza Radix (20 µg/ml)
for 6 or 12 h. Following fixation with 4% paraformaldehyde for 15
min at room temperature, cells were washed once with 1X
immunocytochemistry washing buffer (0.1% Triton X-100 and 1% horse
serum in PBS). Cells were then hybridized with primary antibody
against BrdU (1:400 dilution) overnight at 4°C, followed by
hybridization at 4°C with an FITC-conjugated secondary antibody
(1:1,000 dilution) for 1 h. The final fluorescent image was
captured using a C2 confocal microscope (Nikon Korea, Seoul, South
Korea).
TUNEL assay
PC-3 cells (7.5×104 cells on a coverslip)
were exposed to the acetonitrile extract of S. miltiorrhiza
Radix (20 µg/ml) for 6 h, and a TUNEL assay was conducted using the
DeadEnd™ fluorimetric TUNEL system kit, according to the
manufacturer's protocol.
Measurement of cell cycle arrest and
intracellular reactive oxygen species (ROS) levels by
fluorescence-activated cell sorting (FACS)
Following exposure to the acetonitrile extract of
S. miltiorrhiza Radix (20 µg/ml) for 24, 48 or 72 h, PC-3
cells were washed with 1X PBS and harvested. Equal numbers of cells
(1×106/group) were dispensed into glass tubes and fixed
for 1 h at 37°C with a solution containing 1X PBS and 70% ethanol.
Following washing with 1X PBS, cells were mixed with PI (20 µg/ml)
or DCF-DA (1 µM), and the cell cycle changes and intracellular ROS
levels were measured using a FACSCalibur flow cytometer and
CellQuest Pro software (BD Biosciences).
Western blot analysis
Following exposure to the acetonitrile extract of
S. miltiorrhiza Radix (20 µg/ml) alone or in combination
with 10 µM NAC for >48 h, PC-3 cells were collected by scraper
and centrifugation at 10,000 × g at room temperature. Cells were
then lysed in 200 µl radioimmunoprecipitation assay buffer (50 mM
Tris-HCl (pH 8.0), 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate
and protease inhibitor cocktail (catalog no. P3100; GenDepot,
Seoul, Korea)] on ice for 1 h. Cell lysates were collected and
protein concentrations were determined using the BCA protein assay
kit (catalog no. 23227; Pierce; Thermo Fisher Scientific, Inc.
Waltham, MA, USA), according to the manufacturer's protocol. Equal
amounts (30 µg) of cell lysates were resolved using 8–12% SDS-PAGE
and transferred onto PVDF membranes. The membranes were incubated
in blocking buffer [5% skimmed milk in PBST (1X PBS and 0.1%
Tween-20)] for 1 h at 4°C and hybridized with the aforementioned
primary antibodies (1:1,000 dilution) in 1X PBS containing 3%
bovine serum albumin (BSA; GenDepot, Katy, TX, USA) or 3% skimmed
milk overnight at 4°C. Following washing three times with 1X PBST
for 30 min, the membrane was hybridized with anti-mouse and
anti-rabbit HRP-conjugated secondary antibodies (1:5,000 dilution)
for 1 h at room temperature and washed three times with 1X PBST
solution for 30 min. The membrane was visualized using an enhanced
chemiluminescence detection system (Pierce; Thermo Fisher
Scientific, Inc.) according to the manufacturer's instructions.
β-actin was used as a loading control.
Measurement of 8-OH-G levels
In order to measure changes in 8-OH-G levels, PC-3
cells (75,000 cells/well in 500 µl media) grown on coverslips were
treated with the acetonitrile extract of S. miltiorrhiza
Radix (20 µg/ml) for 6 h alone or in combination with 1 mM NAC, and
incubated with blocking serum (1% BSA) for 30 min at room
temperature. Following washing three times with 1X PBS, cells were
hybridized with primary antibody against 8-OH-G (1:400 dilution)
overnight at 4°C. Following washing three times with 1X PBS, the
coverslips were probed with FITC-conjugated rabbit secondary
antibody (1:1,000 dilution) at room temperature. The fluorescent
images were captured using a C2 confocal microscope.
PC-3 xenograft study
A total of 32 6-week-old, male, immunodeficient mice
(negative control group, 16 mice with a mean weight of 19.9 g; and
for oral administration, 16 mice with a mean weight of 19.7 g) were
purchased from Daehan Biolink Co., Ltd. (Eumseong, South Korea).
Animals were housed in sterile filter-capped microisolator cages
under a 12 h light/12 h dark cycle, and provided with water and an
AIN-76 diet ad libitum. After 1 week of acclimation,
individual mice were subcutaneously injected into the right flank
with PC-3 cells (2×106 cells/0.2 ml suspension medium),
which were previously suspended with 50% Matrigel™ in ice-cold
RPMI-1640 medium. The acetonitrile extract of S.
miltiorrhiza Radix (100 mg/kg in PBS) was administered by oral
gavage for 6 weeks, and the incidence and size of tumors were
measured using a caliper at the end of each week. Tumor volume (V)
was calculated as V=(LxW2)x0.52, where L is the length
and W is the width of a xenograft during the course of the
experiment. At sacrifice by asphyxiation with CO2,
tumors were excised and weighed. All animal experiments were
performed under an Institutional Animal Care and Use
Committee-approved protocol (IACUC-2013-003) from Dongguk
University (Seoul, South Korea).
Statistical analysis
Statistical analysis was performed by Student's
t-test using Microsoft Excel 2013 (Microsoft Corporation, Redmond,
WA, USA). Results are presented as the mean ± standard deviation.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Acetonitrile extract of S.
miltiorrhiza Radix exhibits significant suppressive effects on the
growth of prostate cancer cells
The aim of the present study was to identify novel
traditional medicinal plant extract(s) that exhibit marked
inhibitory effects on the growth of prostate cancer cells. PC-3
cells were exposed to 400 medicinal plant extracts, prepared using
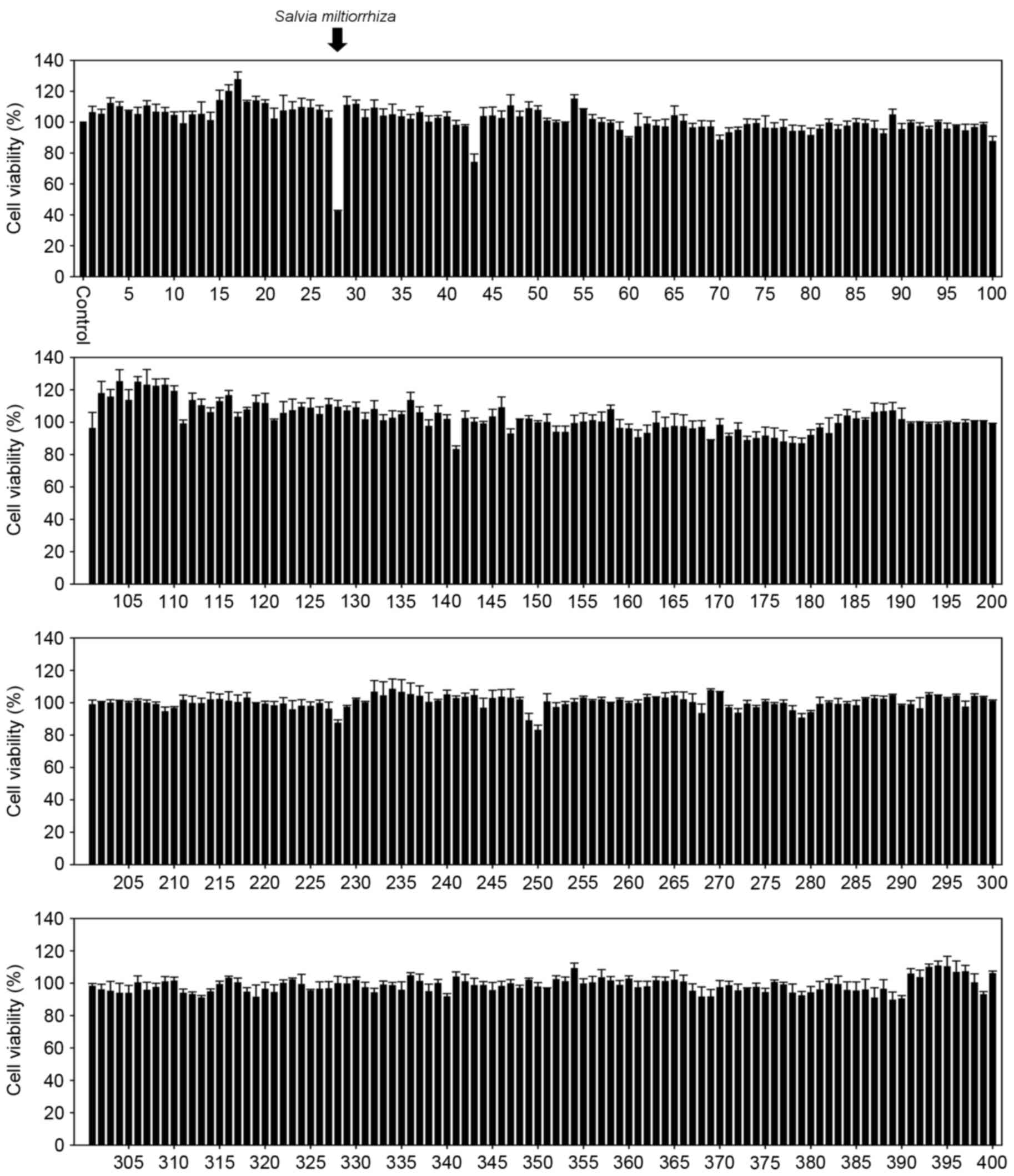
water or acetonitrile, at a concentration of 20 µg/ml for 24 h, and
an MTT assay was performed to measure the cell viability. The
results demonstrated that, whereas none of the water extracts of
traditional medicinal plants affected the growth of PC-3 cells
(data not shown), the acetonitrile extract of S.
miltiorrhiza Radix exerted a marked inhibitory effect on the
growth of PC-3 cells (Fig. 1). In
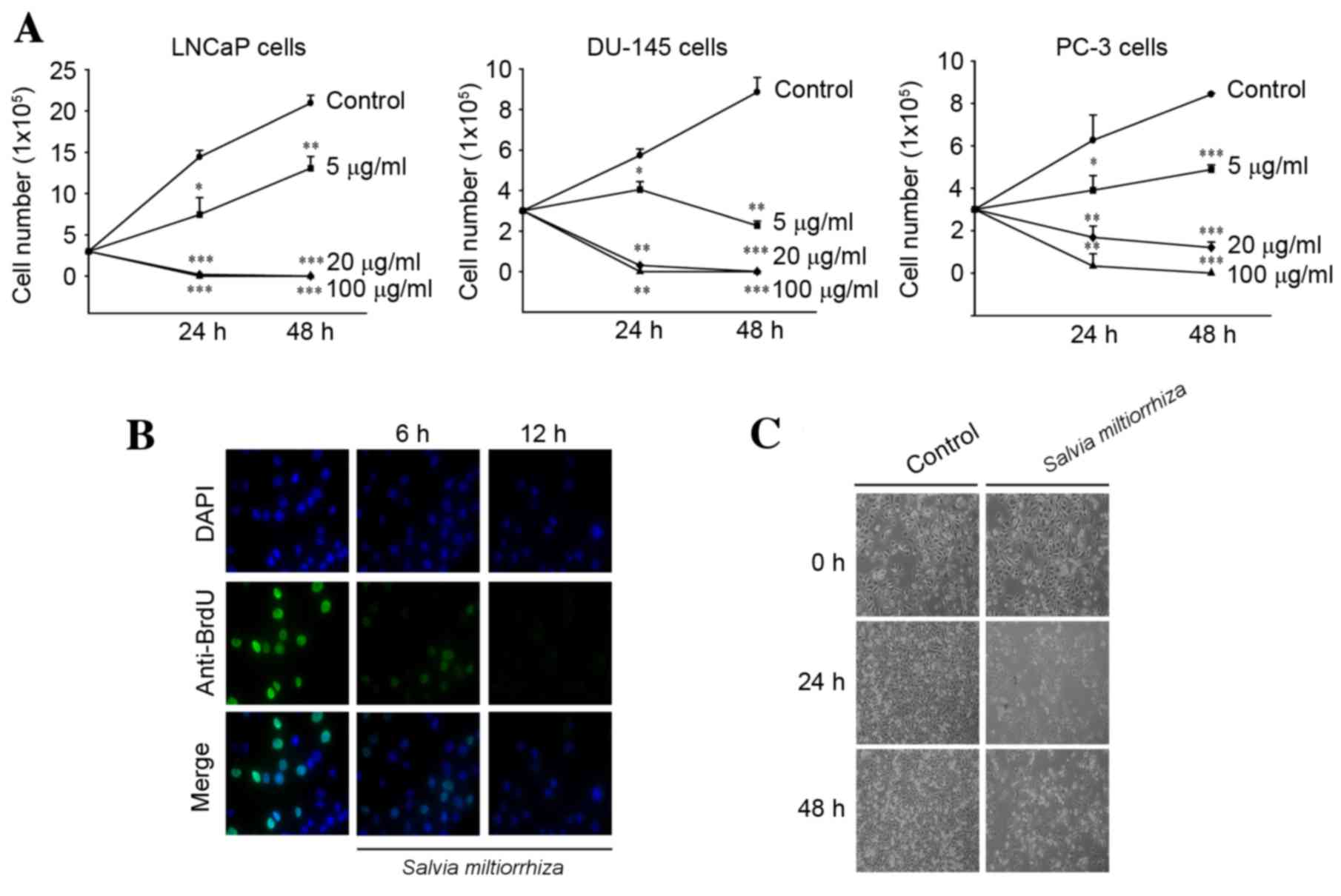
order to acquire the exact cell viability, various concentrations
of the acetonitrile extract of S. miltiorrhiza Radix were
exposed to three prostate cancer cell lines (LNCaP, DU-145 and PC-3
cells) and a trypan blue exclusion assay was performed. The results
demonstrated that the acetonitrile extract of S.
miltiorrhiza Radix significantly inhibited the growth of all
three cell lines in a dose- and time-dependent manner compared with
the untreated control cells (P<0.05; Fig. 2A). In addition, exposure to the
acetonitrile extract of S. miltiorrhiza Radix inhibited BrdU
incorporation (Fig. 2B) and elicited
marked cell death (Fig. 2C) in PC-3
cells compared with the control. These results indicate that the
acetonitrile extract of S. miltiorrhiza Radix exhibited
inhibitory effects on the growth of prostate cancer cells.
Acetonitrile extract of S.
miltiorrhiza Radix induces cell cycle arrest and apoptosis in PC-3
cells
To determine whether the acetonitrile extract of
S. miltiorrhiza Radix may induce cell cycle arrest, PC-3
cells were exposed to the acetonitrile extract of S.
miltiorrhiza Radix for 24, 48 and 72 h, and cell cycle changes
were assessed using FACS analysis. It was observed that the
acetonitrile extract of S. miltiorrhiza Radix significantly
induced cell cycle arrest at G1/S phase compared with the untreated
control (P<0.01; Fig. 3A). Western
blot analysis demonstrated that the acetonitrile extract of S.
miltiorrhiza Radix increased the expression of p21 protein and
decreased cyclin-dependent kinase 2 (CDK2), CDK4 and cyclin D1
protein levels (Fig. 3B). These
results suggest that the induction of cell cycle arrest at G1/S
phase by the acetonitrile extract of S. miltiorrhiza Radix
may be attributable to changes in the levels of G1/S cell cycle
regulator proteins.
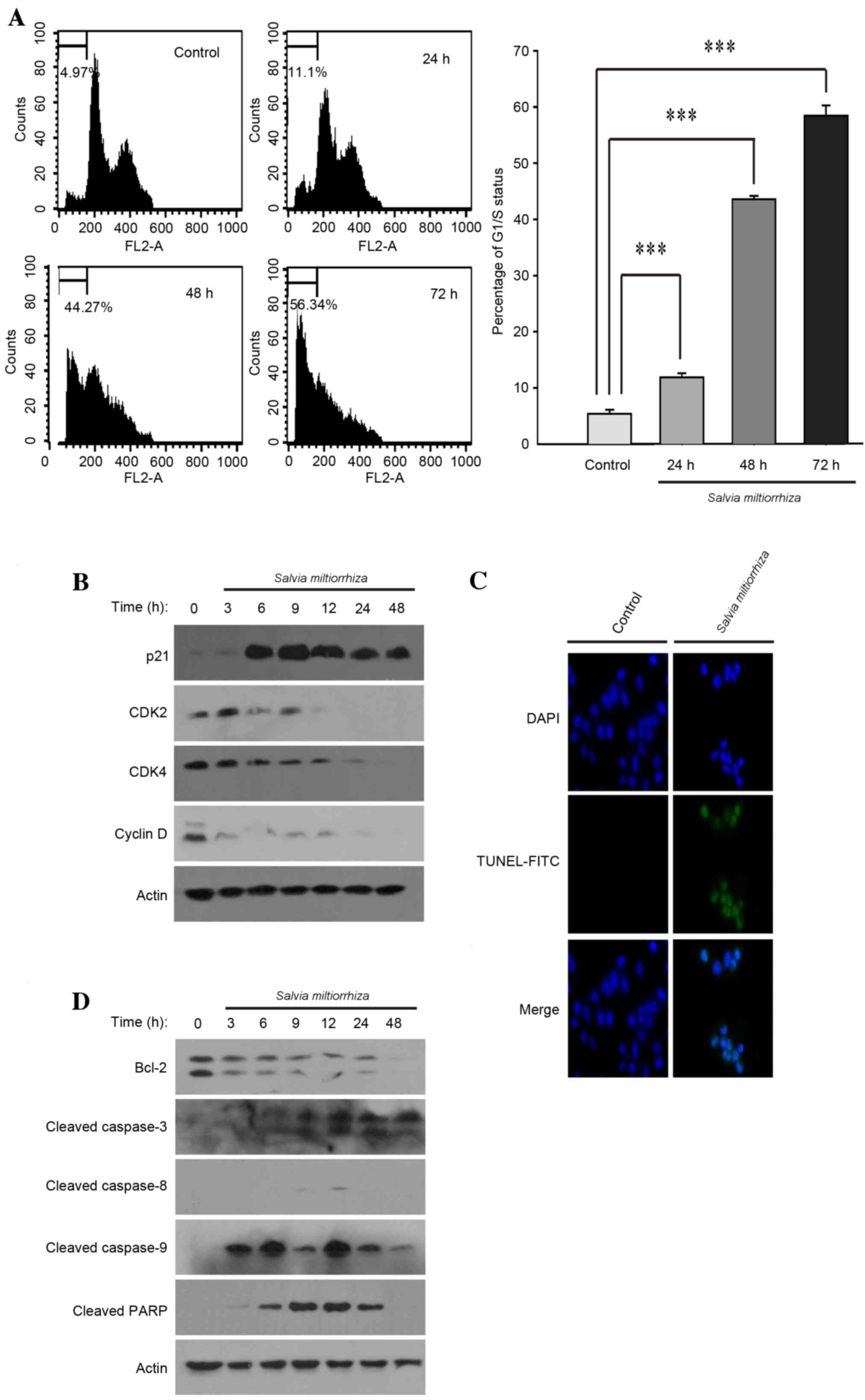 | Figure 3.Acetonitrile extract of Salvia
miltiorrhiza Radix induces cell cycle arrest and apoptosis in
PC-3 cells. (A) PC-3 cells were exposed to the acetonitrile extract
of S. miltiorrhiza Radix and the resulting cell cycle
changes were monitored using fluorescence-activated cell sorting
analysis (left panels). The experiments were performed in
triplicate and the percentage of G1/S cell cycle arrest was
analyzed and depicted (right panel). ***P<0.001 vs. control. (B)
PC-3 cells were exposed to the acetonitrile extract of S.
miltiorrhiza Radix and western blot analysis was performed
using polyclonal antibodies against G1/S cell cycle regulator
proteins (p21, CDK2, CDK4 and cyclin D). β-Actin was used as a
loading control. (C) PC-3 cells, grown on a coverslip, were exposed
to the acetonitrile extract of S. miltiorrhiza Radix and a
TUNEL assay was performed. Representative confocal microscopy
images are presented. (D) PC-3 cells were exposed to the
acetonitrile extract of S. miltiorrhiza Radix and western
blot analysis was performed using polyclonal antibodies against
apoptosis-related proteins (Bcl-2, cleaved caspase-8, cleaved
caspase-9, cleaved caspase-3 and cleaved PARP). β-actin was used as
a loading control. CDK, cyclin-dependent kinase; TUNEL, terminal
deoxynucleotidyltransferase-mediated dUTP nick end labeling; FITC,
fluorescein isothiocyanate; Bcl-2, apoptosis regulator; PARP, poly
(ADP-ribose) polymerase. |
It is well-known that the induction of cell cycle
arrest at G1/S phase, also referred to as a cell cycle checkpoint,
temporarily allows cells to repair the cellular damage caused by
extracellular or intracellular stress (8). Alternatively, the cell cycle checkpoint
may also activate signaling pathways, leading to apoptosis if
cellular damage is extensive and unable to be properly repaired
(9). Therefore, it was investigated
whether exposure to the acetonitrile extract of S.
miltiorrhiza Radix may induce apoptosis in PC-3 cells. It was
observed that the acetonitrile extract of S. miltiorrhiza
Radix induced apoptosis in PC-3 cells, as determined using the
TUNEL assay (Fig. 3C). Western blot
analysis indicated that the acetonitrile extract of S.
miltiorrhiza Radix decreased the expression level of apoptosis
regulator Bcl-2 and activated apoptosis-inducer proteins, including
caspase-9, caspase-3 and poly (ADP-ribose) polymerase (PARP)
(Fig. 3D). It should be noted that
the acetonitrile extract of S. miltiorrhiza Radix exhibited
no significant effect on the activity of caspase-8, a critical
protein that initiates the intrinsic apoptotic signaling pathway
(Fig. 3D). These results suggest that
the induction of apoptosis using the acetonitrile extract of S.
miltiorrhiza Radix may occur exclusively through the activation
of the extrinsic apoptotic signaling pathway in PC-3 cells.
Induction of cell cycle arrest and
apoptosis by the acetonitrile extract of S. miltiorrhiza Radix is
dependent on the generation of intracellular ROS
It is known that the generation of intracellular ROS
regulates various cellular processes, including cell cycle arrest
and apoptosis (10). Based on the
observations that the acetonitrile extract of S.
miltiorrhiza Radix is a potent inducer of cell cycle arrest and
apoptosis (Fig. 3), it was
hypothesized that generation of intracellular ROS may be involved
in these events. Consistently, it was observed that treatment with
the acetonitrile extract of S. miltiorrhiza Radix induced
the generation of intracellular ROS in PC-3 cells (Fig. 4A). In addition, it was observed that a
combinatorial treatment of NAC, an antioxidant peptide that is used
as a precursor for the synthesis of intracellular reduced
glutathione thereby contributing to the detoxification of
intracellular ROS (11), with the
acetonitrile extract of S. miltiorrhiza Radix completely
abrogated the decrease in cellular CDK4 and cyclin D1 levels that
was observed in the absence of NAC (Fig.
4B). These results suggest that intracellular ROS may be
responsible for cell cycle arrest at G1/S. Consistently, the MTT
assay demonstrated that NAC significantly decreased the cell death
induced by the acetonitrile extract of S. miltiorrhiza Radix
compared with in the absence of NAC (P<0.05; Fig. 4C). In addition, NAC prevented the
activation of caspase-3 and PARP proteins induced by the
acetonitrile extract of S. miltiorrhiza Radix (Fig. 4D). It was also observed that NAC
reduced the formation of intracellular 8-OH-G, an oxidative stress
marker induced by the acetonitrile extract of S.
miltiorrhiza Radix in PC-3 cells (Fig. 4E). These results suggest that the
induction of cell cycle arrest and apoptosis by the acetonitrile
extract of S. miltiorrhiza Radix is mediated by the
generation of intracellular ROS.
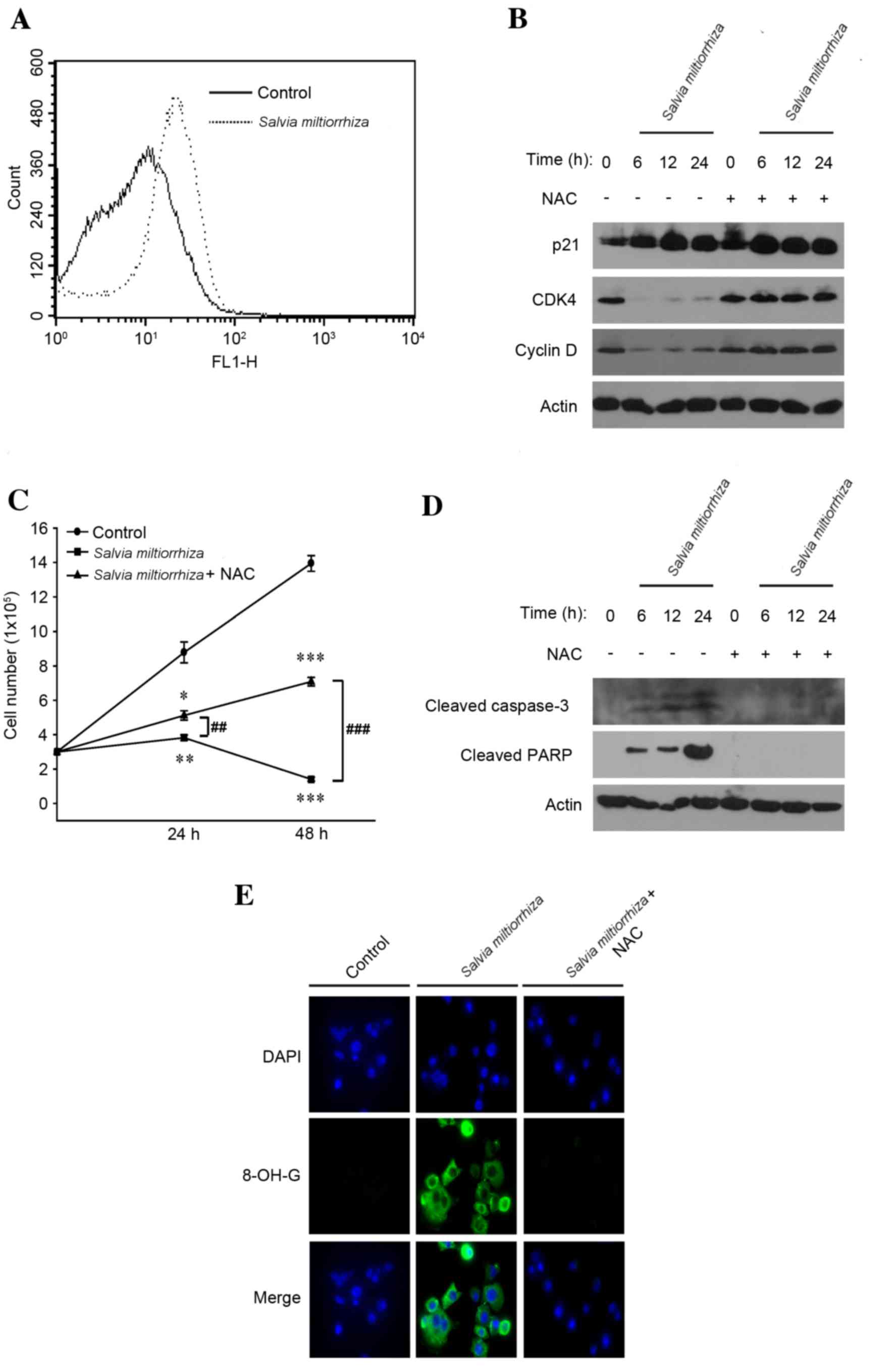 | Figure 4.Induction of cell cycle arrest and
apoptosis by the acetonitrile extract of Salvia miltiorrhiza Radix
is dependent on the generation of intracellular ROS. (A) PC-3 cells
were exposed to the acetonitrile extract of S. miltiorrhiza
Radix for 24 h and stained with 2′,7′-dichlorofluorescin diacetate.
The generation of intracellular ROS was monitored by
fluorescence-activated cell sorting analysis. (B) PC-3 cells were
exposed to the acetonitrile extract of S. miltiorrhiza Radix
alone or in combination with NAC. Western blot analysis was
performed using polyclonal antibodies against G1/S cell cycle
regulator proteins (p21, CDK4 and cyclin D). β-Actin was used as a
loading control. (C) PC-3 cells were exposed to the acetonitrile
extract of S. miltiorrhiza Radix alone or in combination
with NAC and an MTT assay was performed. *P<0.05, **P<0.01,
***P<0.001 vs. control; ##P<0.01,
###P<0.001 vs. treatment with NAC. (D) PC-3 cells
were exposed to the acetonitrile extract of S. miltiorrhiza
Radix alone or in combination with NAC, and western blot analysis
was performed using polyclonal antibodies against apoptosis-related
proteins (cleaved caspase-3 and cleaved PARP). β-Actin was used as
a loading control. (E) PC-3 cells, grown on a coverslip were
exposed to the acetonitrile extract of S. miltiorrhiza Radix
alone or in combination with NAC, and hybridized using primary
antibody against 8-OH-G. Representative confocal microscopy images
of the immunocytochemistry are presented. ROS, reactive oxygen
species; NAC, N-acetylcysteine; CDK4, cyclin-dependent
kinase; PARP, poly (ADP-ribose) polymerase; 8-OH-G,
8′-hydroxyguanosine. |
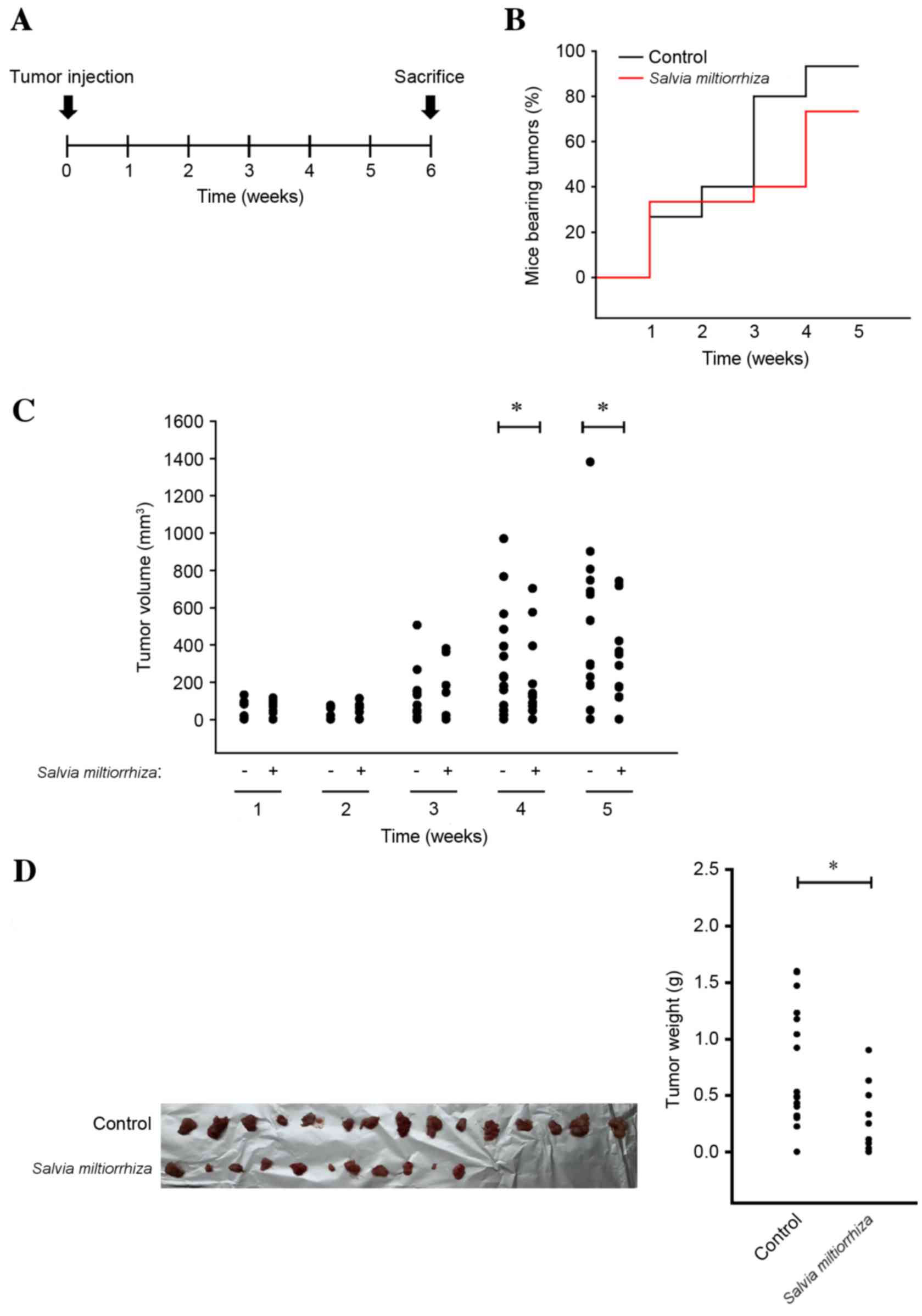
Oral administration of acetonitrile
extract of S. miltiorrhiza Radix suppresses the incidence and
growth of PC-3 tumor xenografts in vivo
As the acetonitrile extract of S.
miltiorrhiza Radix efficiently induced cell cycle arrest and
apoptosis of PC-3 cells in vitro, it was investigated
whether the acetonitrile extract of S. miltiorrhiza Radix
may suppress the growth of PC-3 cells in vivo. PC-3 cells
were injected into the flank of nude mice, which were orally
administered with the acetonitrile extract of S.
miltiorrhiza Radix for 6 weeks (Fig.
5A). It was observed that the overall body weight of mice in
the group of the acetonitrile extract of S. miltiorrhiza
Radix was not affected when compared with that of the control group
(data not shown), implying that oral administration of the
acetonitrile extract of S. miltiorrhiza Radix was tolerable
to mice at this dosage. However, it was observed that the
acetonitrile extract of S. miltiorrhiza Radix significantly
decreased the incidence (Fig. 5B) and
volume (P<0.05; Fig. 5C) of PC-3
xenografts in mice after 4 weeks of administration compared with
the untreated control. At autopsy, it was observed that the
acetonitrile extract of S. miltiorrhiza Radix decreased the
overall incidence (Fig. 5D, left
panel) of PC-3 xenografts in nude mice. Furthermore, the weight of
PC-3 xenografts in nude mice was significantly decreased using the
acetonitrile extract of S. miltiorrhiza Radix compared with
the untreated control (P<0.05; Fig.
5D, right panel). These results indicate that the acetonitrile
extract of S. miltiorrhiza Radix inhibits the incidence and
growth of prostate cancer in vivo.
Discussion
In the present study, an MTT assay with 400
traditional medicinal plants, extracted with water or acetonitrile,
was performed and it was identified that the acetonitrile extract
of S. miltiorrhiza Radix exhibited marked growth-inhibitory
effects on PC-3 cells. In addition, it was observed that the
inhibition of PC-3 cell growth by the acetonitrile extract of S.
miltiorrhiza Radix occurred through the induction of cell cycle
arrest and apoptosis via the generation of intracellular ROS. A
previous study has revealed that >50 chemical ingredients are
present in S. miltiorrhiza Radix, including hydrophilic
phenolic acids and lipophilic tanshionones (12). As S. miltiorrhiza Radix
extracted using acetonitrile, but not using water, exerted
significant growth-inhibitory effects, it may be that lipophilic
constituents, rather than hydrophilic compounds, in S.
miltiorrhiza Radix contributed to the growth inhibition of PC-3
cells. Consistent with this hypothesis, two previous studies have
demonstrated that lipophilic tanshinones exhibited
growth-inhibitory effects on prostate cancer cells, including PC-3
cells (13,14). However, the amount of lipophilic
tanshinones that exist in the acetonitrile extract of S.
miltiorrhiza Radix remains unclear. In addition, it was
observed that induction of apoptosis by the acetonitrile extract of
S. miltiorrhiza Radix occurred exclusively through the
extrinsic apoptotic signaling pathway. Although the underlying
molecular mechanism by which the acetonitrile extract of S.
miltiorrhiza Radix induces apoptosis in PC-3 cells remains
unclear, it may be hypothesized that the acetonitrile extract of
S. miltiorrhiza Radix lacks phytochemicals mimicking
cellular death ligands, including tumor necrosis factor receptor
superfamily members 6 and 10, which are able to initiate a
death-receptor-initiated signaling pathway in PC-3 cells (15).
It has previously been demonstrated that S.
miltiorrhiza Radix, also referred to as Danshen in Chinese,
exhibits a number of beneficial pharmacological activities. In
particular, traditional pharmacological effects of S.
miltiorrhiza Radix have been attributed primarily to promoting
circulation (16). Therefore, phase
III clinical trials for treatment of diabetic retinopathy and heart
disease are currently underway with a compound, the ‘Danshen
dripping pill’ (17). However, the
number of studies that demonstrate anti-tumorigenic effects of
S. miltiorrhiza Radix is fewer, compared with those
illustrating its beneficial cardiovascular effects (18). Following the results of the present
study demonstrating that the acetonitrile extract of S.
miltiorrhiza Radix suppressed the growth of prostate cancer
cells in vitro and in vivo, in-depth biochemical
studies are required to identify novel ingredients in the
acetonitrile extract of S. miltiorrhiza Radix that may be
responsible for its anti-tumorigenic effects in prostate
cancer.
The present study provides convincing preclinical
evidence for anti-tumorigenic effects of the acetonitrile extract
of S. miltiorrhiza Radix against the growth of prostate
cancer in vitro and in vivo. It was demonstrated that
the growth-inhibitory effects of the acetonitrile extract of S.
miltiorrhiza Radix against prostate cancer cells occurred
through the induction of cell cycle arrest and apoptosis via the
generation of intracellular ROS. Considering that a limited number
of chemotherapeutic agents useful for treatment of aggressive
prostate cancer currently exist, the present study justifies the
on-going investigation of the acetonitrile extract of S.
miltiorrhiza Radix as a source of a potential chemotherapeutic
agent for the treatment of prostate cancer.
Acknowledgements
The present study was supported by the Gyoenggi
Regional Research Center Program of Gyeonggi Province (grant no.
GRRC-DONGGUK2015-B02, development and discovery of new therapeutic
target modulators).
References
|
1
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Heidenreich A, Bastian PJ, Bellmunt J,
Bolla M, Joniau S, van der Kwast T, Mason M, Matveev V, Wiegel T,
Zattoni F, et al: EAU guidelines on prostate cancer. part 1.
Screening, diagnosis, and local treatment with curative
intent-update 2013. Eur Urol. 65:124–137. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Heidenreich A, Bastian PJ, Bellmunt J,
Bolla M, Joniau S, van der Kwast T, Mason M, Matveev V, Wiegel T,
Zattoni F, et al: EAU guidelines on prostate cancer. Part II:
Treatment of advanced, relapsing and castration-resistant prostate
cancer. Eur Urol. 65:467–479. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Saad F and Hotte SJ: Guidelines for the
management of castrate-resistant prostate cancer. Can Urol Assoc J.
4:380–384. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sporn MB and Suh N: Chemoprevention of
cancer. Carcinogenesis. 21:525–530. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
William WN Jr, Heymach JV, Kim ES and
Lippman SM: Molecular targets for cancer chemoprevention. Nat Rev
Drug Discov. 8:213–225. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Violette PD and Saad F: Chemoprevention of
prostate cancer: Myths and realities. J Am Board Fam Med.
25:111–119. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Burhans WC and Heintz NH: The cell cycle
is a redox cycle: Linking phase-specific target to cell fate. Free
Radic Biol Med. 47:1282–1293. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pietenpol JA and Stewart ZA: Cell cycle
checkpoint signaling: Cell cycle arrest versus apoptosis.
Toxicology. 181–182:475–481. 2002. View Article : Google Scholar
|
|
10
|
Circu ML and Aw TY: Reactive oxygen
species, cellular redox systems, and apoptosis. Free Radic Biol
Med. 48:749–762. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pompella A, Visvikis A, Paolicchi A, De
Tata V and Casini AF: The changing faces of glutathione, a cellular
protagonist. Biochem Pharmacol. 66:1499–1503. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li MH, Chen JM, Peng Y, Wu Q and Xiao PG:
Investigation of Danshen and related medicinal plants in China. J
Ethnopharmacol. 120:419–426. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gong Y, Li Y, Lu Y, Li L, Abdolmaleky H,
Blackburn GL and Zhou JR: Bioactive tanshinones in Salvia
miltiorrhiza inhibit the growth of prostate cancer cells in
vitro and in mice. Int J Cancer. 129:1042–1052. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang Y, Won SH, Jiang C, Lee HJ, Jeong
SJ, Lee EO, Zhang J, Ye M, Kim SH and Lü J: Tanshinones from
Chinese medicinal herb Danshen (Salvia miltiorrhiza Bunge)
suppress prostate cancer growth and androgen receptor signaling.
Pharm Res. 29:1595–1608. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Elmore S: Apoptosis: A review of
programmed cell death. Toxicol Pathol. 35:495–516. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wu WY and Wang YP: Pharmacological actions
and therapeutic applications of Salvia miltiorrhiza depside
salt and its active components. Acta Pharmacol Sin. 33:1119–1130.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
National Institutes of Health. https://clinicaltrials.gov/ct2/results?term=NCT00797953&Search=Search
|
|
18
|
Su CY, Ming QL, Rahman K, Han T and Qin
LP: Salvia miltiorrhiza: Traditional medicinal uses,
chemistry, and pharmacology. Chin J Nat Med. 13:163–182.
2015.PubMed/NCBI
|