Introduction
Renal Clear Cell Carcinoma (RCC) comprises >80%
of renal malignancies in adults (1).
Common sites of RCC metastasis include the lung, bone, liver and
adrenal glands (2). The thyroid
gland, however, represents a less common metastatic target site for
renal malignancies in general, and RCC in particular (2). The incidence of metastasis to the
thyroid was revealed to occur in 1.25–24% of autopsy cases
(2). According to the US National
Cancer Institute (Rockville, MD, USA), the five-year survival rate
for patients diagnosed with renal cancer is ~73.2%, and this number
decreases to ~11% with proven metastasis (3).
There have been several reports in the literature of
acquired arteriovenous malformations (AVM) being mistaken for RCC
(4). Conversely, RCCs have also been
mistaken for AVMs (5).
Radiologically, an AVM may take numerous forms, with masses ranging
from cystic to solid necrotic, from homogeneous to heterogeneous
and from small to large (6). To the
best of our knowledge, an AVM associated with RCC metastasis to the
brain has never been reported in the literature. The typical
clinical presentation of an AVM includes the cardinal features of
hemorrhage, seizures, progressive neurological deficits or headache
(7). However, recent animal models
have postulated that genetic manipulation and angiogenic
stimulation, including malignancy, are required for AVM development
and are independent of congenital etiologies (8). The current study presents a case of RCC
metastasis to the thyroid gland, with an AVM associated with
metastatic involvement in the brain. This case is unique as the
metastasis to the thyroid was only detected due to the clinical
manifestations of a cerebral AVM.
Case investigations, research and writing were
conducted at the Miami Valley Hospital in Dayton Ohio, a teaching
institution for Wright State University Boonshoft School of
Medicine. The case presented is unique in that it informs the
academic community of a novel presentation of renal cell carcinoma
that has metastasized to the thyroid and brain. Furthermore, it
demonstrates how a presentation of what appears to be an AVM is
anything but. Lastly, the metastasis of RCC to the thyroid is a
rare event that was further unusual in this case in that it was
detected only by diagnosis of the ‘AVM’.
Case report
A 45-year-old African-American female presented to
the Miami Valley Hospital Emergency Department (Dayton, OH, USA) in
November 2015 with left-sided weakness, slurred speech, facial
droop and a grand-mal seizure. The patient's medical history was
notable for a diagnosis of RCC, 2010 American Joint Committee on
Cancer Tumor-Node-Metastasis Stage 1B (T1B, N0, M0) grade III
status post-right robotic-assisted partial nephrectomy 18 months
prior. There was no personal or family history of seizure disorders
or neurologic diseases. A review of subjective symptoms was
negative for vision changes, fevers, chills, weight changes,
unilateral weakness, or decreased sensation. Physical examination
revealed an unresponsive patient with spontaneous movement of the
right side, but flaccid paralysis of the left upper and lower
extremities. No thyromegaly, thyroid nodules, cervical or
submandibular lymph nodes were palpated. Laboratory tests revealed
that blood, liver and kidney function were normal. Magnetic
resonance imaging (MRI) of the cervical, thoracic and lumbar spine
revealed signs of a metastatic lesion. The patient had numerous
episodes of focal status epileptics during hospitalization. After
several days, the patient underwent a right partial parietal
craniotomy with resection of the intra-axial mass; subsequent
pathology was inconclusive for a malignancy and consistent with an
AVM.
Due to the thyroid mass observed on the MRI, a
positron emission tomography (PET)/computed tomography (CT) scan
was performed, which revealed a hypermetabolic area in the left
lobe of the thyroid extending into the left aspect of the isthmus.
A corresponding ultrasound of the thyroid identified a homogeneous
hypervascular lesion throughout the left lobe that extended into
the isthmus and had poorly-defined borders. Fine needle aspiration
of the thyroid and subsequent histopathological analysis revealed
the thyroid tumor to be comprised of clear cell carcinoma cells.
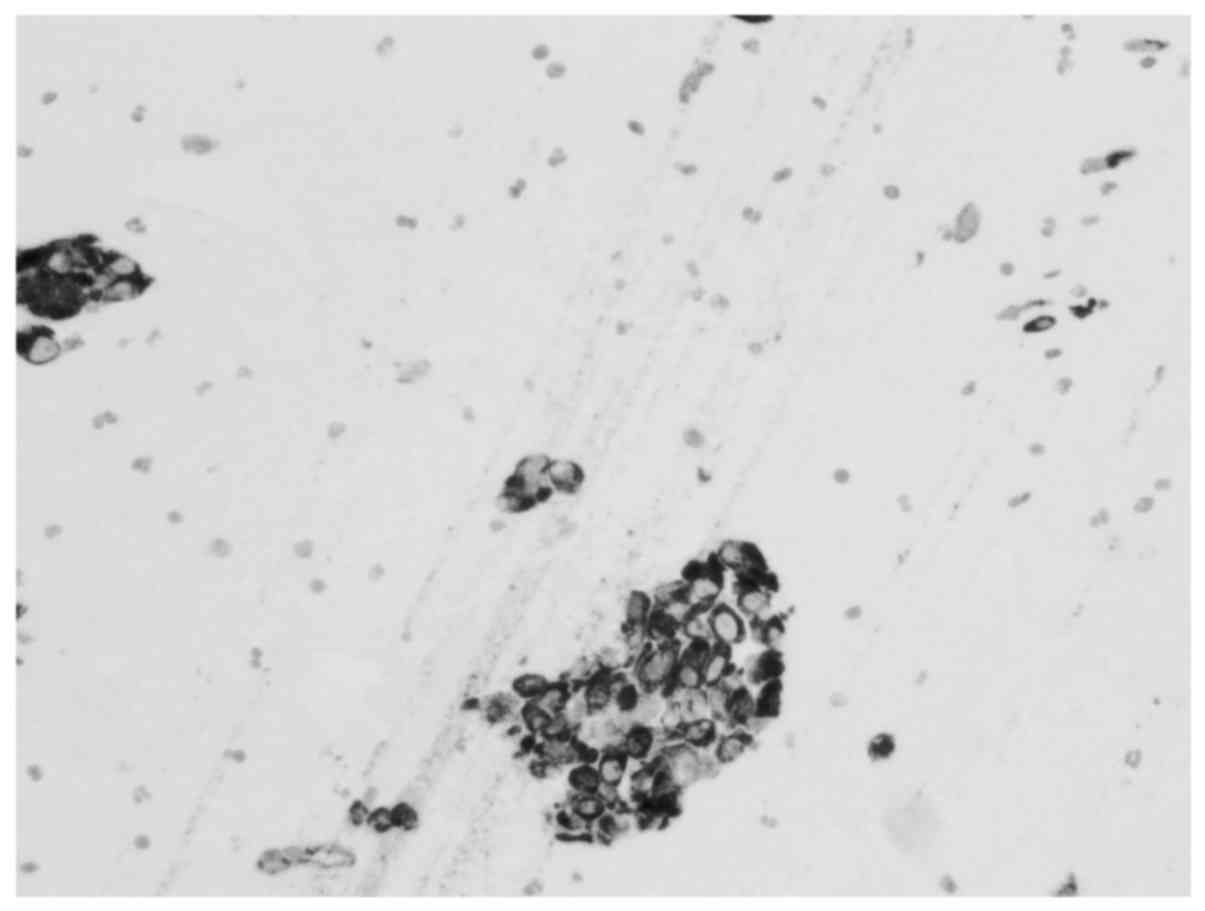
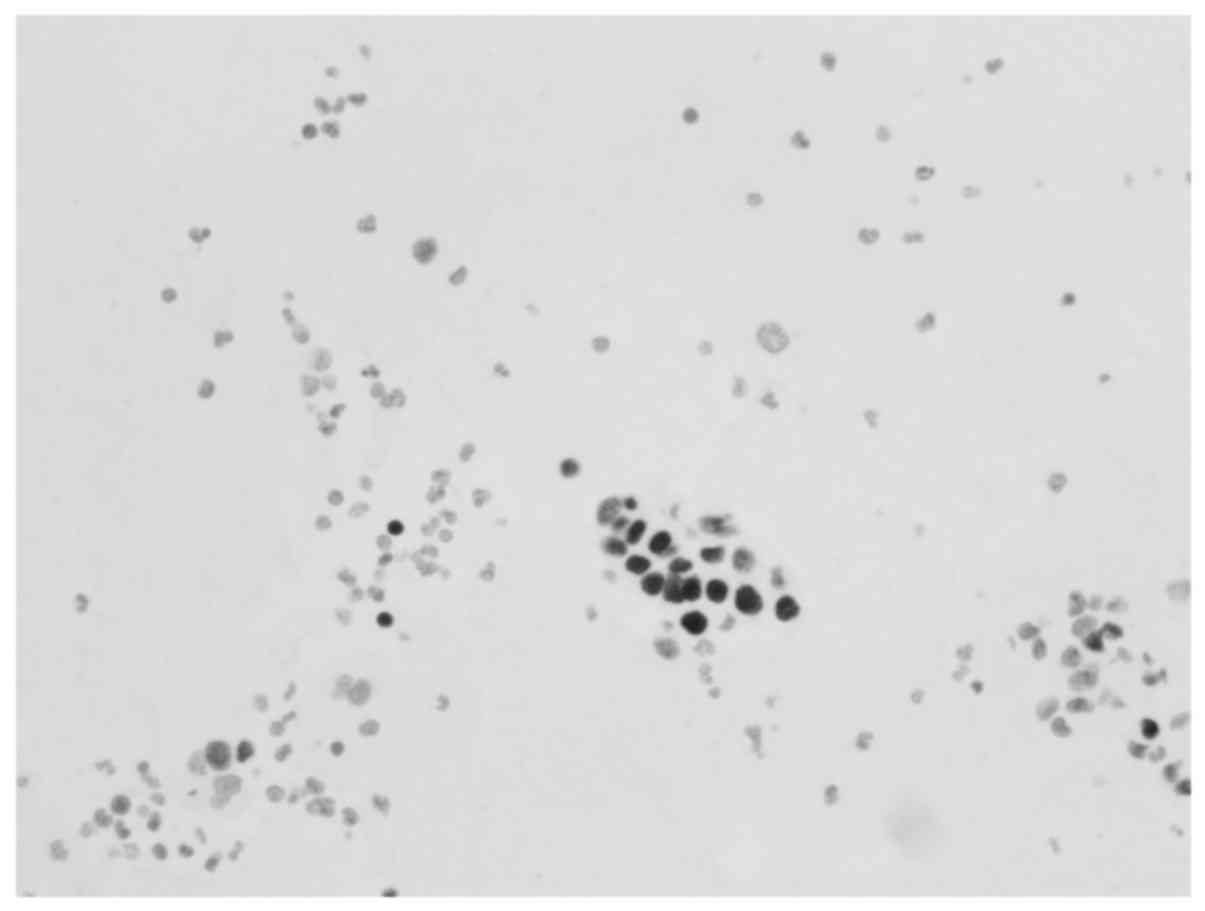
Immunohistochemical analysis (Figs.
1–4) of 10% formalin-fixed
paraffin-embedded aspirate tissue sections (4-µm thick) of the
thyroid tumor revealed the following: Polyclonal cytokeratins 1/3
(AE1/3; 1:450 dilution; catalog no. M3515; Agilent Technologies,
Inc., Santa Clara, CA, USA), positive; vimentin, positive; paired
box gene 8 (PAX8; 1:400 dilution; catalog no. 363M-16; Cell Marque,
Rocklin, CA, USA), positive; renal cell carcinoma (PN-15; 1:300
dilution; catalog no. 329M-97; Cell Marque), positive; cluster of
differentiation (CD) 10 (1:10 dilution; catalog no. AC-0169;
Epitomics, Burlingame, CA, USA), positive; cytokeratin 20 (CK20),
negative; cytokeratin 7 (CK7), negative; thyroglobulin, negative;
and thyroid transcription factor-1, negative. Detection was carried
out using DAKO EnVision™ Flex+ and DAKO Austostainer Link (Dako;
Agilent Technologies, Inc.). All antibody details are illustrated
in Table I. Metastatic involvement of
the thyroid was determined to be associated with the patient's
prior history of grade III RCC. In addition to being referred to
radiation-oncology and neurology-oncology surgery, the patient was
placed on Keppra, remained seizure free and was started on
pazopanib as an outpatient.
 | Table I.Antibodies utilized in
immunohistochemical staining for renal cell carcinoma. |
Table I.
Antibodies utilized in
immunohistochemical staining for renal cell carcinoma.
| Immunohistochemistry
antibody | Clone | Dilution | Manufacturer | Cat. no. |
| AE1/AE3 | AE1 & AE3 | 1:450 | Dakoa | M3515 |
| CD10 | EP195 | 1:10 |
Epitomicsb | AC-0169 |
| PAX8 | MRQ-50 | 1:400 | Cell
Marquec | 363M-16 |
| PN15 | PN-15 | 1:300 | Cell
Marquec | 329M-97 |
| Vimentin | V9 | 1:125 | Dakoa | M072529 |
| Cytokeratin 20 | Ks20.8 | 1:300 | Dakoa | M701901 |
| Cytokeratin 7 | OV-TL 12/30 | 1:500 | Dakoa | M701801 |
| Thyroglobulin | Polyclonal | 1:100,000 | Dakoa | A025102 |
| Thyroid transcription
factor | 8G7G3/1 | 1:400 | Dakoa | M357501 |
| Glial fibrillary
acidic protein | Polyclonal | 1:12,000 | Dakoa | Z033401 |
| MNF116
cytokeratin | MNF116 | 1:150 | Dakoa | M0821 |
Several months after discharge, the patient was
readmitted to the Miami Valley Hospital for worsening headaches,
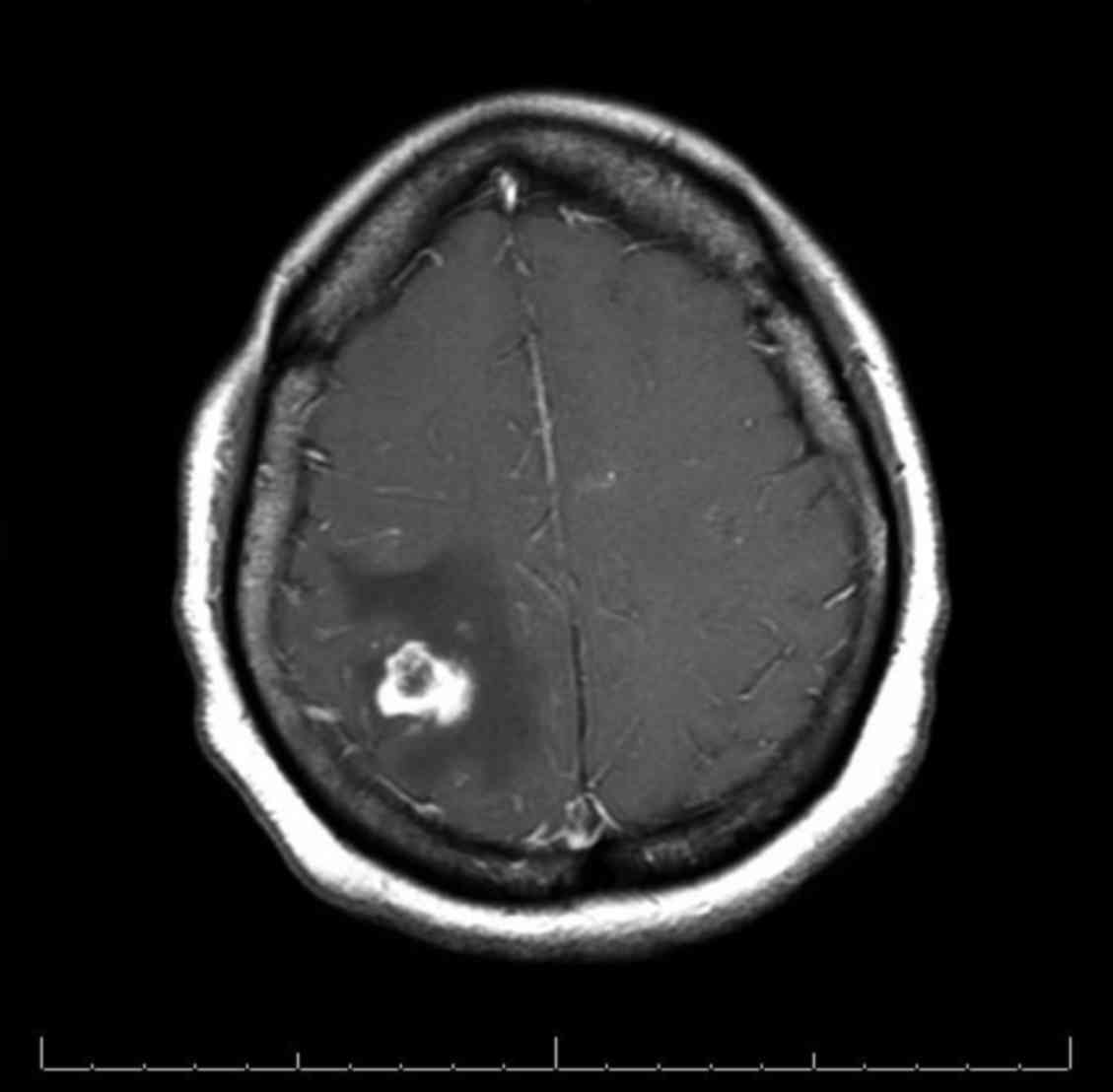
nausea and vomiting. CT and MRI scans of the brain revealed the
recurrence of a 1.9 cm mass in the right parietal lobe with
extensive vasogenic edema (Fig. 5).
Biopsy of the mass at several distinct points indicated a vascular
lesion composed of numerous vessels of varying caliper, suggestive
of AVM. Immunostaining revealed the mass to be AE1/3, cytokeratin
(MNF116; 1:150 dilution; catalog no. M0821; Agilent Technologies),
PAX8 and PN-15 negative. The patient was readmitted again to the
hospital after several months for worsening headaches, nausea and
vomiting. CT and MRI scans of the brain revealed the return of a
3.7 cm mass exhibiting vasogenic edema. Biopsy of this novel mass
did not suggest an AVM, rather the immunohistochemical stains
demonstrated the following: Glial fibrillary acidic protein,
negative; vimentin, CD10, PN-15, PAX-8 and cytokeratin AE1/3,
positive; CK7 and CK20, negative. The findings from the novel
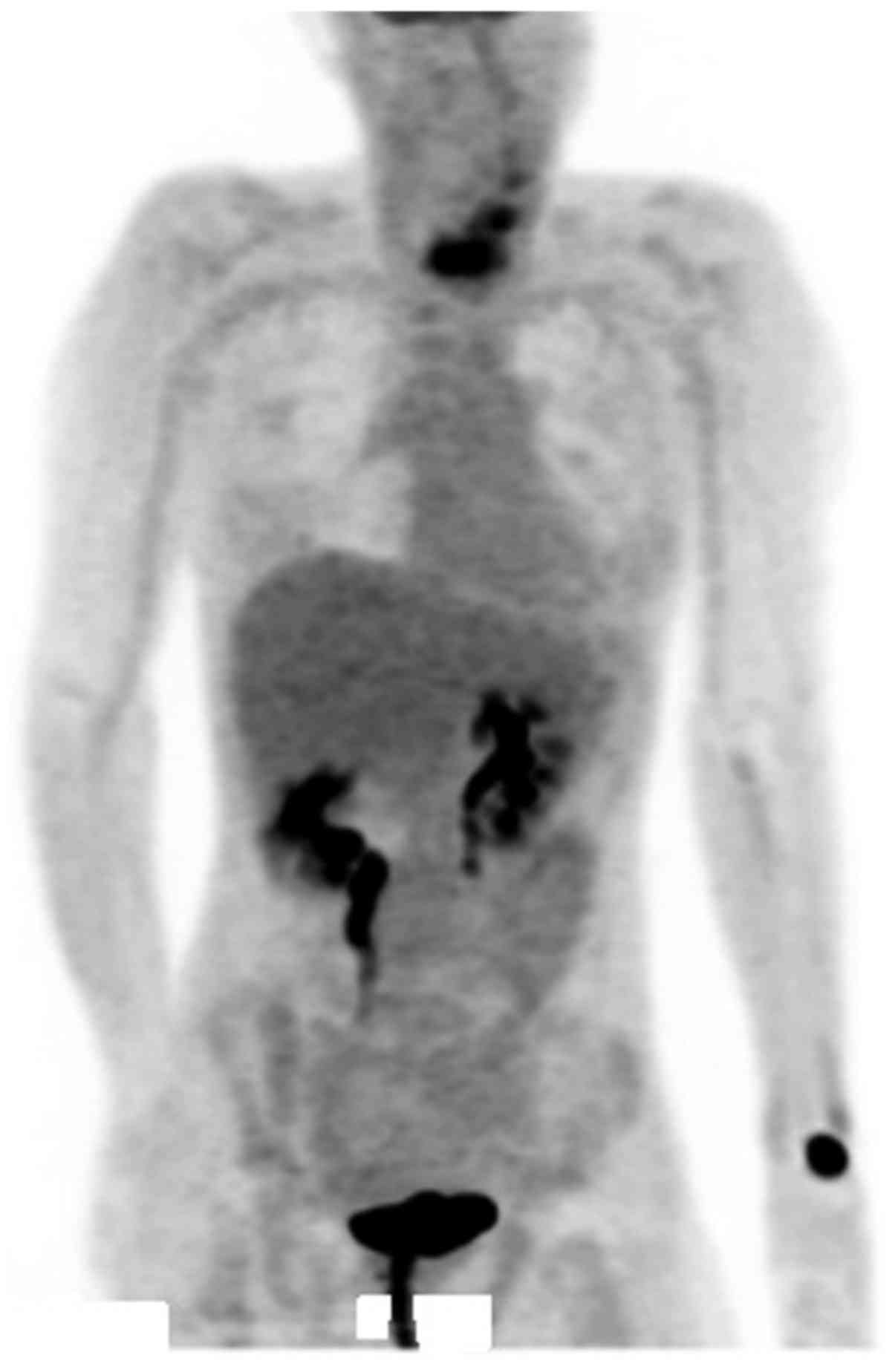
lesion supported a diagnosis of metastatic RCC. Fig. 6 presents PET imaging, which suggested
metastasis to the thyroid.
Discussion
RCC accounts for <5% of all malignant tumors
(9). Metastasis is common in patients
with RCC, with typical sites including the lungs, lymph nodes,
bone, and liver (10). Metastasis to
the head and neck region is less common (11). To the best of our knowledge, RCC
metastasis to the brain associated with AVM formation has not been
reported in the literature to date. More commonly, AVMs associated
with RCC are located in the renal vasculature and present with the
cardinal symptom of flank pain (5).
Recent literature has commented on the association between AVMs and
RCC as they share highly vascular characteristics (5). It was previously considered that various
types of cancer have high circulating expression levels of plasma
vascular endothelial growth factor (VEGF) (12). However, a recent study demonstrated
that free VEGF plasma levels are low or absent in numerous types of
cancer, with the exception of RCC (13). RCC is a vascular tumor that has been
demonstrated to lead to the abnormal expression of various
angiogenesis-promoting growth factors (14). VEGF is the central pro-angiogenic
agent involved in RCC carcinogenesis, and is associated with
mutations in the von Hippel-Lindau tumor suppressor (VHL)
gene (14). Somatic VHL
mutations have been associated with persistently high expression
levels of hypoxia-inducible factor-1α signaling pathway proteins,
and with VEGF expression in patients with metastatic RCC (15).
Recent studies using animal models have postulated
that genetic manipulation and angiogenic stimulation are each
required for AVM development, and are independent of congenital
etiologies (8). It may be theorized
that the dysregulation of pro-angiogenic factors associated with
RCC development contributes to the development of AVMs observed in
this type of malignancy. These angiogenic factors are vital to the
pathophysiological pathway that leads to RCC, and may well explain
the development of AVMs within these neoplasms as demonstrated in
the aforementioned case. DNA sequencing of AVM tissue from a
patient with Proteus-like syndrome has revealed PTEN
mutations that have been previously implicated in Cowden's syndrome
(16). With the use of
immunohistochemical staining on tissue samples from similar areas
of the brain, the progression of negative markers for malignancy in
the setting of an AVM to positive markers for malignancy in the
absence of AVM was demonstrated. It has been noted that certain
pathologists have stated that an AVM and RCC may be regarded as
associated entities, rather than distinct processes, in the context
of surgical histopathology. Conversely, the case has been presented
for serial MR imaging of supposed AVMs in order to distinguish them
from malignancies, specifically RCC (5).
The patient in the current case was further unusual
in that they developed biopsy-confirmed renal metastasis to the
thyroid. Typically, patients with RCC metastasis to the thyroid are
detected following a physical exam, not in the setting of a
coinciding metastasis to the brain (17). Metastases to the thyroid have
generally been revealed to occur several years following
nephrectomy (10). It is unclear why
metastasis to the thyroid is a rare event, as it has a rich
vascular supply (18).
Current guidelines pertaining to metastatic RCC do
not recommend routine brain imaging for surveillance purposes
unless there are concurrent CNS symptoms or other abnormalities
indicating brain involvement (19).
Patients with a history of tobacco abuse and pulmonary metastases
are more likely to have metastasis to the brain; therefore, this
may serve as an indication for surveillance imaging of the brain
(19). With increasing understanding
of the role of angiogenic dysregulation in the pathogenesis of RCC,
novel therapeutic strategies are utilizing targeted anti-angiogenic
agents with varying degrees of success (20).
In conclusion, brain metastasis must be considered
in patients with a positive history of RCC, who present with an
acute onset neurological event and apparent AVM on initial biopsy.
Postoperative distinction between AVM and metastasis to the brain
may be difficult to distinguish, as discussed in the aforementioned
case. A high index of suspicion for brain metastasis, despite
initial negative immunohistochemical testing, should be followed up
by a repeat biopsy of the tissue in question at a later date. This
may assist in guiding palliative therapy should the otherwise
prognostically favorable AVM be determined to be a prognostically
unfavorable metastasis to the brain.
References
|
1
|
Thoenes W, Störkel S, Rumpelt HJ and Moll
R: Cytomorphohogical typing of renal cell carcinoma-a new approach.
Eur Urol. l8:(Suppl 2). S6–S9. 1990.
|
|
2
|
Nakhjavani MK, Gharib H, Goellner JR and
van Heerden JA: Metastasis to the thyroid gland. A report of 43
cases. Cancer. 79:574–578. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
National Cancer Institute: Cancer
statistics: SEER Stat Fact Sheets: Kidney and renal pelvis cancer.
https://seer.cancer.gov/statfacts/html/kidrp.htmlAccessed.
May 1–2016.
|
|
4
|
Wong C, Leveillee RJ, Yrizarry JM and
Kirby K: Arteriovenous malformation mimicking a renal-cell
carcinoma. J Endourol. 16:685–686. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Volin S, Steinberg P and Mittleider D:
Renal cell carcinoma initially presenting as an arteriovenous
malformation: A case presentation and a review of the literature.
Case Rep Urol. 2013:3568192013.PubMed/NCBI
|
|
6
|
Park SB, Cho KS, Lee JH, Jeong YK, Choi
SH, Kang BS and Kim JK: Unusual manifestations of renal cell
carcinoma. Acta Radiol. 49:839–847. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Young AM, Teo M, Martin SC, Phang I,
Bhattacharya JJ and St George EJ: The diagnosis and management of
brain arteriovenous malformations in a single regional center.
World Neurosurg. 84:1621–1688. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen W, Choi EJ, McDougall CM and Su H:
Brain arteriovenous malformation modeling, pathogenesis, and novel
therapeutic targets. Transl Stroke Res. 5:316–329. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ohba K, Miyata Y, Mitsunari K, Matsuo T,
Mochizuki Y and Sakai H: Left atrial metastasis of renal cell
carcinoma: A case report and review of the literature. BMC Res
Notes. 7:5202014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bianchi M, Sun M, Jeldres C, Shariat SF,
Trinh QD, Briganti A, Tian Z, Schmitges J, Graefen M, Perrotte P,
et al: Distribution of metastatic sites in renal cell carcinoma: A
population-based analysis. Ann Oncol. 23:973–980. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rizzo M, Rossi RT, Bonaffini O, Scisca C,
Sindoni A, Altavilla G and Benvenga S: Thyroid metastasis of clear
cell renal carcinoma: Report of a case. Diagn Cytopathol.
37:759–762. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Longo R and Gasparini G: Challenges for
patient selection with VEGF inhibitors. Cancer Chemother Pharmacol.
60:151–170. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Niers TM, Richel DJ, Meijers JC and
Schlingemann RO: Vascular endothelial growth factor in the
circulation in cancer patients may not be a relevant biomarker.
PLoS One. 26:e198732001.
|
|
14
|
Clifford SC, Prowse AH, Affara NA, Buys CH
and Maher ER: Inactivation of the von Hippel-Lindau (VHL) tumour
suppressor gene and allelic losses at chromosome arm 3p in primary
renal cell carcinoma: Evidence for a VHL-independent pathway in
clear cell renal tumourigenesis. Genes Chromosomes Cancer.
22:200–209. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Falcão MS, Vinagre J, Soares P, Lopes JM,
Brandão E and Carneiro AM: A clear cell renal cell carcinoma
inhibiting the response to intravitreal antivascular endothelial
growth factor therapy in wet age-related macular disease. Case Rep
Ophthalmol. 3:443–451. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhou XP, Marsh DJ, Hampel H, Mulliken JB,
Gimm O and Eng C: Germline and germline mosaic PTEN mutations
associated with a Proteus-like syndrome of hemihypertrophy, lower
limb asymmetry, arteriovenous malformations and lipomatosis. Hum
Mol Genet. 9:765–768. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lee MW, Batoroev YK, Odashiro AN and
Nguyen GK: Solitary metastatic cancer to the thyroid: A report of
five cases with fine-needle aspiration cytology. Cytojournal.
4:52007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Medas F, Calò PG, Lai ML, Tuveri M, Pisano
G and Nicolosi A: Renal cell carcinoma metastasis to thyroid tumor:
A case report and review of the literature. J Med Case Rep.
7:2652013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hanzly M, Abbotoy D, Creighton T, Diorio
G, Mehedint D, Murekeyisoni C, Attwood K, Kauffman E, Fabiano AJ
and Schwaab T: Early identification of asymptomatic brain
metastases from renal cell carcinoma. Clin Exp Metastasis.
32:783–788. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bastos DA, Molina AM, Hatzoglou V, Jia X,
Velasco S, Patil S, Voss MH, Feldman DR and Motzer RJ: Safety and
efficacy of targeted therapy for renal cell carcinoma with brain
metastasis. Clin Genitourin Cancer. 13:59–66. 2015. View Article : Google Scholar : PubMed/NCBI
|