Introduction
Lung cancer has increased in incidence in the
developing world since 2008, from 1.8 to 2 million per year, and
this trend is predicted to continue in the future (1). Non-small cell lung cancer (NSCLC) is
among the most malignant types of cancer, and has a high mortality
rate (2). Approximately 80–90% of
lung cancer cases are of NSCLC, and 50–70% of these patients are
diagnosed at the advanced stage of disease (1,3). The
survival time of patients with lung cancer is 5 years in 10–15% of
cases, with the remaining exhibiting poorer survival times
(1). Therefore, the early prevention
of NSCLC metastasis is a key factor in lung cancer prevention and
the development of novel therapeutic agents. A number of studies
have investigated the mechanisms underlying NSCLC metastasis
(4,5);
however, the exact mechanisms underlying this process require
further elucidation.
Raf kinase inhibitor protein (RKIP) is a small
evolutionarily conserved protein, which was initially identified to
function as a physiological inhibitor of the Raf/mitogen-activated
protein kinase kinase (MEK)/extracellular signal-regulated kinase
(ERK) signaling pathway (6). RKIP
inhibits the interaction between RAF1 and MEK, thereby preventing
RAF-mediated MEK phosphorylation, which is required for signal
propagation (7). RKIP is widely
expressed in normal human tissues, indicating that it may function
in a variety of physiological processes (8). Previous studies have suggested that RKIP
may be a suppressor of cancer metastasis, as its loss of or reduced
expression levels are highly associated with cancer malignancy and
aggressiveness (9,10). Overexpression of RKIP protein has a
limited impact on the proliferation of prostate cancer cell
proliferation and angiogenesis in mouse models; however, cancer
cell invasion and metastasis is significantly reduced upon RKIP
overexpression (11), indicating its
potential tumor suppressor role in cancer metastasis. In a previous
study, RKIP mRNA expression levels were demonstrated to be
significantly downregulated in NSCLC, and lower mRNA levels were
correlated with poorer differentiation and advanced
tumor-node-metastasis stage in patients with NSCLC (12). However, the clinical significance of
RKIP and its associations with metastasis in NSCLC, as well as its
downstream mechanisms and targets, remain to be established.
Signal transducer and activator of transcription 3
(STAT3) is activated by various growth factors, cytokines and
oncogenic proteins, as well as being constitutively activated in
various types of malignancy (13). A
previous study reported that STAT3 functions downstream of the
Raf/ERK signaling pathway, and is associated with cancer metastasis
(14). However, whether RKIP
participates in the phosphorylation and activation of STAT3 during
NSCLC cell metastasis remains to be established. A previous study
reported that increased RKIP level and Raf/ERK/STAT3 pathway
activation were found in metastatic NSCLC patients (15). The current study examined the role of
RKIP and STAT3 phosphorylation in NSCLC, based on clinical sample
analysis and in vitro and in vivo experiments.
Materials and methods
Cell lines and reagents
The A549 and NCI-H1299 human NSCLC cell lines were
purchased from the American Type Culture Collection (Manassas, VA,
USA). Cells were maintained in Dulbecco's modified Eagle's medium
(DMEM; Thermo Fisher Scientific, Inc., Waltham, MA, USA),
supplemented with 10% fetal bovine serum (Gibico; Thermo Fisher
Scientific, Inc.), 100 U/ml penicillin and 100 µg/ml streptomycin
(Sigma-Aldrich; Merck Millipore, Darmstadt, Germany) at 37°C in a
humidified atmosphere containing 5% CO2. Antibodies
against RKIP (cat. no. 13006), STAT3 (cat. no. 9139),
phosphorylated (p)STAT3 (cat. no. 9145), ERK (cat. no. 9102) and
pERK (cat. no. 4370) were purchased from Cell Signaling Technology,
Inc. (Danvers, MA, USA) and β-actin (cat. no. 7210) antibody was
purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX,
USA).
Patient tissue samples and
pathological diagnosis
Between March 2015 and August 2015, 100 patients
with NSCLC were recruited for the present study at The First
Affiliated Hospital of Bengbu Medical College (Bengbu, China). All
patients underwent curative surgical resection and none had
received preoperative chemotherapy or radiotherapy. All patients
were diagnosed by pathological methods. The current study was
performed with the approval of the Bengbu Medical College Ethics
Committee, and informed consent was obtained from all patients that
were recruited.
Immunohistochemical staining
Immunohistochemical staining for RKIP and
phosphorylated (p)STAT3 was performed according to previous
protocol (16). Briefly, antigen
retrieval was performed by boiling tissue samples in an autoclave
for 20 min in 1X antigen retrieval Citra solution (BioGenex,
Fremont, CA, USA). The tissue samples were fixed with 4%
formaldehyde at room temperature for 24 h. After fixation, samples
were sectioned at a thickness of 5 µm for further analysis.
Sections were blocked with PBS containing 5% goat serum
(Sigma-Aldrich; Merck Millipore) and 0.1% Triton X-100, followed by
incubation with anti-RKIP antibody (dilution, 1:200) or pSTAT3
antibody (dilution, 1:500) at 4°C in a humidified chamber for 12 h.
Following washes with PBS, the tissue samples were incubated at
room temperature with horseradish peroxidase (HRP)-labeled
secondary antibody (cat. no. 8114, 1:1,000 dilution; Cell Signaling
Technology, Inc.) for 2 h and visualized with a
3,3′-diaminobenzidine kit (Vector Laboratories, Inc., Burlingame,
CA, USA). The sections were observed and recorded using a Leica
DM3000 microscope.
Western blotting
siRNA transfected cells were lysed in ice-cold lysis
buffer (Bio-Rad Laboratories, Inc., Hercules, CA, USA) for 30 min,
supplemented with protease and phosphatase inhibitors
(Sigma-Aldrich; Merck Millipore). Following centrifugation for 15
min at 12,000 × g at 4°C, the supernatant was collected and the
protein concentration was determined using a protein assay kit
(Bio-Rad Laboratories, Inc.). Total protein (50 µg/lane) was
separated by 10% SDS-PAGE and transferred to Hybond-C
nitrocellulose membranes (GE Healthcare Life Sciences, Chalfont,
UK). The membranes were incubated with primary antibodies RKIP,
pSTAT3 or β-actin (1:2,000) overnight at 4°C, respectively, and
subsequently probed with HRP-conjugated secondary antibodies (cat.
no. 8114; 1:2,500 dilution; Cell Signaling Technology, Inc.) for 1
h at room temperature. Chemiluminescent signals were developed
using LumiGLO® reagent (Cell Signaling Technology, Inc.)
and detected using the ChemiDoc™ XRS+ gel documentation system
(Bio-Rad Laboratories, Inc.). In grayscale quantification,
Photoshop version 8.0 (Adobe Systems, Inc., San Jose, CA, USA) was
used, setting actin grayscale as control.
Small interfering (si)RNA
interference
The experiment was performed according to previous
protocol (17). Briefly,
3×105 A549 and NCI-H1299 cells/2 ml were seeded in
6-well plates 1 day prior to transfection. 10 µM siRNAs (cat. no.
sc-36430; Santa Cruz Biotechnology, Inc.) were transfected into the
cells with the aid of FuGENE® transfection reagent
(Promega Corporation, Madison, WI, USA). Three days later, cells
were collected using lysis assay buffer, and incubated on ice for
30 min with protease inhibitors cocktail (Sigma-Aldrich; Merck
Millipore).
Transwell assay
The migration of NCI-H1299 cells in vitro was
determined using Transwell assays (pore size, 8-µm; Corning
Incorporated, Corning, NY, USA). Briefly, cell-free DMEM (0.8 ml)
with or without human interleukin-6 (10 ng/ml; BD Biosciences, San
Jose, CA, USA) was placed in the lower chamber. Here, IL-6 was used
as chemoattractant to induce cells migration form upper chamber to
the lower plate. Microglial suspension (0.1 ml; 5×104
cells/well) was placed in the upper chamber and the plates were
incubated for 24 h at 37°C. The inserts were subsequently removed
and the cells on the upper surface were removed with cotton pads.
Cells on the lower surface were air dried at room temperature and
stained with hematoxylin and eosin (H&E) method after 15 min
fixation at room temperature. Migration ability was quantified and
compared by counting the number of migrated cells in the lower
chamber. Five random fields at ×40 magnification were counted for
each condition under a phase-contrast microscope. Each experiment
was repeated three times. Results were presented as the number of
cells counted per field.
Lentivirus construction and
infection
Lentivirus particles containing the RKIP gene were
constructed using the pLenti7.3⁄V5-DEST™ Gateway® Vector
kit (Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. The lentiviral vectors were co-transfected
with virus packing vectors to form mature lentivirus particles in
293FT cells (Thermo Fisher Scientific, Inc.). The viral supernatant
was harvested to determine the viral titer as previously described
(18), and added to the A549 and
NCI-H1299 cells for target gene expression.
Xenograft metastatic in vivo
study
The current animal experiment was approved by the
Bengbu Medical College Ethic Committee (Bengbu, China) in 2015. A
total of 15 nude mice were randomly divided into 2 groups. Nude
mice (6 weeks old, 11 male and 4 female, weight 15–18 g) were
purchased from the Animal Center of Bengbu Medical College (Bengbu,
China) and kept in the Animal Center for SPF level feeding at 25°C,
with a 12 h day/night cycle. Group one (six mice) received
parenteral injection via tail vein with A549 cells as controls.
Group two (nine mice) were injected with pLenti7.3-RKIP infected
and overexpressed stable cells, with ≥90% positively expressing
RKIP, using a modified tail vein injection transplantation model
from a previous study (19). The
transduction efficiency was determined by green fluorescent
protein-positive counting under a fluorescent microscope. Briefly,
5- to 6-week-old BALB/c nude mice were injected with
1×106 A549 cells per mouse through the tail vein. At six
weeks following injection, mice were examined using Xenogen IVIS
imaging (PerkinElmer, Inc., Waltham, MA, USA) to detect tumor
metastasis status. Subsequently, the mice were sacrificed using the
cervical dislocation method. Lung tissue was collected for analysis
and the tumor number and volume within the lung was measured. One
part of tumor tissue was fixed for H&E staining analysis, and
the remaining was stored in a liquid nitrogen tank for subsequent
studies.
Statistical analysis
Statistical analysis was performed using SPSS
version 13.0 (SPPS, Inc., Chicago, IL, USA). Continuous variables
were expressed as the mean ± standard error and analyzed using the
Student's t-test (two-tailed), U test, χ2 test and
Fisher's exact test. Spearman's rank correlation analysis was
performed to determine the correlation between two rank-order
variables. P<0.05 was considered to indicate a statistically
significant difference.
Results
RKIP expression levels are reduced in
NSCLC cancerous tissues
Following obtaining ethical approval and informed
patient consent, 100 patients with NSCLC were recruited to the
present study. The clinicopathological information of patients is
summarized Table I. There were 21
cases of TNM stage I/II and 79 cases of stage III/IV NSCLC. The
variation in levels of RKIP protein expression between these two
groups was statistically significant (P=0.008), with reduced RKIP
expression levels in patients with late-stage NSCLC. A total of 82
patients had lymph node metastasis, whereas 18 were negative for
metastasis. Of these, 47 (57.32%) patients with metastasis
exhibited low RKIP expression levels and 9 (50.0%) of the patients
without metastasis had high RKIP expression levels, a difference
that was statistically significant (P=0.001). When NSCLC distant
metastasis was compared, 63 patients (65.08%) had developed distant
metastasis and exhibited lower levels of RKIP expression, whereas
27 patients (72.97%) had intra-lung metastasis with significantly
higher levels of RKIP expression (P=0.018).
 | Table I.Clinicopathological characteristics of
patients with NSCLC and RKIP expression levels. |
Table I.
Clinicopathological characteristics of
patients with NSCLC and RKIP expression levels.
|
|
| RKIP expression
levels |
|
|---|
|
|
|
|
|
|---|
| Clinical
characteristics | Number of cases | Low (%) | Moderate (%) | High (%) | P-value |
|---|
| Gender |
| Male | 76 | 40 (52.63) | 23 (30.26) | 13 (17.11) | 0.341 |
|
Female | 24 | 9
(37.50) | 11 (45.83) | 4
(16.67) |
|
| Age |
| ≥60 | 56 | 18 (32.14) | 23 (41.07) | 15 (26.79) | 0.452 |
|
<60 | 44 | 19 (43.18) | 13 (29.55) | 12 (27.27) |
|
| TNM stage |
| I/II | 21 | 12 (57.14) | 7 (33.33) | 2 (9.52) | 0.008 |
|
III/IV | 79 | 41 (51.90) | 23 (29.11) | 15 (18.99) |
|
| Lymph node
metastasis |
|
Positive | 82 | 47 (57.32) | 22 (26.83) | 13 (15.85) | 0.001 |
|
Negative | 18 | 5
(27.78) | 4 (22.22) | 9
(50.00) |
|
| Distant
metastasis |
|
Within-lung | 37 | 3 (8.11) | 7 (18.92) | 27 (72.97) | 0.018 |
| Long
distance | 63 | 41 (65.08) | 17 (26.98) | 5 (7.94) |
|
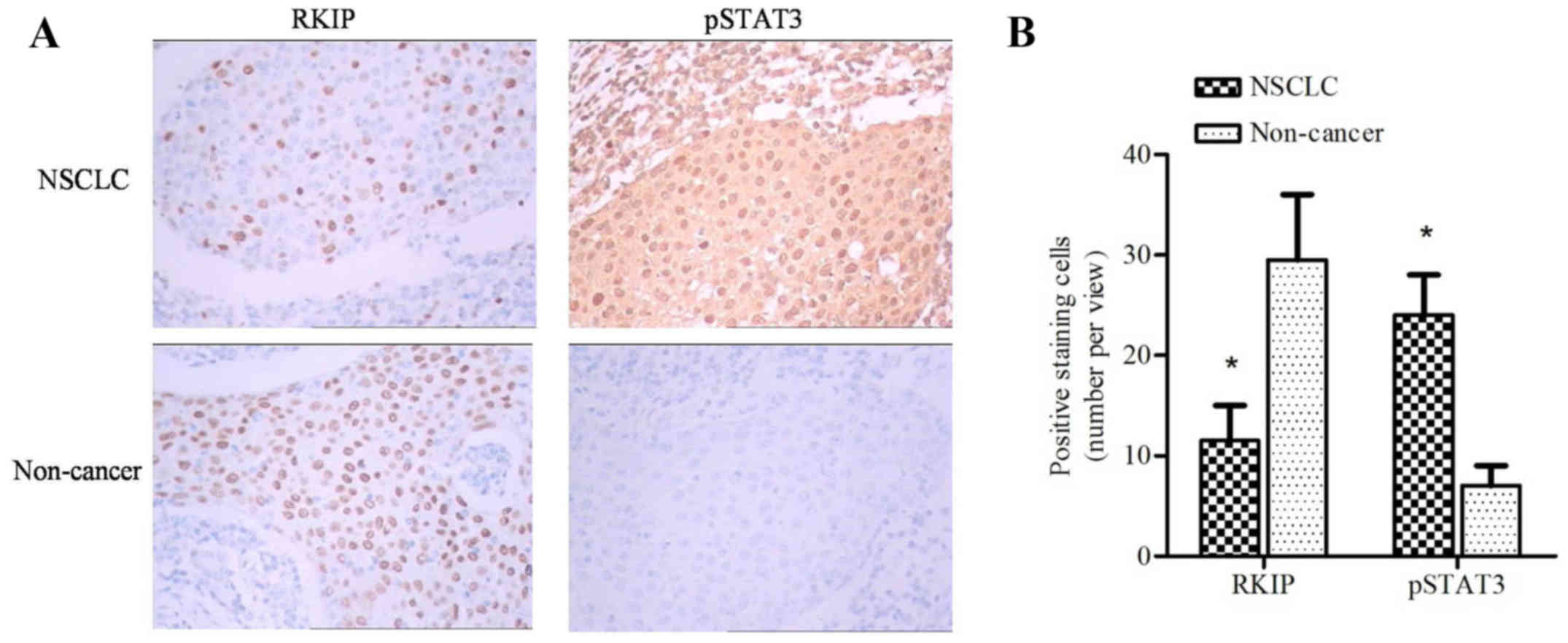
Histological sections of cancer and non-cancer
tissues were stained with anti-RKIP or anti-pSTAT3 antibodies.
Negative or weak staining of RKIP was observed in the majority of
cancerous tissues compared with intense staining in non-cancerous
tissues (Fig. 1A). However, intense
pSTAT3 staining was observed in cancerous tissues (Fig. 1A). Bar chart quantification of RKIP
and pSTAT3 positive staining revealed a significant difference
between cancerous and non-cancerous tissues (Fig. 1B; P=0.00256; paired t-test).
Furthermore, patients with long distance metastasis exhibited lower
RKIP expression levels compared with patients with within-lung
metastasis (P=0.018; Table I).
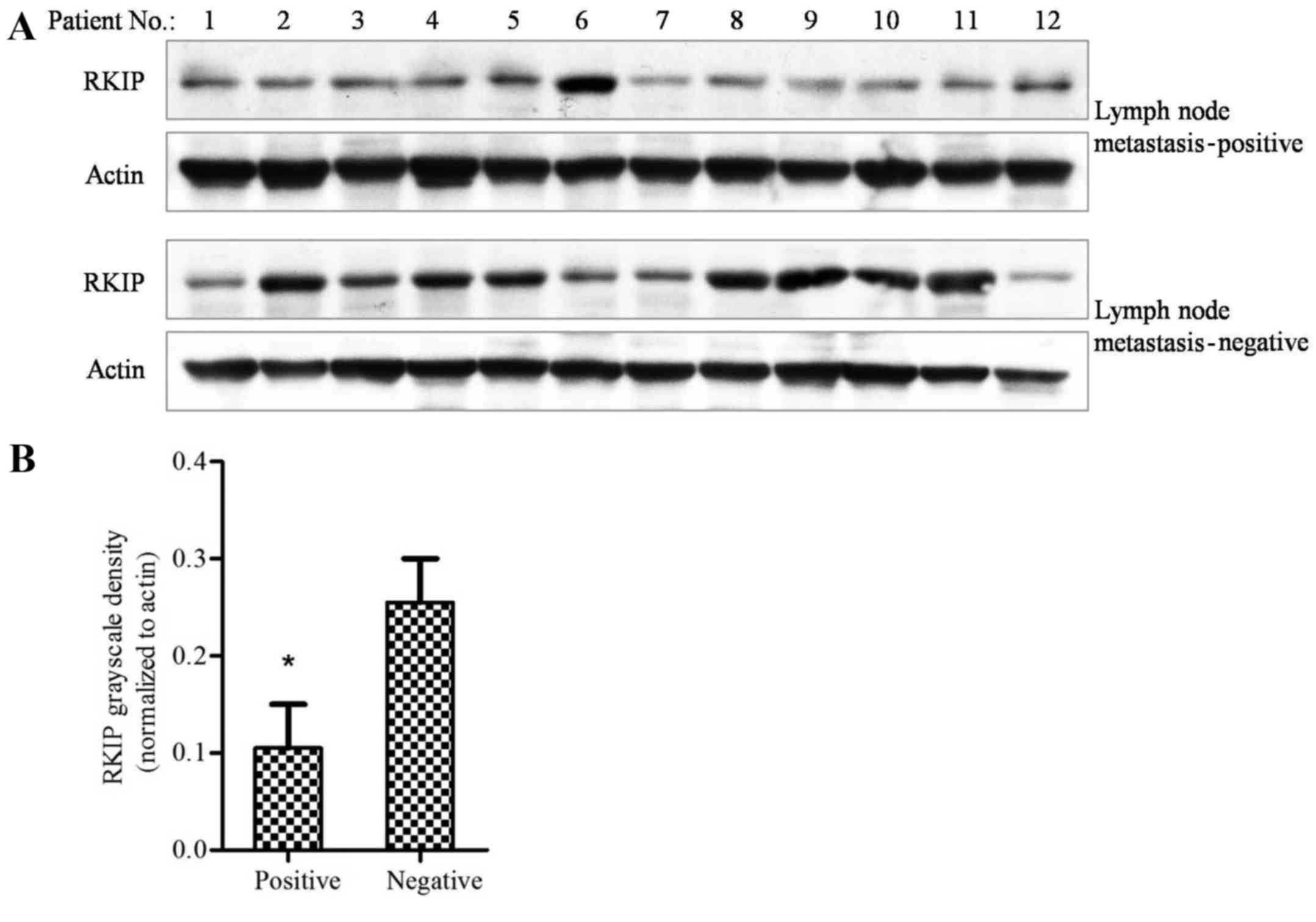
Reduced RKIP expression levels are
associated with NSCLC intra-lung or long distance metastasis
Tissues obtained from patients that were lymph node
metastasis-positive and -negative were examined using western
blotting. The results revealed that patients that were positive for
lymph node metastasis had lower levels of RKIP protein expression,
as compared with tissues from patients that were negative for
metastasis (Fig. 2A). The grayscale
density of each band was evaluated using Photoshop software, and
calculated in Prism 5.0 software. The results revealed that tissue
from patients with lymph node metastasis had significantly lower
levels of RKIP expression compared with that of patients without
metastasis (Fig. 2B; P=0.0013;
χ2 test). This finding indicated that NSCLC metastasis
may be associated with RKIP expression.
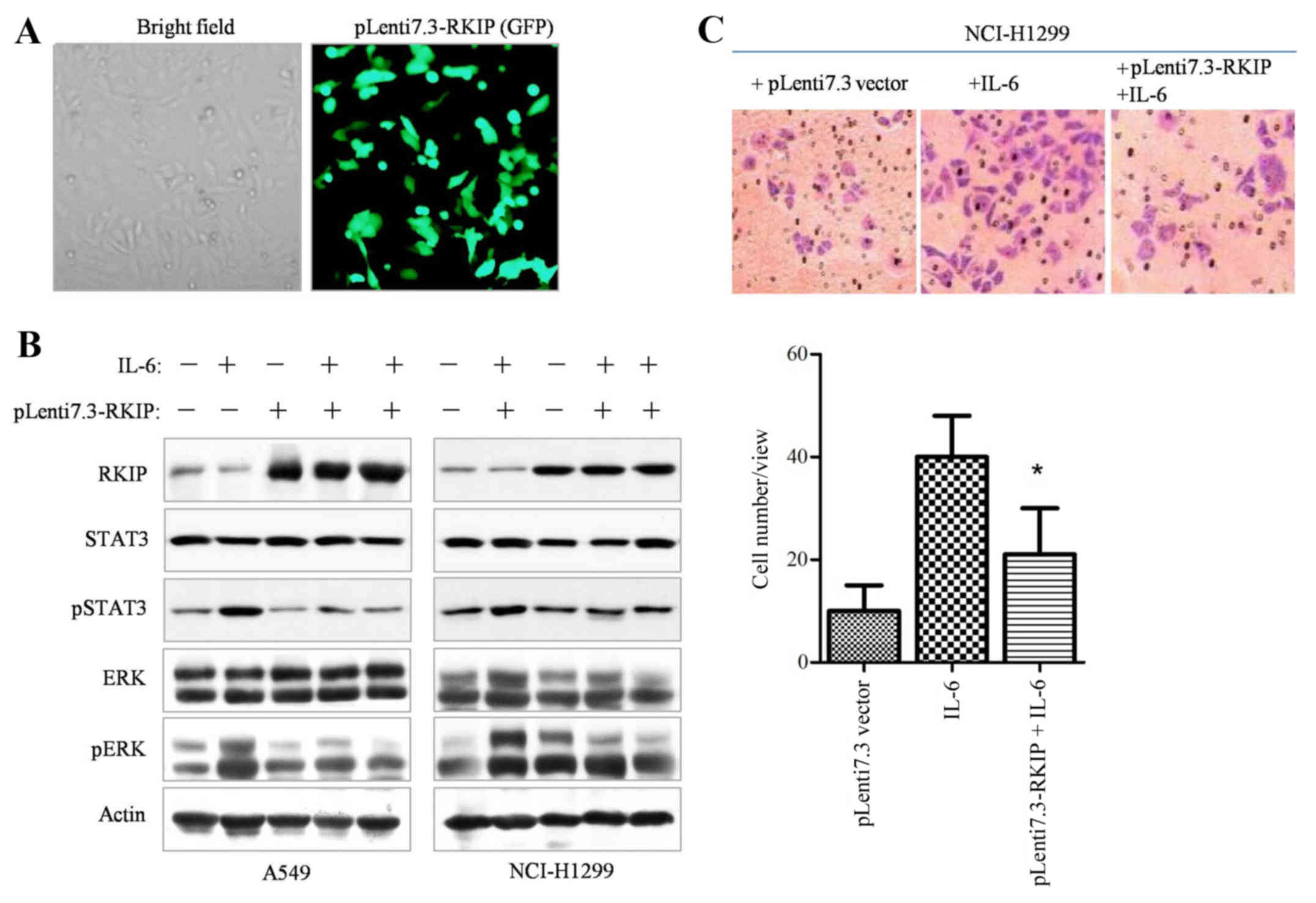
RKIP blocks IL-6-induced
phosphorylation-mediated STAT3 activation and inhibits NSCLC cells
metastasis
In order to elucidate whether RKIP overexpression is
able to inhibit STAT3 phosphorylation in NSCLC cells, the RKIP gene
was constructed into pLenti6.3/V5-DEST lentiviral vectors.
Initially, the infective efficiency of the constructed RKIP
lentivirus vector was assessed by examining GFP fluorescence,
indicating that ≥90% of the cancer cells were infected with viral
vectors (Fig. 3A), and western blot
analysis also demonstrated RKIP overexpression (data not shown).
The western blotting results revealed that overexpression of RKIP
significantly suppressed IL-6-dependent ERK and STAT3
phosphorylation (Fig. 3B, P=0.028).
Upon overexpression of RKIP through Lenti7.3-RKIP infection, the
Transwell invasive ability of NSCLC cells decreased (Fig. 3C, P=0.034). Therefore, the results
demonstrated that RKIP overexpression was able to inhibit STAT3
phosphorylation and cell migration in NSCLC cell lines.
RKIP knockdown promotes STAT3
phosphorylation and NSCLC-cell metastasis
A549 and NCI-H1299 NSCLC cells were seeded into
6-well plates 24 h prior to RKIP siRNA interference. Transfection
reagent-mediated siRNA interference was administered to cancer
cells. At 48 h following incubation, cells were collected and
analyzed. Western blot results revealed that STAT3 phosphorylation
was decreased in the presence of IL-6 stimulation in the two
transfected cell lines, compared with the control group (Fig. 4A and B).
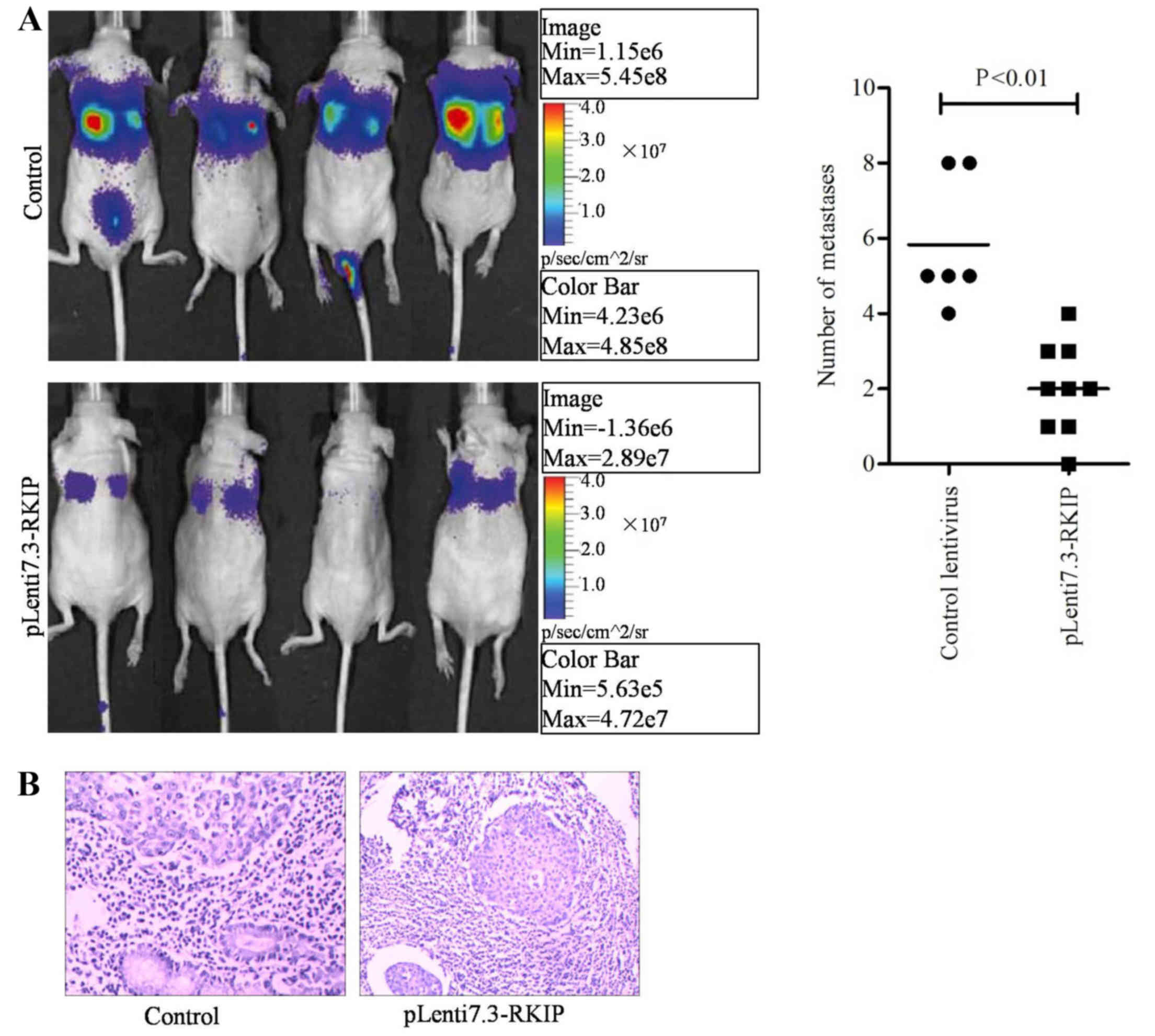
RKIP overexpression suppressed
xenograft tumor metastasis in nude mice
The in vivo study was designed to mimic the
progressive growth of lung metastatic tumors through tail vein
injection of infected or control A549 NSCLC cells at a density of
1×106 cells/mouse. At 6 weeks following transplantation,
bioluminescence was utilized to conduct the lung cancer
cell-derived tissue metastasis assay. Fluorescence signals from
mice were detected using the Xenogen IVIS imaging system. The
results indicated that the RKIP overexpression group had lower
numbers of metastatic tumors within the lung or the whole body,
compared with the control group (Fig.
5A; P=0.0063; Fisher's exact test). Hematoxylin and eosin
staining was performed on mouse tissues, revealing that the lungs
exhibited reduced tumor mass in the RKIP overexpression group,
compared with the control group (Fig.
5B). The experimental results indicated the overexpression of
RKIP may block A549 cancer cell metastasis and growth through the
blockade of downstream signaling pathways, including the
RAF/ERK/STAT3 pathway.
Discussion
The poor prognosis and shorter survival time of
patients with NSCLC are closely associated with tumor cell
aggressive and long distance metastasis (2). The biological behavior of NSCLC cells
may also impact the efficacy of therapeutic agents, due to its
malignancy (2,20). In the present study, based on the data
from 100 patients with NSCLC, negative or weak staining of RKIP was
observed in the majority of cancerous tissues, compared with an
intense signal in non-cancerous tissues (Fig. 1A). Patients at an increased clinical
stage (III/IV) exhibited lower levels of RKIP expression, whereas
stage I/II patients had higher levels of RKIP expression and an
improved prognosis. In total, 57.32% patients with lymph node
metastasis exhibited low levels of RKIP expression, but 50% of
lymph node metastasis-negative patients had high levels of RKIP
expression. Additionally, the statistical data revealed that 65% of
long-distance metastasis patients had low levels of RKIP
expression, whereas >72% of patients with within-lung metastasis
exhibited high levels of RKIP expression (P=0.018, Table I). Taken together, these findings
reveal that NSCLC metastasis is inversely correlated with RKIP
expression levels. This result is concordant with a previous study
examining NSCLC and RKIP expression (12).
A previous study revealed that silenced Raf
expression promoted tumor invasive ability, whereas its
upregulation suppressed tumor invasive ability (21). Other previous studies have indicated
that Raf kinase activated transcriptional activity via activation
of the ERK signaling pathway (22,23). RKIP
is a negative regulator of the RAS-mitogen-activated protein kinase
and ERK signaling pathway (24). A
previous study indicated using immunohistochemistry that RKIP
expression levels were detectable in all non-cancerous prostate
tissues and were decreased to low or undetectable levels in all
prostate cancer metastases (25).
Similar results have been demonstrated in malignant melanoma,
colorectal, breast, thyroid and liver cancer (26–28).
Concordant with the results from previous studies, the current
study also revealed that RKIP may be associated with the
Raf/MEK/ERK/STAT3 pathway, and clinical data from 100 patients with
NSCLC also supported the hypothesis that RKIP expression levels are
associated with NSCLC metastasis. Patients with positive metastatic
lymph nodes, with intra-lung or long-distance metastasis, had lower
RKIP protein levels (Figs. 1 and
2; Table
I), and lower RKIP expression levels were observed with
increasing TNM stage. Previous findings have demonstrated that
pRKIP is a predictive indicator of lung cancer survival (24). Therefore, future studies may further
examine the role of RKIP in NSCLC, as well as investigating pRKIP,
its kinase and underlying mechanisms in this form of cancer.
In conclusion, the present study suggests an
important role for RKIP in NSCLC on the basis of in vitro
data, in vivo metastatic experiments and clinical statistic
analysis. The findings strengthen the hypothesis that RKIP
suppresses NSCLC cell metastasis through blocking of the
Raf/ERK/STAT3 signaling pathway.
Acknowledgements
The current study was supported by the Natural
Science Foundation of Anhui Province (grant no. 1408085MH144).
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Black RC and Khurshid H: NSCLC: An update
of driver mutations, their role in pathogenesis and clinical
significance. R I Med J (2013). 98:25–28. 2015.PubMed/NCBI
|
|
3
|
Alberg AJ, Brock MV, Ford JG, Samet JM and
Spivack SD: Epidemiology of lung cancer: Diagnosis and management
of lung cancer, 3rd ed: American College of Chest Physicians
evidence-based clinical practice guidelines. Chest. 143:(5 Suppl).
e1S–e29S. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tang Z, Li J, Shen Q, Feng J, Liu H, Wang
W, Xu L, Shi G, Ye X, Ge M, et al: Contribution of upregulated
dipeptidyl peptidase 9 (DPP9) in promoting tumoregenicity,
metastasis and the prediction of poor prognosis in non-small cell
lung cancer (NSCLC). Int J Cancer. Dec 10–2016.(Epub ahead of
print).
|
|
5
|
Goyette MA and Côté JF: NSCLC metastasis:
Going with ELMO3. Oncotarget. 5:5850–5851. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Escara-Wilke J, Yeung K and Keller ET: Raf
kinase inhibitor protein (RKIP) in cancer. Cancer Metastasis Rev.
31:615–620. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Deiss K, Kisker C, Lohse MJ and Lorenz K:
Raf kinase inhibitor protein (RKIP) dimer formation controls its
target switch from Raf1 to G protein-coupled receptor kinase (GRK)
2. J Biol Chem. 287:23407–23417. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Farooqi AA, Li Y and Sarkar FH: The
biological complexity of RKIP signaling in human cancers. Exp Mol
Med. 47:e1852015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yun J, Frankenberger CA, Kuo WL, Boelens
MC, Eves EM, Cheng N, Liang H, Li WH, Ishwaran H, Minn AJ and
Rosner MR: Signalling pathway for RKIP and Let-7 regulates and
predicts metastatic breast cancer. EMBO J. 30:4500–4514. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jia B, Liu H, Kong Q and Li B: RKIP
expression associated with gastric cancer cell invasion and
metastasis. Tumour Biol. 33:919–925. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fu Z, Kitagawa Y, Shen R, Shah R, Mehra R,
Rhodes D, Keller PJ, Mizokami A, Dunn R, Chinnaiyan AM, et al:
Metastasis suppressor gene Raf kinase inhibitor protein (RKIP) is a
novel prognostic marker in prostate cancer. Prostate. 66:248–256.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang Q, Wu X, Wu T, Li GM and Shi Y:
Clinical significance of RKIP mRNA expression in non-small cell
lung cancer. Tumour Biol. 35:4377–4380. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tan FH, Putoczki TL, Stylli SS and Luwor
RB: The role of STAT3 signaling in mediating tumor resistance to
cancer therapy. Curr Drug Targets. 15:1341–1353. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Saxena NK, Sharma D, Ding X, Lin S, Marra
F, Merlin D and Anania FA: Concomitant activation of the JAK/STAT,
PI3K/AKT, and ERK signaling is involved in leptin-mediated
promotion of invasion and migration of hepatocellular carcinoma
cells. Cancer Res. 67:2497–2507. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jin HO, Lee YH, Park JA, Kim JH, Hong SE,
Kim HA, Kim EK, Noh WC, Kim BH, Ye SK, et al: Blockage of Stat3
enhances the sensitivity of NSCLC cells to PI3K/mTOR inhibition.
Biochem Biophys Res Commun. 444:502–508. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gorgisen G, Ozes D, Pehlivanoglu S,
Erdogan A, Dertsiz L, Ozbilim G, Ozbudak IH, Savas B and Ozes ON:
Differential expression and activation of epidermal growth factor
receptor 1 (EGFR1), ERK, AKT, STAT3, and TWIST1 in nonsmall cell
lung cancer (NSCLC). Exp Lung Res. 39:387–398. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ye CG, Chen GG, Ho RL, Merchant JL, He ML
and Lai PB: Epigenetic upregulation of Bak by ZBP-89 inhibits the
growth of hepatocellular carcinoma. Biochim Biophys Acta.
1833:2970–2979. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang G, Liu F, Chen XY, Fang WY, Li HL,
Huang ZP, Chu HJ, Wang X and Zhao T: Construction and
identification of a recombinant lentivirus harboring small
interfering RNA targeting mouse CD99 antigen-like 2 gene. Nan Fang
Yi Ke Da Xue Xue Bao. 29:228–231. 2009.(In Chinese). PubMed/NCBI
|
|
19
|
Zheng J, Wang J, Sun X, Hao M, Ding T,
Xiong D, Wang X, Zhu Y, Xiao G, Cheng G, et al: HIC1 modulates
prostate cancer progression by epigenetic modification. Clin Cancer
Res. 19:1400–1410. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Reinmuth N, Stumpf P, Stumpf A, Muley T,
Kobinger S, Hoffmann H, Herth FJ, Schnabel PA, Bischoff H and
Thomas M: Characteristics of lung cancer after a previous
malignancy. Respir Med. 108:910–917. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chatterjee D, Sabo E, Tavares R and
Resnick MB: Inverse association between Raf kinase inhibitory
protein and signal transducers and activators of transcription 3
expression in gastric adenocarcinoma patients: Implications for
clinical outcome. Clin Cancer Res. 14:2994–3001. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ulivi P, Arienti C, Amadori D, Fabbri F,
Carloni S, Tesei A, Vannini I, Silvestrini R and Zoli W: Role of
RAF/MEK/ERK pathway, p-STAT-3 and Mcl-1 in sorafenib activity in
human pancreatic cancer cell lines. J Cell Physiol. 220:214–221.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Aziz MH, Hafeez BB, Sand JM, Pierce DB,
Aziz SW, Dreckschmidt NE and Verma AK: Protein kinase Cvarepsilon
mediates Stat3Ser727 phosphorylation, Stat3-regulated gene
expression, and cell invasion in various human cancer cell lines
through integration with MAPK cascade (RAF-1, MEK1/2, and ERK1/2).
Oncogene. 29:3100–3139. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Huerta-Yepez S, Yoon NK, Hernandez-Cueto
A, Mah V, Rivera-Pazos CM, Chatterjee D, Vega MI, Maresh EL,
Horvath S, Chia D, et al: Expression of phosphorylated raf kinase
inhibitor protein (pRKIP) is a predictor of lung cancer survival.
BMC Cancer. 11:2592011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yousuf S, Duan M, Moen EL, Cross-Knorr S,
Brilliant K, Bonavida B, LaValle T, Yeung KC, Al-Mulla F, Chin E
and Chatterjee D: Raf kinase inhibitor protein (RKIP) blocks signal
transducer and activator of transcription 3 (STAT3) activation in
breast and prostate cancer. PLoS One. 9:e924782014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cross-Knorr S, Lu S, Perez K, Guevara S,
Brilliant K, Pisano C, Quesenberry PJ, Resnick MB and Chatterjee D:
RKIP phosphorylation and STAT3 activation is inhibited by
oxaliplatin and camptothecin and are associated with poor prognosis
in stage II colon cancer patients. BMC Cancer. 13:4632013.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Datar I, Qiu X, Ma HZ, Yeung M, Aras S, de
la Serna I, Al-Mulla F, Thiery JP, Trumbly R, Fan X, et al: RKIP
regulates CCL5 expression to inhibit breast cancer invasion and
metastasis by controlling macrophage infiltration. Oncotarget.
6:39050–39061. 2015.PubMed/NCBI
|
|
28
|
Zhang XM, Gu H, Yan L and Zhang GY: RKIP
inhibits the malignant phenotypes of gastric cancer cells.
Neoplasma. 60:196–202. 2013. View Article : Google Scholar : PubMed/NCBI
|