Introduction
Hypoxia is an established characteristic of
high-grade breast tumors (1) and is
considered to be associated with increased therapy resistance, poor
disease-free survival (DFS) and a high rate of metastasis (2–6). The
hypoxia-inducible factor family members are key elements in the
hypoxic signaling pathway (7).
Intratumoral hypoxia is caused by abnormal microvasculature in
proliferating tumor tissues, and may induce proteomic changes,
allowing tumors to adapt to or overcome the nutrient-deprived state
(8–11). This is accomplished with enhanced
glycolysis, inhibition of apoptosis and the increased expression of
certain proteins that are associated with tumor invasiveness
(12,13). HIF-1α is a cytoplasmic protein that is
involved in the cellular response to alterations in oxygen levels,
and is important in the expression of hypoxia-inducible genes
(14). HIF-1α protein is expressed
and continuously degraded under normoxic conditions due to binding
to the von Hippel-Lindau protein in the cytoplasm (15). Under hypoxic conditions, HIF-1α
subunits translocate to the nucleus and heterodimerize with HIF-1β
subunits (2). HIF-1α overexpression
and signaling result in adaptive responses, which may enhance
vascular endothelial growth factor-mediated angiogenesis in order
to meet the energy requirements of the cell (16). Basal-like breast carcinoma differs
from the luminal type due to its negative expression of certain
hormone receptors and associated genes, and positive expression of
cytokeratin 5/6 (CK5/6) (17).
Basal-like breast carcinoma lesions frequently exhibit increased
hypoxia and a high tumor grade (18,19), which
triggers further necrosis and aggressive behavior, as compared with
the luminal type (20), and tumor
necrosis is considered a consequence of hypoxia (11,21). The
current study hypothesized that these tumors may have altered
hypoxic responses and metabolic requirements.
Another important mechanism underlying necrosis is a
lack of gluconeogenesis in the tumor cell environment, in which
cells grow at a rate that outstrips the rate of energy production
(22). Upregulation of the HIF-1α
gene and its downstream target genes may result in glycolysis
(23). Fructose 1,6-bisphophatase is
a rate-limiting enzyme that functions during gluconeogenesis
(24,25) to convert fructose-1,6-bisphophate
(FBP1) into fructose-6-phosphate and inorganic phosphates. FBP1 is
recognized as a gluconeogenesis regulatory enzyme (24,26).
Previous studies have indicated that an increase in FBP1 expression
levels predicts an improved outcome across a spectrum of neoplastic
diseases, including kidney (27,28),
stomach and lung carcinoma (29).
These results suggest that the epigenetic regulation of FBP1 is
important in modulating glucose metabolism in cancer. Li et
al (28) identified that FBP1
limits clear-cell renal cell carcinoma proliferation by inhibiting
the function of nuclear HIF via a direct interaction with the HIF
inhibitory domain.
The present study hypothesized that FBP1 may possess
anticancer properties in breast cancer cell lines, potentially due
to the suppression of HIF-1α expression levels. Therefore, the
expression levels of HIF-1α and FBP1 were investigated using
immunohistochemical analysis in human luminal and basal-like breast
cancer tissues. Subsequently, the association between clinical
characteristics and the expression levels of HIF-1α and FBP1 was
analyzed.
Materials and methods
Patient selection and
clinicopathological analysis
Tumor tissue samples from patients with breast
cancer were obtained by resection between September 2004 and
September 2008 at The Tumor Hospital, Harbin Medical University
(Harbin, China). Paraffin-embedded tissue samples were obtained
retrospectively from the archives of the Department of Pathology.
Informed patient consent for the anonymous use of the remainder of
tumor material was obtained as part of the standard treatment
agreement. All tissue specimens had been fixed for ≤24 h in neutral
buffered 4% formaldehyde and classified according to the World
Health Organization (30). All
patients had operable breast carcinoma and were not diagnosed with
metastatic disease at the time of presentation. Information
regarding patient characteristics, including patient age at initial
diagnosis, tumor size, nuclear grade, histology and nodal status,
were obtained from the clinical and pathological records. The mean
age of the patients was 53 years (range, 25–70). In total, 43% of
the tumors were invasive ductal of no specific type, 37% were
invasive lobular carcinoma and 20% were of other histological
classifications. Histological classification revealed 38 luminal
type and 26 basal-like type cases. Tumors were graded using the
Elston criteria, as grade 1 (n=22), grade 2 (n=22) or grade 3
(n=20) (31). Nodal disease was
present in 55% of patient tissue samples. None of the patients
received preoperative chemotherapy, hormonal treatment or
radiotherapy. Adjuvant systemic treatment (chemotherapy for
premenopausal and tamoxifen for postmenopausal patients) was
administered according to the established guidelines of the
National Comprehension Cancer Network (32). Estrogen receptor (ER) status was
determined routinely by immunohistochemistry (33). The follow-up period was 16–84 months
(mean, 60) for surviving patients. During follow-up, 42 patients
developed loco-regional recurrence (n=9) or distant metastases
(n=33), leading to a total of 33 disease-associated mortalities.
Four additional patients succumbed to unrelated conditions and were
removed from the survival analysis. Approval for the analyses
conducted in the present study was received from The Ethics
Committee of Harbin Medical University.
DFS was evaluated as the time from the date of the
initial curative surgery to the date of the first loco-regional or
systemic relapse, or mortality in the absence of relapse.
Immunohistochemistry was performed on 3-µm thick tissue sections.
Table I presents all antibodies,
dilutions, antigen-retrieval methods, incubation times and methods
of detection used. Tissue sections were deparaffinized with xylene
and rehydrated with ethanol solutions. The optimal primary antibody
incubation times and concentrations were determined via serial
dilution for each immunohistochemical assay using an identically
fixed and embedded tissue block. The slides were counterstained
with Harris hematoxylin. The degree of staining was determined by
two pathologists using a multiview light microscope.
 | Table I.Antibodies and experimental
conditions for immunohistochemistry. |
Table I.
Antibodies and experimental
conditions for immunohistochemistry.
| Catalog number | Specificity | Source | Dilution | Antigen-retrieval
method | Incubation
time |
|---|
| ab85886 | HIF-1α | Abcam (Cambridge,
UK) | 1:500 | Microwave, citrate
buffer, 95°C, 30 min | 30 min, RT |
| ab196556 | FBP1 | Abcam | 1:200 | Microwave, citrate
buffer, 95°C, 20 min | Overnight, 4°C |
| ab86974 | CK5/6 | Abcam | 1:50 | Pepsin enzyme, RT,
30–60 sec | 30 min, RT |
Immunohistochemical staining
Tumor sections were placed in ice-cold 4% formalin
containing a phosphatase-inhibiting reagent (4% paraformaldehyde
for 24–48 h), dehydrated using ethanol (100, 95, 85 and 75%),
paraffin embedded, and stained (Table
I). Samples were incubated with 3% hydrogen peroxide solution
(Sinopharm Chemical Reagent Co., Ltd., Shanghai, China) at room
temperature for 15 min to eliminate endogenous peroxidase activity.
Samples were washed with PBS three times. Samples were blocked with
goat serum (Hyclone, GE Healthcare Life Sciences, Logan, UT, USA)
for 60 min at room temperature. Samples were subsequently incubated
with the primary antibodies presented in Table I. Samples were washed with PBS three
times. Samples were incubated with biotin-labeled secondary
anti-rabbit antibody (cat. no. ab6721; dilution, 1:200; Abcam,
Cambridge, UK) at 37°C for 60 min. Samples were washed with PBS
three times. Samples were incubated with horseradish
peroxidase-labeled chain mildew avidin or alkaline phosphatase
(ZSGB-Biology Co., Ltd., Beijing, China) at 37°C for 30 min.
Samples were washed with PBS three times. Samples were stained with
diaminobenzidine (ZSGB-Biology Co., Ltd.) for between 3 and 10 min
in darkness and observed using an inverted microscope,
magnification, ×4-10. Samples were washed twice with
H2O. Samples were counterstained with hematoxylin
(Western Biology Co., Ltd., Chongqing, China) for 1 min. Samples
were washed with H2O. Samples were dehydrated in an
ascending alcohol (Sinopharm Chemical Reagent Co., Ltd.) series of
75, 85, 95 and 100% for 3 min. Xylene (Sinopharm Chemical Reagent
Co., Ltd.) was used to deparaffinize the specimens for 3 min.
Neutral gum (ZSGB-Biology Co., Ltd.,) was used to seal the
samples.
Evaluation of immunohistochemical
staining
All immunohistochemical markers (HIF-1α, FBP1 and
CK5/6) were assessed using light microscopy. Scoring of the
immunostained slides was performed according to the proportion of
tumor cells that exhibited nuclear (HIF-1α and FBP1) staining.
HIF-1α staining was considered positive when an immunohistochemical
signal was observed in ≥5% of nuclei, according to the cut-off
value previously utilized by Bos et al (34). FBP1 expression was considered positive
when >25% of the tumor cell nuclei were stained.
Molecular classification of breast
cancer according to immunohistochemistry
According to the results of immunohistochemistry,
breast cancer types were classified into basal-like type (CK5/6
positive and/or EGFR positive) or luminal type [ER positive and/or
progestin receptor (PR) positive] (30). The expression levels of ER and PR were
designated as positive when ≥1% of the tumor nuclei exhibited
positive staining. Human epidermal growth factor receptor-2 (HER2)
expression levels were also classified using immunohistochemical
staining based on the HercepTest™ (Dako; Agilent Technologies,
Inc., Santa Clara, CA, USA) (35). A
tumor was considered to be HER2-positive if the tissue specimen
scored ≥3 for the intensity of membrane staining in the tumor
cells.
Statistical analysis
Data were analyzed using SPSS version 19.0 for
Windows (IBM SPSS, Armonk, NY, USA). Student's t-tests and Fisher's
exact tests were used for continuous and categorical variables,
respectively. DFS rates periods following surgery were calculated
using the Kaplan-Meier method, and the variation between survival
curves was assessed using the log-rank test. Multivariate
regression analysis was performed using the Cox proportional
hazards model. Pearson correlation analysis was used to compare the
associations between FBP1 and HIF-1α expression levels. Values are
presented as the mean ± standard deviation. P<0.05 was
considered to indicate a statistically significant difference.
Results
Clinical data
Of the 60 tumor tissue samples, 36 (60%) were
luminal-type tumors (Table II),
whereas 24 tissue samples (40%) were basal-like tumors.
 | Table II.Patient characteristics in various
types of breast cancer. |
Table II.
Patient characteristics in various
types of breast cancer.
|
Characteristics | All (n=60) | Luminal (n=36) | Basal-like
(n=24) |
P-valuea |
|---|
| Mean age,
years | 53±8.7 (25-70) | 55±9.0 (30-68) | 49±9.0 (25-70) | 0.932 |
| Menopausal
status |
|
|
| 0.312 |
|
Premenopausal | 22 | 15 (68.2) | 7
(31.8) |
|
|
Postmenopausal | 38 | 21 (55.3) | 17 (44.7) |
|
| Stage |
|
|
| 0.098 |
| I | 20 | 14 (70.0) | 6
(30.0) |
|
| II | 28 | 17 (60.7) | 11 (39.3) |
|
|
III | 12 | 5
(41.7) | 7
(58.3) |
|
| T stage |
|
|
| 0.132 |
| T1 | 30 | 24 (80.0) | 6
(20.0) |
|
| T2 | 18 | 8
(44.4) | 10 (55.6) |
|
| T3 | 12 | 4
(33.3) | 8
(66.7) |
|
| Nodal status |
|
|
| 0.050 |
|
Negative | 27 | 17 (63.0) | 10 (37.0) |
|
|
Positive | 33 | 19 (57.6) | 14 (42.4) |
|
| Nuclear grade |
|
|
| 0.191 |
| I | 22 | 18 (81.8) | 4
(18.2) |
|
| II | 19 | 12 (65.5) | 7
(34.5) |
|
|
III | 19 | 9
(47.4) | 10 (52.6) |
|
| Histology |
|
|
| 0.203 |
| Ductal
carcinoma | 26 | 16 (61.5) | 10 (38.5) |
|
| Lobular
carcinoma | 22 | 19 (86.4) | 3
(13.6) |
|
|
Other | 12 | 1
(8.30) | 11 (91.7) |
|
Expression of HIF-1α
Increased expression of HIF-1α was primarily
identified in the nuclei of tumor cells (Fig. 1A and B). Using a cut-off value of ≥5%
of nuclei, positive HIF-1α expression was identified in 20/60
(33.3%) cases (Table III). Of
these, 8 tumors (40%) were luminal-type and 12 tumors (60%) were
basal-type. No association was observed between HIF-1α expression
levels and patient age (P=0.124), nuclear grade (P=0.732) or
advanced tumor stage (P=0.411; Table
IV). Increased expression levels of HIF-1α were associated with
a high degree of lymph node metastasis (P=0.007; Table IV).
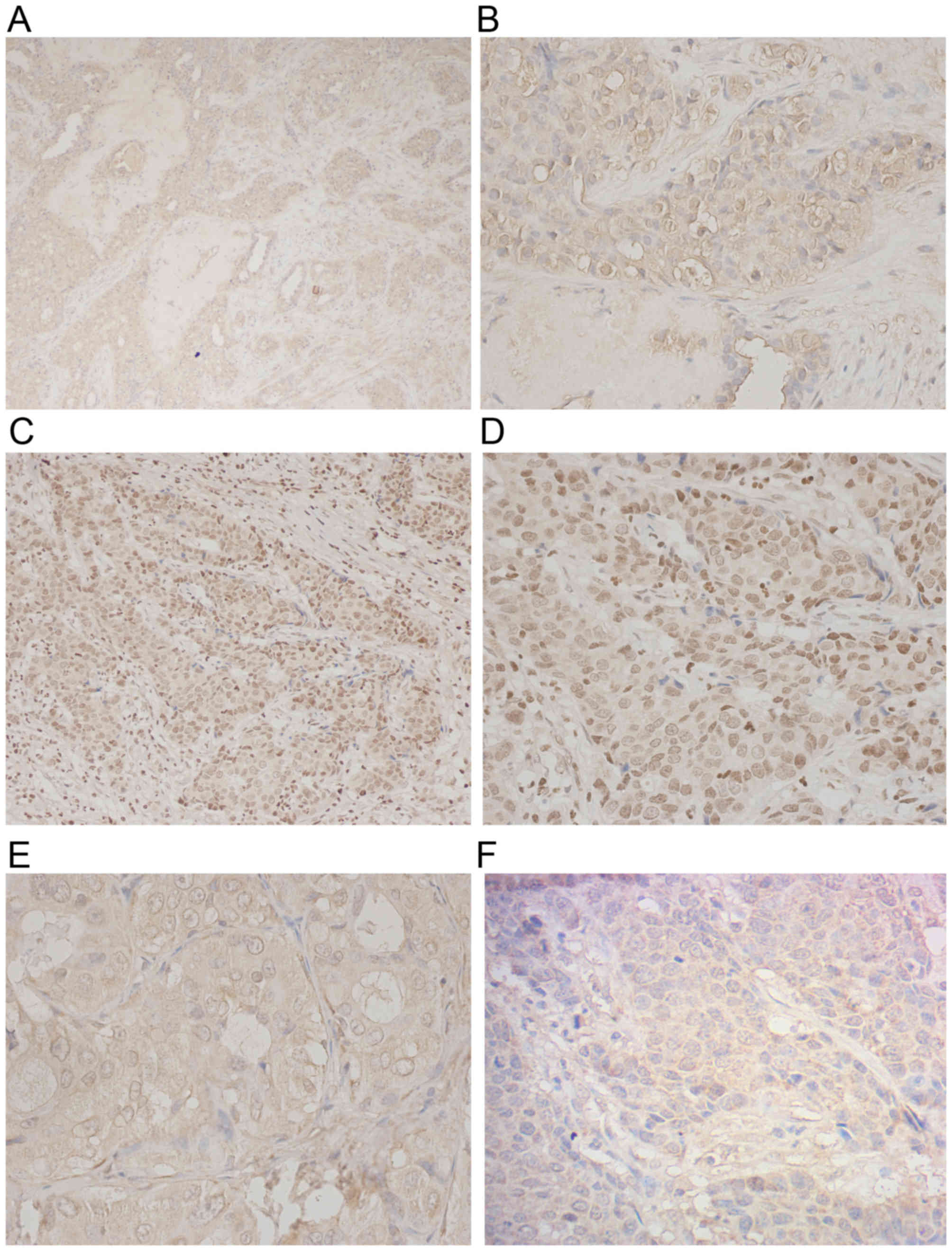 | Figure 1.Immunohistochemical analysis of
HIF-1α, CK5/6 and FBP1 expression levels in human breast cancer.
(A) HIF-1α expression was observed in invasive breast cancer
(original magnification, ×100). (B) High expression of HIF-1α was
detected in tumor cell nuclei (original magnification, ×400). CK5/6
expression was observed within basal-like breast cancer tissue at
(C) original magnification, ×200 and (D) original magnification,
×400. (E) The expression of FBP1 in luminal breast cancer tissue
(original magnification, ×400). (F) Low nuclear expression of FBP1
was detected in basal-like breast cancer tissue (original
magnification, ×400). HIF-1α, hypoxia-inducible factor-1α; FBP1,
liver fructose-1,6-bisphophatase; CK5/6, cytokeratin 5/6. |
 | Table III.Immunohistochemical characteristics
of cancer cells according to breast cancer phenotype. |
Table III.
Immunohistochemical characteristics
of cancer cells according to breast cancer phenotype.
| Antibodies | Total, n=60
(%) | Basal-like, n=24
(40%) | Luminal, n=36
(60%) | P-value |
|---|
| HIF-1α |
|
|
| 0.056 |
|
Negative | 40 (66.7) | 12 (30.0) | 28 (70.0) |
|
|
Positive | 20 (33.3) | 12 (60.0) | 8 (40.0) |
|
| FBP1 |
|
|
| 0.008a |
|
Negative | 27 (45) | 18 (66.7) | 9 (33.3) |
|
|
Positive | 33 (55) | 6 (18.2) | 27 (81.8) |
|
| CK5/6 |
|
|
| 0.043a |
|
Negative | 36 (60) | 0 (0.0) | 36 (100.0) |
|
|
Positive | 24 (40) | 24 (100.0) | 0 (0.0) |
|
 | Table IV.Correlation between the expression
levels of metabolism genes and clinicopathological factors. |
Table IV.
Correlation between the expression
levels of metabolism genes and clinicopathological factors.
|
| HIF-1α | FBP1 |
|---|
|
|
|
|
|---|
| Parameters | (−) | (+) | P-value | (−) | (+) | P-value |
|---|
| Age, years |
|
| 0.124 |
|
| 0.475 |
|
≤35 | 10 | 1 |
| 13 | 7 |
|
|
>35 | 34 | 15 |
| 14 | 26 |
|
| Nuclear grade |
|
| 0.732 |
|
| 0.017a |
|
I/II | 28 | 13 |
| 10 | 31 |
|
|
III | 16 | 3 |
| 17 | 2 |
|
| T stage |
|
| 0.411 |
|
| 0.012a |
| T1 | 25 | 5 |
| 6 | 24 |
|
|
T2-3 | 19 | 11 |
| 21 | 9 |
|
| N stage |
|
| 0.007a |
|
| 0.864 |
| N0 | 25 | 2 |
| 6 | 21 |
|
|
N1-3 | 19 | 14 |
| 21 | 12 |
|
Correlation between FBP1 expression
levels and clinicopathological parameters in invasive breast
carcinoma
Localization of FBP1 staining in the tissues was
examined using conventional light microscopy. The levels of
immunohistochemical staining in the nuclei (Fig. 1E and F) were divided into positive and
negative FBP1 expression tumor groups. FBP1 was positive in 33/60
tumors (55%; Table III). The
majority of these tumors (27/36; 75%) were of the luminal category.
FBP1 in basal-like breast adenocarcinomas exhibited low or absent
expression levels in 6/24 (18.2%) cases (P=0.008). FBP1 expression
was significantly correlated with small tumor size (P=0.012) and
high nuclear grade (P=0.017; Table
IV). No correlation was observed between patient age (P=0.475)
and nodal status (P=0.864; Table
IV).
Correlation between the expression
levels of HIF-1α and FBP1
The expression levels of CK5/6 were higher in the
basal-like carcinoma tissues, whereas luminal cases exhibited low
CK5/6 expression levels (P=0.043; Fig. 1C
and D). In addition, increased HIF-1α expression levels were
positively correlated with low or absent expression levels of FBP1
in invasive breast carcinoma, and this aspect was statistically
significant (r=−0.711; P<0.001). In luminal breast carcinoma,
increased HIF-1α expression levels were significantly correlated
with low or absent levels of FBP1 expression (r=−0.772;
P<0.001). In basal-like breast carcinoma, HIF-1α expression
levels were significantly negatively correlated with the levels of
FBP1 expression (r=−0.577; P=0.003).
Survival analysis
In univariate survival analysis, patients with tumor
tissues that did not express FBP1 had a significantly poorer DFS
(P<0.001; data not shown). There was a significant difference in
the DFS of patients with any type of breast carcinoma, when
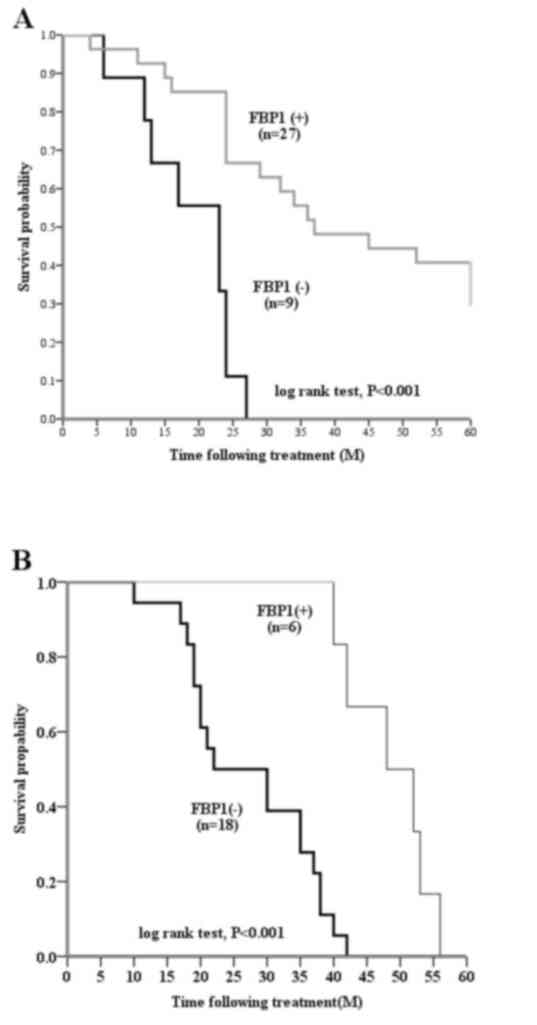
stratified by the levels of FBP1 expression (P<0.001; Fig. 2A and B). Patients with FBP1-positive
basal-like breast carcinomas had a significantly longer DFS,
compared with patients with FBP1-negative basal-like breast
carcinomas (P<0.001; Fig. 2B).
Significant differences in DFS were observed among the luminal
subtypes (P<0.001), and when basal-like carcinoma types were
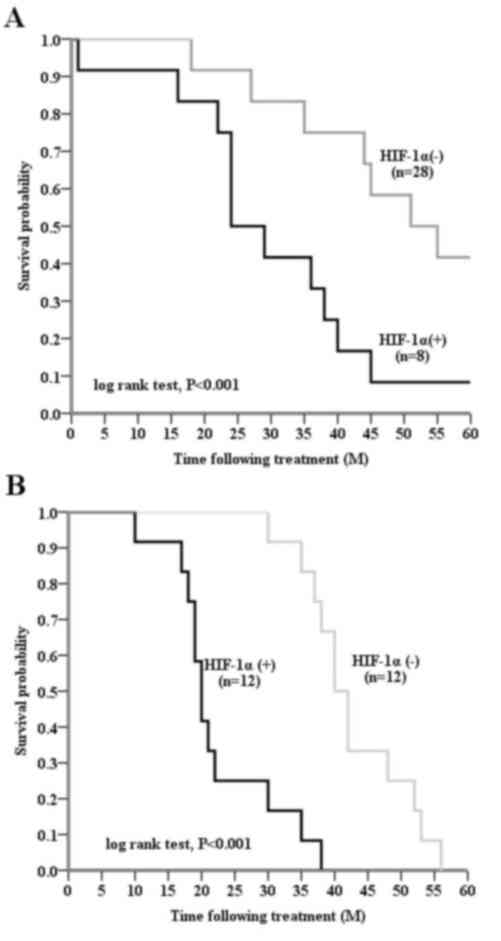
stratified according to HIF-1α expression levels (P<0.001).
HIF-1α-positive tumor tissue samples were significantly associated
with a shorter DFS (P<0.001), compared with HIF-1α-negative
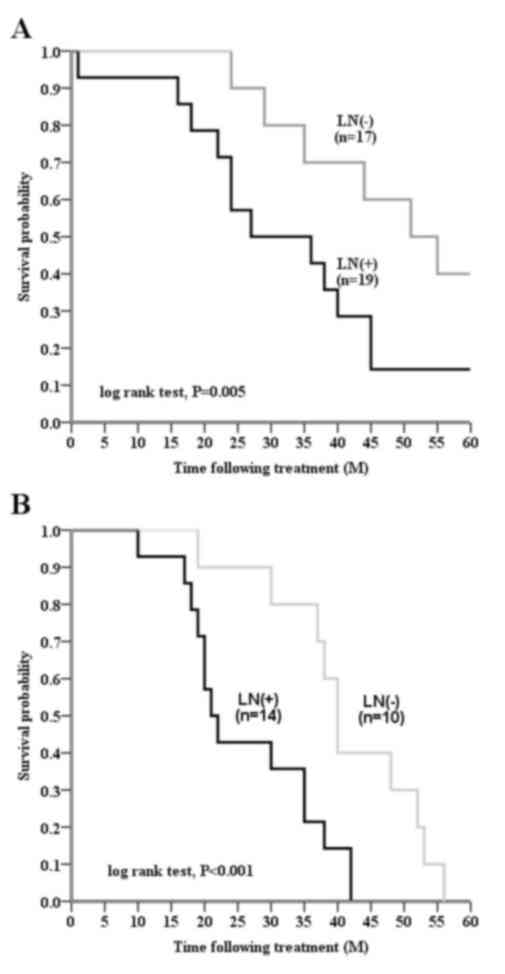
tissue samples, in patients with invasive breast cancer (Fig. 3A and B). Node-positive patients
exhibited a shorter DFS, as compared with node-negative patients
(P=0.001; Fig. 4; Table V). In a multivariate Cox regression
model, FBP1 and tumor size were identified as independent
prognostic factors (Table VI).
 | Table V.Univariate analysis of
clinicopathological and immunohistological results and disease-free
survival. |
Table V.
Univariate analysis of
clinicopathological and immunohistological results and disease-free
survival.
|
|
| Disease-free
survival |
|---|
|
|
|
|
|---|
| Parameters | Number of patients
(n=60) | Mean survival,
months (95% CI) | P-value |
|---|
| T stage |
|
| 0.013a |
| T1 | 23 | 40.9
(35.4-46.4) |
|
|
T2-3 | 37 | 29.1
(24.7-33.5) |
|
| N stage |
|
|
<0.001a |
| N0 | 27 | 41.9
(36.4-47.4) |
|
|
N1-3 | 33 | 26.8
(23.1-30.6) |
|
| Age |
|
| 0.124 |
|
≤35 | 11 | 28.3
(22.1-33.7) |
|
|
>35 | 49 | 49.5
(41.6-55.8) |
|
| Histological
grade |
|
| 0.667 |
|
I/II | 41 | 41.5
(35.4-44.7) |
|
|
III | 19 | 31.5
(26.1-35.1) |
|
| HIF-1α |
|
|
<0.001a |
|
Negative | 40 | 40.5
(36.6-44.4) |
|
|
Positive | 20 | 19.8
(16.7-22.9) |
|
| FBP1 |
|
|
<0.001a |
|
Negative | 27 | 23.6
(20.0-27.3) |
|
|
Positive | 33 | 41.8
(37.3-46.2) |
|
 | Table VI.Multivariate analysis of prognostic
markers. |
Table VI.
Multivariate analysis of prognostic
markers.
| Variable | Odds ratio | 95% CI | P-value |
|---|
| FBP1 (−) vs.
(+) | 0.289 | 0.103-0.734 | 0.010a |
| HIF-1α (−) vs.
(+) | 1.012 | 0.407-2.395 | 0.797 |
| T-stage (<2 cm)
vs. (>2 cm) | 1.672 | 1.158-3.310 | 0.012a |
| LN (−) vs. (+) | 1.158 | 0.634-2.163 | 0.613 |
Discussion
Livasy et al (36) reported that the most frequent
immunophenotype of basal-like breast adenocarcinoma consists of
negative expression of ER and HER2, and positive expression of
CK5/6, CK8/18 and vimentin. CK5/6 is considered to be the most
common basal marker of metabolism-associated adaptation in
basal-like breast cancer (37). The
results of the present study revealed an association between low
expression levels of FBP1 and CK5/6 positivity.
In the current study, increased HIF-1α expression
levels did not differ significantly between luminal and basal-like
breast carcinoma. In these forms of breast carcinoma, expression of
HIF-1α was primarily detected in the tumor cell nuclei. In
addition, the overexpression of HIF-1α was detected in 20/60
(33.3%) invasive breast cancer tissues, when this was defined as
≥5% nuclear HIF-1α immunoreactivity. Yamamoto et al
(38) observed increased HIF-1α
expression levels in invasive breast cancer. A number of studies
have suggested that HIF-1α protein overexpression is a marker of
poor prognosis in patients with primary breast cancer (39,40). In
the current study, total HIF-1α expression was negatively
correlated with DFS. There is an urgent requirement to predict the
factors that may be associated with HIF-1α expression in clinical
tissue samples. The present study demonstrated associations between
HIF-1α expression levels, and lymph node metastasis and FBP1
expression.
A significant association between positive node
status and intense, diffuse HIF-1α staining in breast tumor tissues
was observed during the current study, and HIF-1α expression levels
were markedly higher in lymph node metastatic tumors. These results
are concordant with the findings of previous studies (40,41), which
demonstrated that the expression levels of HIF-1α were high in the
majority of patients with positive axilla lymph nodes and served as
a predictor of poor prognosis. Furthermore, Schoppmann et al
(42) identified a significant
association between HIF-1α expression and the degree of peritumoral
lymphangiogenesis in patients with breast cancer. Consequently,
this finding suggests that HIF-1α-positive tumors possess a high
degree of lymph node metastasis, as compared with HIF-1α-negative
tumors.
No significant association between HIF-1α expression
and large tumors and a more advanced nuclear grade was observed in
the present study. Conversely, Kronblad et al (43) noted a significant positive correlation
between these factors, particularly in tumors with diameters >5
cm. It has been hypothesized that the levels of HIF-1α expression
increase with tumor growth, as large tumors are generally more
hypoxic, compared with those of a small size (38). The correlation between HIF-1α
expression and nuclear grade also remains to be established. Bos
et al (34) demonstrated that
increased levels of HIF-1α expression were positively associated
with nuclear grade. Kronblad et al (43) evaluated 564 patients and observed a
positive correlation between increased histological grade and
HIF-1α expression levels. It is established that hypoxia induces
genetic alterations in tumor cells that allow them to adapt to a
hypoxic environment (44–46). Such genetic alterations also induce
morphological changes in tumor cells and their nuclei. No
significant correlation between HIF-1α expression levels and
nuclear grade was observed in the present study, which may be due
to the limited sample size and a majority of the HIF-1α positive
patients within the basal-like group.
To the best of our knowledge, the current study
demonstrated for the first time that FBP1 expression levels
correlate with the clinicopathological characteristics of tumors in
patients with basal-like breast carcinoma. FBP1 localizes to the
nuclei of proliferating cells (47,48) and is
recognized to be important for the regulation of gluconeogenesis
(49,50). The FBP1 gene is also expressed in
certain cancer cells; however, its expression levels are reduced by
comparison with non-malignant tissues (27,29). Bhide
(51) also observed that the
activities of gluconeogenic enzymes were lowest in tumor tissues.
The loss of FBP activity in tumors may therefore result in loss of
the gluconeogenic capacity of malignant tissues. In the present
study, FBP1 expression levels decreased progressively in basal-like
breast carcinoma, as compared with luminal cell lines; FBP1 levels
are correlated with nuclear grade and tumor stage, and are
indicative of extended DFS. These results are concordant with an
earlier study from Liu et al (27), which observed that high FBP1
expression levels in cells from patients with gastric carcinoma are
predictive of survival. The significant positive association
between FBP1 and DFS may occur as FBP1 inhibits HIF-1α and
glycolytic metabolism (28).
Alternatively, this effect may be due to the downstream targets of
HIF-1α, carbonic anhydrase IX and glucose transporter 1, being
inhibited by FBP1 (28).
The association between HIF-1α and FBP1 expression
was also examined in the current study, revealing negative
correlations between HIF-1α and FBP1 expression levels in
basal-like breast carcinoma tissues. This result is supported by a
study from Li et al (28),
which observed the same correlation between these two parameters in
renal cell carcinoma. It is established that the HIF-1α increases
the level of glycolysis, and the stability and signaling of this
protein is primarily involved in glucose metabolism,
neovascularization and survival (52,53). As a
result, the gluconeogenic process is inhibited and the levels of
FBP1-limited enzyme expression are reduced (28). Consequently, the findings of the
present study suggest that changes in the expression of FBP1 may
result in the differential expression of HIF-1α, and FBP1 may be a
target of HIF-1α in basal-like breast carcinoma.
In the present study, the effects of adjuvant
(chemotherapy or hormone) treatment on survival, and its
interactions with the expression levels of HIF-1α and FBP1 were not
examined due to the limited number of patients in each subgroup.
Further studies with a larger sample size are therefore required to
examine this effect.
In conclusion, the results of the current study
suggest that FBP1 is negatively correlated with DFS in patients
with basal-like breast carcinoma, potentially due to the expression
of FBP1 inhibiting the nuclear levels of HIF-1α. Therefore, further
study of the expression levels of FBP1 using immunoprecipitation
analysis may aid understanding of the interaction between FBP1 and
HIF-1α in breast cancer. Furthermore, it may be valuable to examine
the FBP1 status of patients with breast cancer, to enable the
selection of novel therapeutic strategies for breast cancer in the
future.
References
|
1
|
Vaupel P, Schlenger K, Knoop C and Höckel
M: Oxygenation of human tumors: Evaluation of tissue oxygen
distribution in breast cancers by computerized O2 tension
measurements. Cancer Res. 51:3316–3322. 1991.PubMed/NCBI
|
|
2
|
Vaupel P: The role of hypoxia-induced
factors in tumor progression. Oncologist. 9:(Suppl 5). S10–S17.
2004. View Article : Google Scholar
|
|
3
|
Fisher ER, Anderson S, Redmond C and
Fisher B: Pathologic findings from the national surgical adjuvant
breast project protocol B-06. 10-year pathologic and clinical
prognostic discriminants. Cancer. 71:2507–2514. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hockel M and Vaupel P: Tumor hypoxia:
Definitions and current clinical, biologic, and molecular aspects.
J Natl Cancer Inst. 93:266–276. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Garcia S, Dalès JP, Charafe-Jauffret E,
Carpentier-Meunier S, Andrac-Meyer L, Jacquemier J, Andonian C,
Lavaut MN, Allasia C, Bonnier P and Charpin C: Poor prognosis in
breast carcinomas correlates with increased expression of
targetable CD146 and c-Met and with proteomic basal-like phenotype.
Hum Pathol. 38:830–841. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kallergi G, Markomanolaki H, Giannoukaraki
V, Papadaki MA, Strati A, Lianidou ES, Georgoulias V, Mavroudis D
and Agelaki S: Hypoxia-inducible factor-1alpha and vascular
endothelial growth factor expression in circulating tumor cells of
breast cancer patients. Breast cancer Res. 11:R842009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Karlin KL, Mondal G, Hartman JK, Tyagi S,
Kurley SJ, Bland CS, Hsu TY, Renwick A, Fang JE, Migliaccio I, et
al: The oncogenic STP axis promotes triple-negative breast cancer
via degradation of the REST tumor suppressor. Cell Rep.
9:1318–1332. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gleadle JM and Ratcliffe PJ: Induction of
hypoxia-inducible factor-1, erythropoietin, vascular endothelial
growth factor, and glucose transporter-1 by hypoxia: Evidence
against a regulatory role for Src kinase. Blood. 89:503–509.
1997.PubMed/NCBI
|
|
9
|
Price BD and Calderwood SK: Gadd45 and
Gadd153 messenger RNA levels are increased during hypoxia and after
exposure of cells to agents which elevate the levels of the
glucose-regulated proteins. Cancer Res. 52:3814–3817.
1992.PubMed/NCBI
|
|
10
|
Moulder JE and Rockwell S: Tumor hypoxia:
Its impact on cancer therapy. Cancer Metastasis Rev. 5:313–341.
1987. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Giaccia AJ: Hypoxic stress proteins:
Survival of the Fittest. Semin Radiat Oncol. 6:46–58. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kunz M and Ibrahim SM: Molecular responses
to hypoxia in tumor cells. Mol Cancer. 2:232003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Harris AL: Hypoxia-a key regulatory factor
in tumour growth. Nat Rev Cancer. 2:38–47. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Semenza GL: HIF-1, O(2), and the 3 PHDs:
How animal cells signal hypoxia to the nucleus. Cell. 107:1–3.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jaakkola P, Mole DR, Tian YM, Wilson MI,
Gielbert J, Gaskell SJ, von Kriegsheim A, Hebestreit HF, Mukherji
M, Schofield CJ, et al: Targeting of HIF-alpha to the von
Hippel-Lindau ubiquitylation complex by O2-regulated prolyl
hydroxylation. Science. 292:468–472. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Semenza GL: Hypoxia, clonal selection, and
the role of HIF-1 in tumor progression. Crit Rev Biochem Mol Biol.
35:71–103. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kreike B, van Kouwenhove M, Horlings H,
Weigelt B, Peterse H, Bartelink H and van de Vijver MJ: Gene
expression profiling and histopathological characterization of
triple-negative/basal-like breast carcinomas. Breast Cancer Res.
9:R652007. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rakha EA, Elsheikh SE, Aleskandarany MA,
Habashi HO, Green AR, Powe DG, El-Sayed ME, Benhasouna A, Brunet
JS, Akslen LA, et al: Triple-negative breast cancer: Distinguishing
between basal and nonbasal subtypes. Clin Cancer Res. 15:2302–2310.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tan EY, Yan M, Campo L, Han C, Takano E,
Turley H, Candiloro I, Pezzella F, Gatter KC, Millar EK, et al: The
key hypoxia regulated gene CAIX is upregulated in basal-like breast
tumours and is associated with resistance to chemotherapy. Br J
Cancer. 100:405–411. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tsuda H, Takarabe T, Hasegawa F, Fukutomi
T and Hirohashi S: Large, central acellular zones indicating
myoepithelial tumor differentiation in high-grade invasive ductal
carcinomas as markers of predisposition to lung and brain
metastases. Am J Surg Pathol. 24:197–202. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Riva C, Chauvin C, Pison C and Leverve X:
Cellular physiology and molecular events in hypoxia-induced
apoptosis. Anticancer Res. 18:4729–4736. 1998.PubMed/NCBI
|
|
22
|
Vaupel P, Kallinowski F and Okunieff P:
Blood flow, oxygen and nutrient supply, and metabolic
microenvironment of human tumors: A review. Cancer Res.
49:6449–6465. 1989.PubMed/NCBI
|
|
23
|
Semenza GL, Roth PH, Fang HM and Wang GL:
Transcriptional regulation of genes encoding glycolytic enzymes by
hypoxia-inducible factor 1. J Biol Chem. 269:23757–23763.
1994.PubMed/NCBI
|
|
24
|
Mizunuma H and Tashima Y:
Fructose-1,6-biphosphatase of the small intestine. Purification and
comparison with liver and muscle fructose-1,6-bisphosphatases. J
Biochem. 84:327–336. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tillmann H and Eschrich K: Isolation and
characterization of an allelic cDNA for human muscle
fructose-1,6-bisphosphatase. Gene. 212:295–304. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tejwani GA: Regulation of
fructose-bisphosphatase activity. Adv Enzymol Relat Areas Mol Biol.
54:121–194. 1983.PubMed/NCBI
|
|
27
|
Liu X, Wang X, Zhang J, Lam EK, Shin VY,
Cheng AS, Yu J, Chan FK, Sung JJ and Jin HC: Warburg effect
revisited: An epigenetic link between glycolysis and gastric
carcinogenesis. Oncogene. 29:442–450. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li B, Qiu B, Lee DS, Walton ZE, Ochocki
JD, Mathew LK, Mancuso A, Gade TP, Keith B, Nissim I and Simon MC:
Fructose-1,6-bisphosphatase opposes renal carcinoma progression.
Nature. 513:251–255. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bigl M, Jandrig B, Horn LC and Eschrich K:
Aberrant methylation of human L- and M-fructose 1,6-bisphosphatase
genes in cancer. Biochem Biophys Res Commun. 377:720–724. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Viale G: Histopathology of primary breast
cancer 2003. Breast. 12:391–396. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Elston CW and Ellis IO: Pathological
prognostic factors in breast cancer. I. The value of histological
grade in breast cancer: Experience from a large study with
long-term follow-up. Histopathology. 41:154–161. 2002.PubMed/NCBI
|
|
32
|
Carlson RW: NCCN breast cancer clinical
practice guidelines in oncology: An update. J Natl Compr Canc Netw.
1:(Suppl 1). S61–S63. 2003.PubMed/NCBI
|
|
33
|
Zhou M, Zhao Y, Ding Y, Liu H, Liu Z,
Fodstad O, Riker AI, Kamarajugadda S, Lu J, Owen LB, et al: Warburg
effect in chemosensitivity: Targeting lactate dehydrogenase-A
re-sensitizes taxol-resistant cancer cells to taxol. Mol Cancer.
9:332010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bos R, van der Groep P, Greijer AE,
Shvarts A, Meijer S, Pinedo HM, Semenza GL, van Diest PJ and van
der Wall E: Levels of hypoxia-inducible factor-1alpha independently
predict prognosis in patients with lymph node negative breast
carcinoma. Cancer. 97:1573–1581. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jacobs TW, Gown AM, Yaziji H, Barnes MJ
and Schnitt SJ: Specificity of HercepTest in determining HER-2/neu
status of breast cancers using the United States Food and Drug
Administration-approved scoring system. J Clin Oncol. 17:1983–1987.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Livasy CA, Karaca G, Nanda R, Tretiakova
MS, Olopade OI, Moore DT and Perou CM: Phenotypic evaluation of the
basal-like subtype of invasive breast carcinoma. Mod Pathol.
19:264–271. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Nielsen TO, Hsu FD, Jensen K, Cheang M,
Karaca G, Hu Z, Hernandez-Boussard T, Livasy C, Cowan D, Dressler
L, et al: Immunohistochemical and clinical characterization of the
basal-like subtype of invasive breast carcinoma. Clin Cancer Res.
10:5367–5374. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yamamoto Y, Ibusuki M, Okumura Y, Kawasoe
T, Kai K, Iyama K and Iwase H: Hypoxia-inducible factor 1alpha is
closely linked to an aggressive phenotype in breast cancer. Breast
Cancer Res Treat. 110:465–475. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Dales JP, Garcia S, Meunier-Carpentier S,
Andrac-Meyer L, Haddad O, Lavaut MN, Allasia C, Bonnier P and
Charpin C: Overexpression of hypoxia-inducible factor HIF-1alpha
predicts early relapse in breast cancer: Retrospective study in a
series of 745 patients. Int J Cancer. 116:734–739. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Schindl M, Schoppmann SF, Samonigg H,
Hausmaninger H, Kwasny W, Gnant M, Jakesz R, Kubista E, Birner P
and Oberhuber G: Austrian Breast and Colorectal Cancer Study Group:
Overexpression of hypoxia-inducible factor 1alpha is associated
with an unfavorable prognosis in lymph node-positive breast cancer.
Clin Cancer Res. 8:1831–1837. 2002.PubMed/NCBI
|
|
41
|
Giatromanolaki A, Koukourakis MI,
Simopoulos C, Polychronidis A, Gatter KC, Harris AL and Sivridis E:
c-erbB-2 related aggressiveness in breast cancer is hypoxia
inducible factor-1alpha dependent. Clin Cancer Res. 10:7972–7977.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Schoppmann SF, Fenzl A, Schindl M,
Bachleitner-Hofmann T, Nagy K, Gnant M, Horvat R, Jakesz R and
Birner P: Hypoxia inducible factor-1alpha correlates with VEGF-C
expression and lymphangiogenesis in breast cancer. Breast Cancer
Res Treat. 99:135–141. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kronblad A, Jirstrom K, Rydén L,
Nordenskjöld B and Landberg G: Hypoxia inducible factor-1alpha is a
prognostic marker in premenopausal patients with intermediate to
highly differentiated breast cancer but not a predictive marker for
tamoxifen response. Int J Cancer. 118:2609–2616. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Semenza GL: HIF-1 and tumor progression:
Pathophysiology and therapeutics. Trends Mol Med. 8:(4 Suppl).
S62–S67. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhong H, De Marzo AM, Laughner E, Lim M,
Hilton DA, Zagzag D, Buechler P, Isaacs WB, Semenza GL and Simons
JW: Overexpression of hypoxia-inducible factor 1alpha in common
human cancers and their metastases. Cancer Res. 59:5830–5835.
1999.PubMed/NCBI
|
|
46
|
Page EL, Robitaille GA, Pouysségur J and
Richard DE: Induction of hypoxia-inducible factor-1alpha by
transcriptional and translational mechanisms. J Biol Chem.
277:48403–48409. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Gizak A, Rakus D and Dzugaj A: Nuclear
localization of fructose 1,6-bisphosphatase in smooth muscle cells.
J Mol Histol. 36:243–248. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Mamczur P, Mazurek J and Rakus D:
Ubiquitous presence of gluconeogenic regulatory enzyme,
fructose-1,6-bisphosphatase, within layers of rat retina. Cell
Tissue Res. 341:213–221. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Nordlie RC, Foster JD and Lange AJ:
Regulation of glucose production by the liver. Annu Rev Nutr.
19:379–406. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Radziuk J and Pye S: Hepatic glucose
uptake, gluconeogenesis and the regulation of glycogen synthesis.
Diabetes Metab Res Rev. 17:250–272. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Bhide SV: Enzyme studies on tumour cell
suspensions. Br J Cancer. 24:869–874. 1970. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Pouysségur J, Dayan F and Mazure NM:
Hypoxia signalling in cancer and approaches to enforce tumour
regression. Nature. 441:437–443. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Ryan HE, Poloni M, McNulty W, Elson D,
Gassmann M, Arbeit JM and Johnson RS: Hypoxia-inducible
factor-1alpha is a positive factor in solid tumor growth. Cancer
Res. 60:4010–4015. 2000.PubMed/NCBI
|