Introduction
Oral cancer (OC) is one of the most common cancers,
comprising ~5% of all annual cancer diagnoses worldwide (1). Previous studies reported that the
occurrence of OC is associated with alcohol consumption (2,3), smoking
(3,4),
betel nut chewing (5), and human
papilloma virus infection (6).
Despite improved clinical interventions, including surgery,
radiotherapy, chemotherapy and chemo-radiotherapy, the 5-year
survival rate for patients with OC has remained poor over the
previous several decades (7).
Typically, OC includes types of cancer formed on the lips, oral
cavity and oropharynx (8). Clinical
interventions for patients with OC may produce alterations in
appearance or oral function (9).
Previously, to reduce side effects and to synergistically enhance
radiotherapeutic efficacy in patients with OC, chemotherapy has
been considered to be a clinical strategy for the treatment of OC
(10). Although chemotherapy has
beneficial effects, including the induction of cancer cell death,
and the retardation of cancer cell migration or metastasis, it has
several side effects including high toxicity to normal cells and
low efficacy in cancer cells, and it may lead to drug resistance
(10).
Previous studies on the development of
chemotherapeutic agents have been focused on inducing
cancer-specific cell death through the modulation of apoptosis, or
programmed cell death. Furthermore, to overcome the side effects of
current chemotherapeutic agents, studies have focused on the
anti-cancer activity of natural compounds purified from herbal
plants (7,11–15). As a
result of these studies, the US Food and Drug Administration
approved the clinical use of a medicinal herbal plant-derived
natural compound with anti-cancer activity for cancer therapy
(16).
Licorice, the common name for roots from plants of
the Glycyrrhiza genus, including Glycyrrhiza glabra,
Glycyrrhiza inflate, and Glycyrrhiza uralensis, is a
herbal plant extract used in traditional folk medicine in Asia.
Licorice has been used as a medication for stomach ulcers,
bronchitis and sore throats (7,17).
Furthermore, the anticancer activity of licorice has been reported
in breast (18,19), oral (11), colon (12) and prostate cancer (13). Licorice inhibits metastasis (18), has anti-proliferative properties
(19), increases apoptosis and may
cause cell cycle arrest (14).
Recently, the authors demonstrated that licochalcone-A, a natural
phenolic chalconoid purified from licorice (15), induces caspase-dependent apoptotic
FaDu cell death through the extrinsic and intrinsic apoptotic
signaling pathways.
Lico-E has recently been isolated from the roots of
Glycyrrhiza species (20) and
has been demonstrated to possess anti-inflammatory properties
(21), antimicrobial activity
(22), antioxidant activity (23), antidiabetic effects (24) and anticancer properties (25). However, the biological functions of
Lico-E have not been completely examined.
Therefore, the present study aimed to determine
whether Lico-E functions as a chemotherapeutic agent against OC.
Furthermore, the potential apoptotic effect of Lico-E on OC was
evaluated and the associated apoptotic signaling pathway was
elucidated.
Materials and methods
Preparation of Lico-E
The Glycyrrhiza roots were purchased from the
Chonnam Herb Association (Gwangju, Korea). A voucher specimen
(MNUYG-003) was deposited at the College of Pharmacy, Mokpo
National University (Mokpo, Korea). The air-dried, powdered
Glycyrrhiza species roots (600 g) were extracted twice with 4 liter
100% methanol using sonication for 3 h. Following filtration with
filter paper (Advantec, Osaka, Japan), the methanol extract was
evaporated and suspended in distilled water and then defatted with
1 liter n-hexane. The aqueous layer was partitioned with methylene
chloride (3×1 liter). The evaporation residue (5 g) was subjected
to flash silica gel chromatography, using an n-hexane:ethyl
acetate:methanol solvent system (2:1:0.1, 1.5:1:10.1, 1:1:0.1 and
100% methanol), to afford 10 fractions. Fractions were subjected to
further flash silica gel chromatography, with a chloroform:methanol
(100:1) eluent system, to afford Lico-E (5 mg). Lico-E was further
purified by column chromatography using RP18 (YMC Co., Ltd., Kyoto,
Japan) to an analytically acceptable purity.
Cell culture
Normal human oral keratinocytes (hNOKs) were
purchased from ScienCell Research Laboratories, Inc. (Carlsbad, CA,
USA). The hNOKs were maintained in Dulbecco's modified Eagle's
medium (Thermo Fisher Scientific, Inc., Waltham, MA, USA)
containing 10% fetal bovine serum (FBS; Thermo Fisher Scientific,
Inc.).
FaDu, a human pharyngeal squamous carcinoma cell
line, was obtained from the American Type Culture Collection
(Manassas, VA, USA) and cultured according to the protocol
provided. FaDu cells were maintained in minimum essential medium
(Thermo Fisher Scientific, Inc.) containing 10% FBS. Cells were
grown in a humidified incubator at 37°C containing 5%
CO2.
Cell viability assay
FaDu cells and hNOKs were seeded at a density of
5×105 cells/ml in 96-well plates, and allowed to attach
to the well overnight. Following incubation, the cultured cells
were treated with 12.5, 25 or 50 µM Lico-E in triplicate, and
incubated at 37°C for 24 h. 20 µl of 5 mg/ml MTT was subsequently
added to each well and cells were incubated for an additional 4 h
at 37°C. In order to dissolve the resulting formazan, the cells
were resuspended in 200 µl dimethyl sulfoxide, and the optical
density (OD) of the solution was determined using a spectrometer at
an incident wavelength of 570 nm. The experiments were repeated
three times, independently. The mean OD ± standard deviation (SD)
for each group of replicates was calculated. The inhibitory rate of
cell growth was calculated using the following equation: % growth
inhibition=[(1-OD extract treated)/(OD negative control)]x100.
Cell survival assay
Cell survival was measured, as previously described
(7), using calcein-AM to stain the
live cells and ethidium bromide homodimer 1 to stain the dead
cells. These reagents were obtained from Molecular Probes (Eugene,
OR, USA). For the cell survival assay, FaDu cells and hNOKs were
plated at a density of 2×104 cells in an 8-well chamber
slide, incubated with 12.5, 25 or 50 µM Lico-E for 24 h, and
subsequently stained with green calcein-AM and ethidium homodimer-1
for 30 min at room temperature, according to the manufacturer's
protocol. The cells were observed and images were captured using
inverted phase contrast microscopy (Eclipse TE2000; Nikon
Corporation, Tokyo, Japan).
Nucleus staining using DAPI
FaDu cells and hNOKs that had been treated with
Lico-E and incubated for 24 h were fixed with 4% paraformaldehyde
at 4°C for 10 min, prior to washing with PBS. The washed cells were
stained with 1 mg/ml DAPI; Roche Diagnostics (Basel, Switzerland)
for 20 min. Nuclear condensation was observed by fluorescence
microscopy (Eclipse TE2000).
Western blot analysis
FaDu cells (density of 5×106 cells/ml)
were plated on culture dishes and incubated for 24 h in a
humidified incubator at 37°C. Cultured FaDu cells were treated with
Lico-E for 24 h. Cells were harvested, lysed using a cell lysis
buffer (cat no. #9803; Cell Signaling Technology, Danvers, MA, USA)
containing protease and phosphatase inhibitor cocktails, and
incubated for 1 h at 4°C. Lysates were centrifuged at 14,000 × g
for 10 min at 4°C. The supernatant was used as the cytosolic
fraction. Total protein concentrations of the cell lysates were
determined by bicinchoninic acid protein assays (Thermo Fisher
Scientific, Inc.). Loading buffer (5X; #S2002; Biosesang, Sungnam,
Korea) was added to equal amounts (40 µg) of protein and the
mixture was boiled at 90°C for 10 min. Total proteins were
separated using 10% SDS-PAGE and transferred to nitrocellulose
membranes. Following blocking for 2 h with 5% bovine serum albumin
(BSA; Sigma-Aldrich; Merck KGaA) in Tris-buffered saline with
Tween-20 (TBST) at room temperature, membranes were incubated with
primary antibody at 4°C overnight and then incubated with
horseradish peroxidase-conjugated secondary antibody (dilution in
TBST with 5% BSA, 1:20,000; #31,460; Thermo Fisher Scientific,
Inc.) at room temperature for 2 h. The antibodies used to study the
apoptotic signaling pathways included antibodies against Fas ligand
(FasL; 40 kDa; #4273), cleaved caspase-3 (17 and 19 kDa; #9661),
cleaved caspase-8 (18 kDa, #9496), cleaved caspase-9 (37 kDa;
#7237), poly(ADP-ribose) polymerase (PARP; preform, 116 kDa and
cleaved form, 85 kDa; #9542), p53 (53 kDa; #2527), apoptosis
regulator Bcl-2 (Bcl-2; 26 kDa; #3498), Bcl-2-like protein 1
(Bcl-xL; 30 kDa; #2762), Bcl-2 associated X (Bax; 20 kDa; #2772),
Bcl-2-associated agonist of cell death (Bad; 23 kDa, #9292),
apoptotic protease-activating factor 1 (Apaf-1; 135 kDa, #87,233)
and β-actin (45 kDa, #4970), which were purchased from Cell
signaling Technology, Inc. (Danvers, MA, USA) and diluted to
1:1,000 in TBST with 5% BSA for western blotting. The
immunoreactive bands were visualized using an Enhanced
Chemiluminescent System (GE Healthcare Life Sciences, Chalfont, UK)
and were exposed on radiographic film.
Caspase-3/−7 activity assay
The apoptotic activity of the executioner caspases 3
and 7 was determined using the cell-permeable fluorogenic
substrate, PhiPhiLux-G1D2 (OncoImmunin, Inc.,
Gaithersburg, MD, USA), according to the manufacturer's
protocol.
Caspase-dependent cell survival
assay
Cells were plated at a density of 1×105
cells/ml in 96-well plates and allowed to adhere for 24 h in a
humidified incubator at 37°C. Following incubation, cultured cancer
cells were treated with Lico-E in the presence or absence of 50 µM
caspase-3 inhibitor (Z-VAD-FMK or N-Benzyloxycarbonyl-Val-Ala-Asp
(O-Me) fluoromethyl ketone; Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) and were incubated for 24 h at 37°C. Following incubation,
cytotoxicity was measured with an MTT assay as previously
described.
Statistical analysis
Statistical analysis of the data was performed using
SPSS version 19 (SPSS, Chicago, IL, USA). Values are presented as
the mean ± SD of three independent experiments performed in
triplicate. Statistical analysis was performed using the Student's
t-test. P<0.05 was considered to indicate a statistically
significant difference.
Results
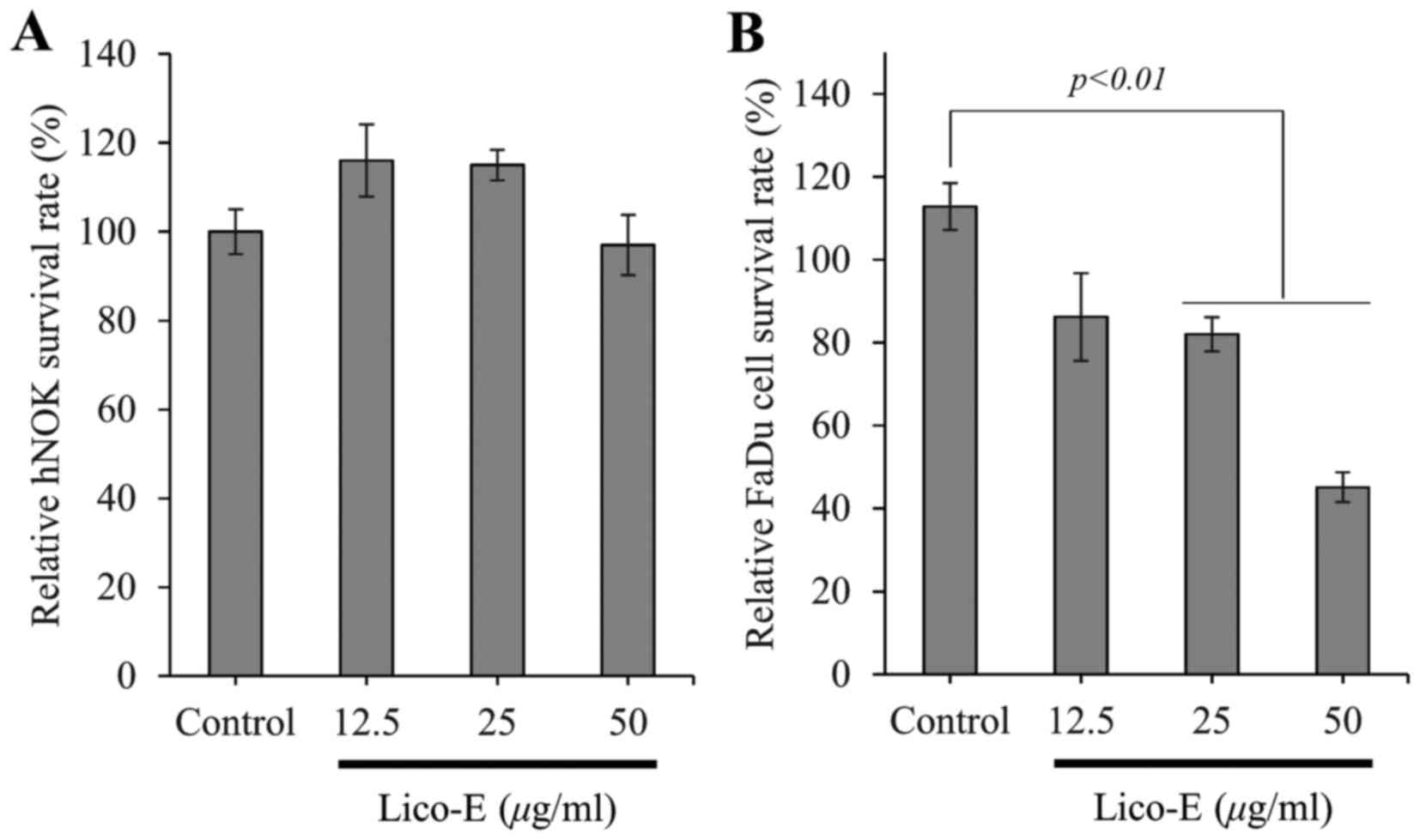
Treatment with Lico-E increases the
cytotoxicity of FaDu cells
Incubation of FaDu and hNOK cells with Lico-E for 24
h, followed by the MTT assay to measure cytotoxicity, demonstrated
that exposure to 12.5, 25 or 50 µM Lico-E did not affect the cell
viability of hNOK cells, which were used as a model of normal cells
(Fig. 2A). By contrast, cytotoxicity
was increased to 17.98±4.1 (P<0.01) and 54.9 ± (P<0.01) for
FaDu cells treated with 25 and 50 µM Lico-E compared to an
untreated control, respectively (Fig.
2B). The IC50 value of Lico-E was ~50 µM for FaDu
cells. These results demonstrate that Lico-E is cytotoxic to FaDu
cells without affecting the viability of the normal hNOKs.
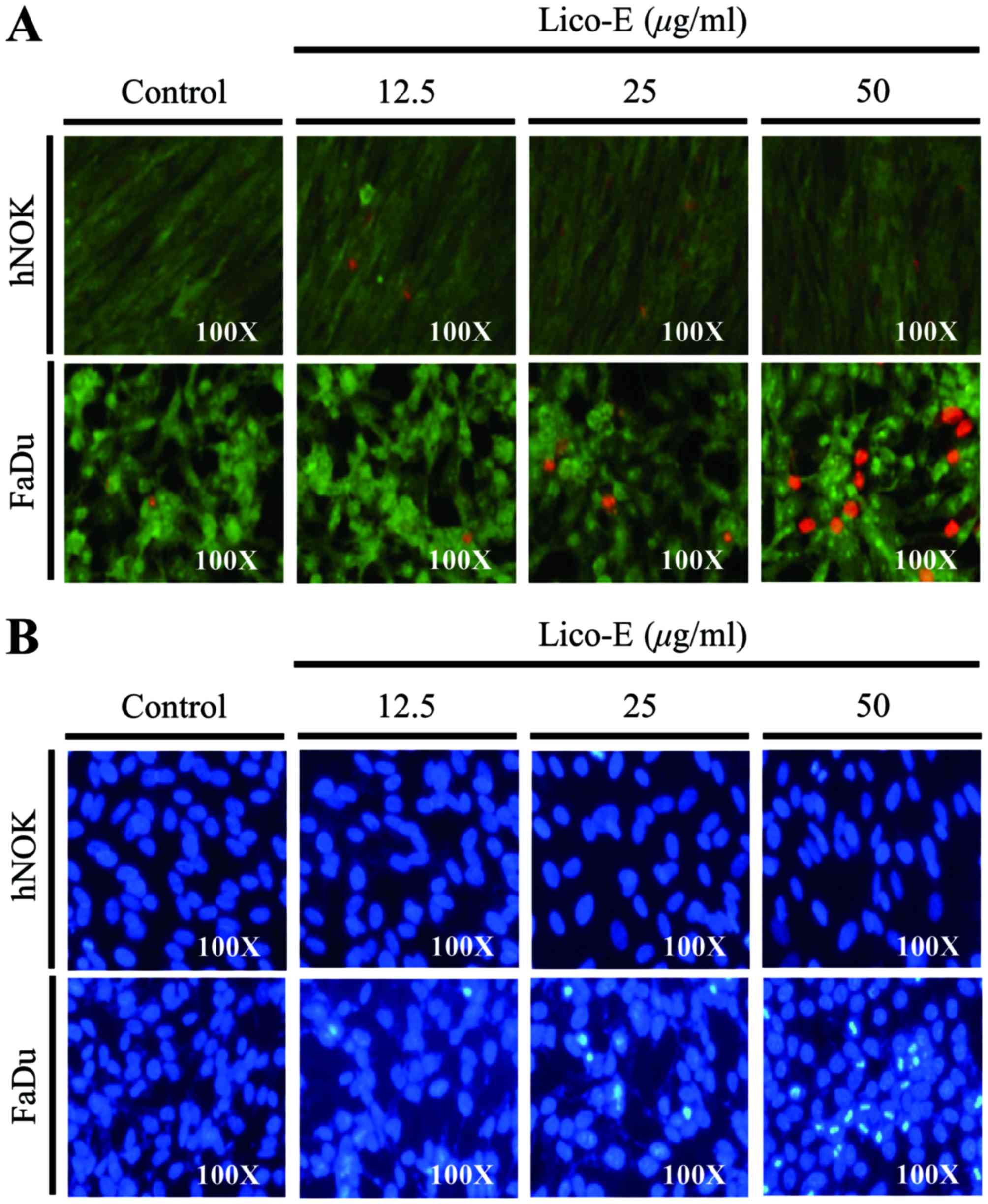
Lico-E induces FaDu cell death
To verify the Lico-E-induced cytotoxicity, FaDu
cells and hNOKs were treated with Lico-E for 24 h, and live cell
and dead cell assays were performed. As demonstrated in Fig. 3A, few dead cells were observed when
hNOKs were treated with 12.5, 25 or 50 µM Lico-E for 24 h. By
contrast, the number of dead FaDu cells was increased following
treatment with Lico-E. These results are consistent with the
hypothesis that Lico-E induces FaDu cell death through cytotoxicity
without affecting normal cells.
Lico-E-induced FaDu cell death is
mediated by apoptosis
Chromatin condensation is a representative feature
of apoptosis. To determine whether Lico-E-induced cell death
involves apoptosis, DAPI staining of cultured cells treated with
Lico-E for 24 h was performed to observe the morphology of the
nucleus. As demonstrated in Fig. 3B,
nuclear condensation was not observed when hNOKs were treated with
Lico-E. When FaDu cells were treated with Lico-E, the number of
cells with morphological alteration of the nucleus or a condensed
nucleus increased significantly. These data indicate that
Lico-E-induced FaDu cell death is mediated by apoptosis.
Lico-E-induced apoptosis is mediated
by death receptor-dependent extrinsic and mitochondrial-dependent
intrinsic apoptotic signaling pathways
As demonstrated in Fig.
4A, the expression of FasL (48 kDa), a representative death
receptor ligand and trigger of the apoptotic signaling pathway, was
increased by Lico-E in FaDu cells in a concentration- dependent
manner. Sequentially, expression of cleaved caspase-8 (18 kDa), a
downstream target molecule associated with the death
receptor-dependent apoptotic signaling pathway, was increased in
the FaDu cells treated with Lico-E in a concentration-dependent
manner. Furthermore, the expression of Bcl-2 (26 kDa) and Bcl-xL
(26 kDa), anti-apoptotic factors associated with the
mitochondrial-dependent intrinsic apoptotic signaling pathway, was
decreased in the FaDu cells treated with Lico-E, as demonstrated in
Fig. 4B. In addition, the expression
of Bax (26 kDa), Bad (23 kDa), Apaf-1 (130 kDa) and cleaved
caspase-9 (37 kDa), pro-apoptotic factors associated with the
mitochondrial-dependent intrinsic apoptotic signaling pathway, was
increased by treatment with Lico-E in FaDu cells in a
concentration-dependent manner. Furthermore, the expression of
tumor suppressor p53 was increased in the FaDu cells treated with
Lico-E. The expression of cleaved caspase-3 (17 and 19 kDa), a
downstream target molecule of caspase-8 and −9, and PARP (89 kDa),
a downstream target molecule of caspase-3, was increased in the
FaDu cells treated with Lico-E (Fig.
4C). Therefore, these results demonstrate that Lico-E-induced
apoptosis of FaDu cell is mediated by the death receptor-dependent
extrinsic and mitochondrial-dependent intrinsic apoptotic signaling
pathways.
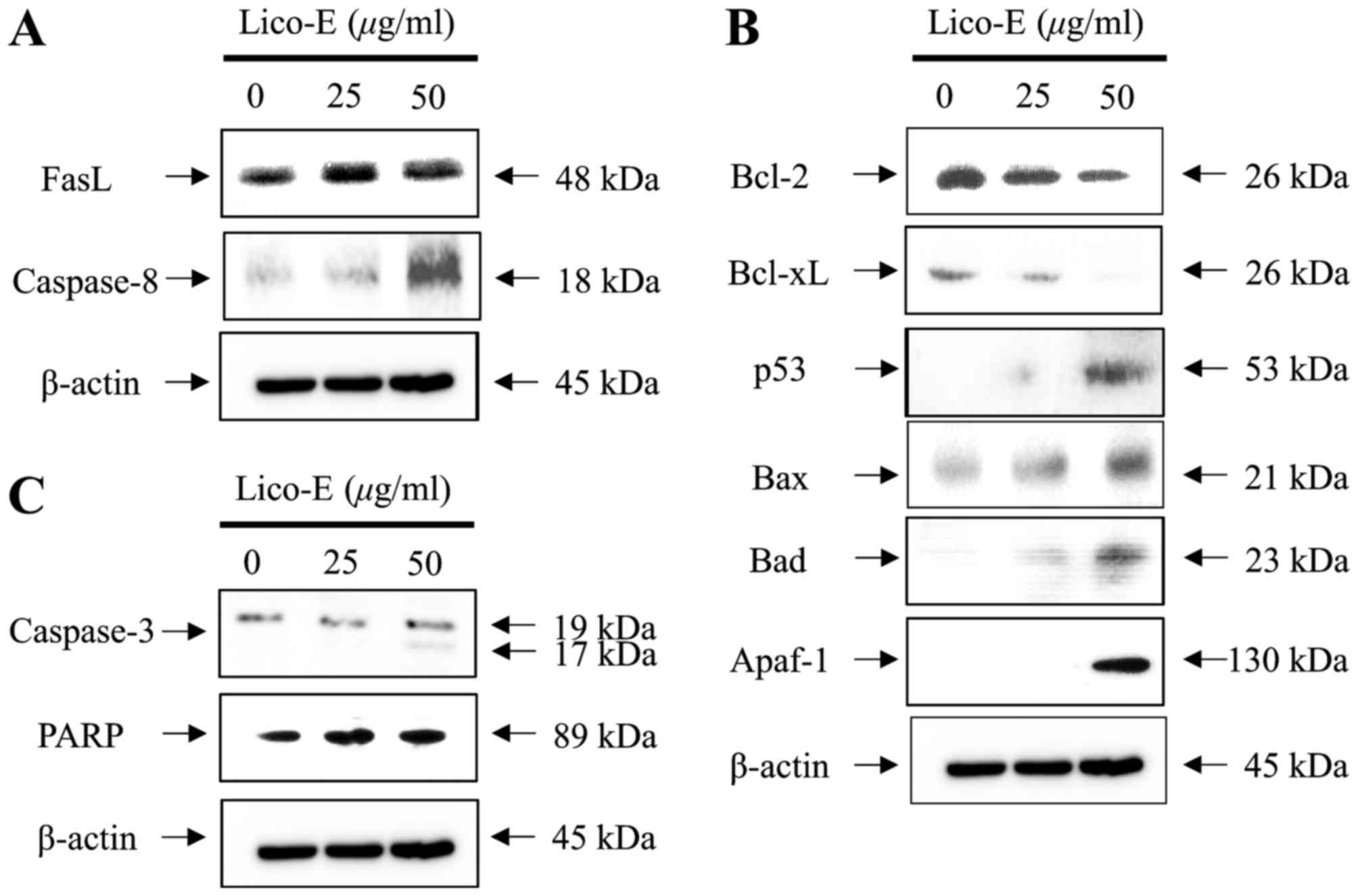 | Figure 4.Lico-E-induced apoptosis is mediated
by the death receptor-dependent extrinsic and
mitochondrial-dependent intrinsic apoptotic signaling pathways in
FaDu cells. (A) Western blotting of FasL and caspase-8. (B) Western
blotting of Bcl-2, Bcl-xL, p53, Bax, Bad and Apaf-1. (C) Western
blotting of caspase-3 and PARP. Lico-E, licochalcone-E; FasL, Fas
ligand; Bcl-2, apoptosis regulator Bcl-2; Bcl-xL, Bcl-2-like
protein 1; Bax, apoptosis regulator BAX; Bad, Bcl-2-associated
agonist of cell death; Apaf-1, apoptotic protease-activating factor
1; PARP, poly (ADP-ribose) polymerase. |
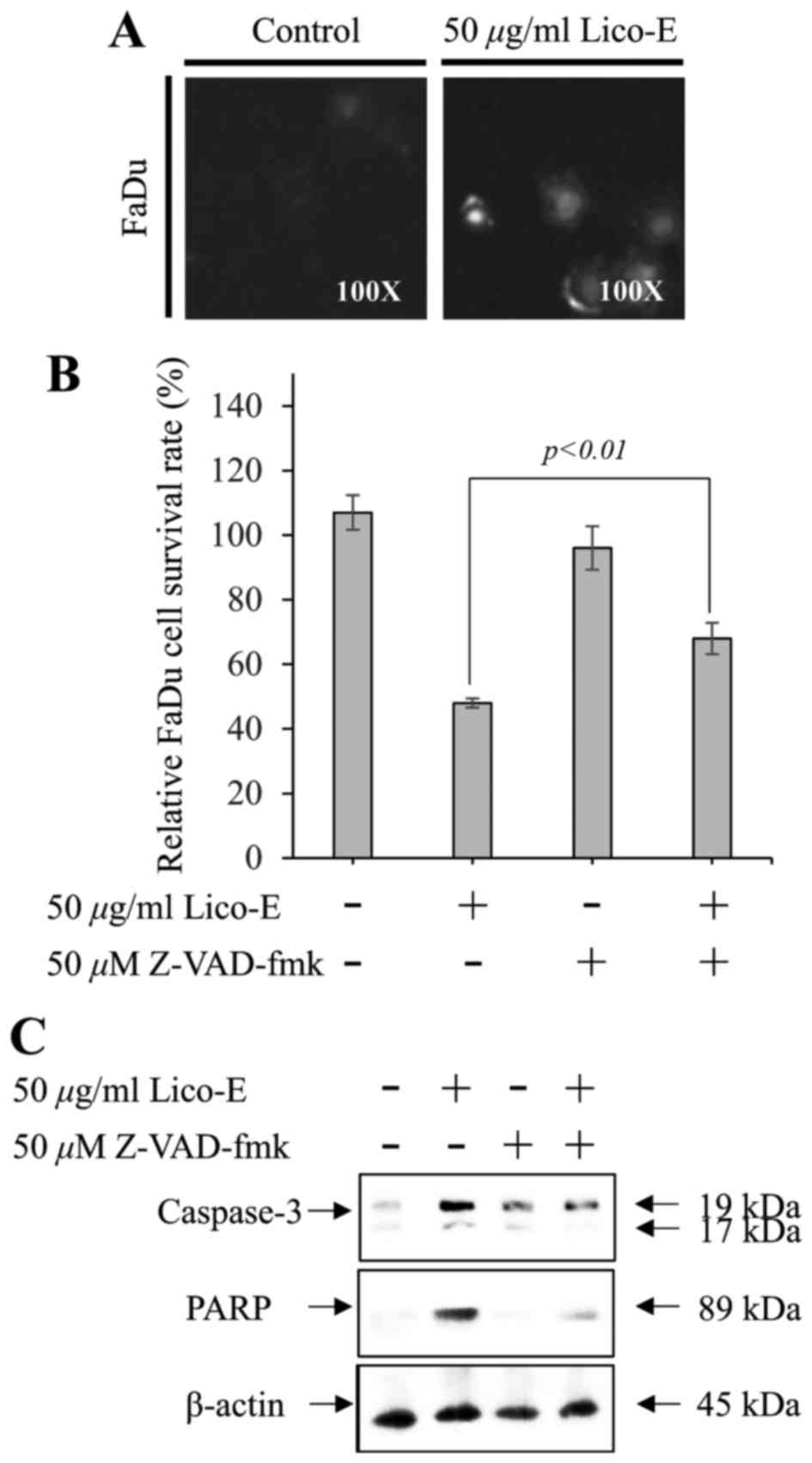
Lico-E-induced apoptosis requires the
activation of caspases in FaDu cells
To confirm the activation of caspase-3 in FaDu cells
treated with Lico-E, the caspase-3/−7 activity assay was performed
using the cell-permeable fluorogenic substrate
PhiPhiLux-G1D2. The cleavage of
PhiPhiLux-G1D2 by activated caspase-3 was
significantly upregulated in FaDu (Fig.
5A) cells treated with 25 and 50 µM Lico-E. Furthermore, the
pan caspase inhibitor Z-VAD-FMK partially inhibited Lico-E-induced
FaDu cytotoxicity (Fig. 5B).
Furthermore, the activation of caspase-3 and its downstream target
molecule PARP in the FaDu cells treated with Lico-E was suppressed
partially by Z-VAD-FMK (Fig. 5C).
These data suggest that Lico-E-induced apoptosis depends upon the
activation of caspases in FaDu cells.
Discussion
Although clinical cancer interventions including
surgery, radiotherapy and chemotherapy have advanced rapidly, the
5-year survival rate of patients with OC has not increased over the
previous decade (26). In particular,
surgical treatment of OC may lead to more severe side effects,
including oral cavity dysfunction, and aesthetic and psychological
problems, compared with the surgical treatment of other types of
cancer (27). Therefore, to reduce
the risk of side effects from OC surgery, the tumor size is
required to be reduced by chemotherapy or radiotherapy, prior to
surgical intervention. Therefore, chemotherapy has been considered
to be the primary clinical treatment of OC.
Current clinical chemotherapeutic agents have
adverse side effects, including a low efficacy to produce
cancer-specific cell death, high toxicity to normal cells,
anorexia, nausea and vomiting (28).
Therefore, there is a need for chemotherapeutic agents with
increased levels of biological safety and efficacy for producing
cancer-specific cell death. The development of chemotherapeutic
agents for cancer have been focused on the anticancer activities of
natural compounds isolated from herbal plants that have had their
biological safety verified in folk and traditional medicine
(29).
Notably, the roots of licorice (Glycyrrhiza)
have been used to treat inflammation, gastric ulcers and
atherosclerosis, in folk and traditional herbal medicine in Eastern
Asian countries, including Korea, Japan and China (30,31).
Licorice root contains alkaloids, polysaccharides, and flavonoids
(32). Previous studies have reported
licorice-induced cancer-specific cell death in various cancer cell
types, including oral (7,11), prostate (13), colon (33) and breast cancer (34,35),
through the inhibition of proliferation (11) and metastasis (18), cell-cycle arrest (13,14), and
apoptotic cell death (14).
Licochalcone-A, a chalconoid and a type of natural phenol, has been
isolated from the root of the licorice plant and has exhibited
various pharmacological effects, including antimalarial,
anticancer, antibacterial and antiviral properties (7). Furthermore, treatment with
licochalcone-A induced apoptosis in various cancer cell types,
including oral (36), bladder
(37,38), lung (39), gastric (40) and prostate cancer cells (41). In addition, Lico-E, a retrochalone,
with various pharmacological effects, including antiparasitic,
antibacterial, antioxidative and superoxide-scavenging properties,
has been isolated from the root of licorice (24). Although Kwon et al (25) reported that Lico-E suppressed lung
metastasis in 4T1 mammary orthotopic cancer, Lico-E-induced
anticancer properties remain unknown in OC. Therefore, in the
present study it was demonstrated that treatment with Lico-E
induced apoptotic cell death in OC cells, including head and neck
squamous carcinoma FaDu cells, compared with hNOK cells used as
normal cells.
The biological safety of Lico-E was demonstrated
through the measurement of cytotoxicity in the hNOKs, which were
used as a normal. Chang et al (42) have reported that Lico-E induced cell
death in ECV 302 cells, which are an immortalized human umbilical
vein endothelial cell line. However, in the present study, Lico-E
did not affect the cytotoxicity of the primary cultured hNOKs,
whereas cytotoxicity was increased in the FaDu cells treated with
Lico-E. Consistent with these results, the number of dead cells was
increased in the FaDu cells treated with Lico-E. Furthermore,
Lico-E did not increase the number of dead cells in the hNOKs.
These results indicate that Lico-E induces OC death and may have a
lower side effect spectrum compared with other chemotherapeutic
agents.
To induce cancer-specific cell death, many studies
have focused on the modulation of the apoptotic signaling pathway
(10). In the present study, the
apoptosis of FaDu cells treated with Lico-E was observed. Chromatin
condensation is indicative of apoptosis (43). Chromatin condensation was
significantly increased in the FaDu cells treated with Lico-E.
Chromatin condensation was not observed when hNOK cells were
treated with Lico-E. Therefore, these data suggest that
Lico-E-induced FaDu cell death is mediated by apoptosis and that
Lico-E may modulate the apoptotic signaling pathway. The apoptotic
signaling pathway is primarily classified as the death
receptor-dependent extrinsic apoptotic signaling pathway or the
mitochondrial-dependent intrinsic signaling pathway. The death
receptor-dependent extrinsic apoptotic signaling pathway is
activated by the binding of expressed death ligands, including FasL
(44) and tumor necrosis
factor-related apoptosis-inducing ligand (45), with the death receptors on the cell
surface. Subsequently, Fas-associated protein with death domain, a
Fas receptor adaptor molecule, initiates cell death through the
cleavage of caspase-8, caspase-3 and PARP in sequence (46). Cleaved caspase-8 of the death
receptor-dependent extrinsic apoptotic pathway initiates the
mitochondrial-dependent intrinsic apoptotic signaling pathway
through the cleavage of cytosolic BH3-interacting domain death
agonist (BID) to truncated BID (47).
Truncated BID promotes the loss of mitochondrial transmembrane
potential through insertion of Bax into the outer mitochondrial
membrane (47). Simultaneously,
anti-apoptotic factors, including Bcl-2 and Bcl-xL are
downregulated, while pro-apoptotic factors, including Bax and Bad,
are upregulated and initiate the cleavage of caspase-9 (48). Cleaved caspase-9 induces cell death
through the cleavage of caspase-3 and PARP in the
mitochondrial-dependent intrinsic apoptotic pathway. Modulation of
apoptosis and its signaling pathways have emerged as a critical
target for developing chemotherapeutic agents based on natural
compounds isolated from medicinal herbal plants (7). Previously, Kwon et al (25) have reported that Lico-E induces
apoptosis through the upregulation of Bax and cleaved caspase-3 and
the downregulation of Bcl-2 in tumor tissues of animals xenografted
with 4T1 mammary carcinoma cells. In the present study, treatment
with Lico-E increased the expression of the death receptor ligand
FasL in FaDu cells in a concentration-dependent manner.
Subsequently, expressed FasL triggered the death receptor-dependent
extrinsic and mitochondrial-dependent intrinsic apoptotic signaling
pathways in the FaDu cells treated with Lico-E. Furthermore,
apoptosis depends on the activation of caspases, including
caspase-8, −7, −9 and −3. In the present study, it was demonstrated
that the pan-caspase inhibitor Z-VAD-FMK partially suppressed
Lico-E-induced apoptosis, through the inhibition of caspase
cleavage in FaDu cells. The results of the present study suggest
that Lico-E-induced cell death in FaDu cells is dependent on the
activation of caspases involved in the intrinsic and extrinsic
apoptotic pathways triggered by FasL expression.
In conclusion, the results of the present study
suggest that Lico-E, a potential therapeutic compound derived from
natural herbal plants, may be used in the clinical chemotherapy of
OC.
Acknowledgements
The present study was supported by the 2016 research
fund from Chosun University Dental Hospital (Gwangju, Korea).
Glossary
Abbreviations
Abbreviations:
|
Apaf-1
|
apoptotic protease-activating factor
1
|
|
Bad
|
Bcl-2-associated agonist of cell
death
|
|
Bax
|
apoptosis regulator BAX
|
|
Bcl-2
|
apoptosis regulator Bcl-2
|
|
Bcl-xL
|
Bcl-2-like protein 1
|
|
FasL
|
Fas ligand
|
|
hNOKs
|
human normal oral keratinocytes
|
|
Lico-E
|
licochalcone-E
|
|
OC
|
oral cancer
|
|
OD
|
optical density
|
|
PARP
|
poly (ADP-ribose) polymerase
|
|
SD
|
standard deviation
|
References
|
1
|
Sturgis EM and Miller RH: Second primary
malignancies in the head and neck cancer patient. Ann Otol Rhinol
Laryngol. 104:946–954. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rothman KJ: The effect of alcohol
consumption on risk of cancer of the head and neck. Laryngoscope.
88:(1 Pt 2 Suppl 8). S51–S55. 1978.
|
|
3
|
Marron M, Boffetta P, Zhang ZF, Zaridze D,
Wünsch-Filho V, Winn DM, Wei Q, Talamini R, Szeszenia-Dabrowska N,
Sturgis EM, et al: Cessation of alcohol drinking, tobacco smoking
and the reversal of head and neck cancer risk. Int J Epidemiol.
39:182–196. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hashibe M, Hunt J, Wei M, Buys S, Gren L
and Lee YC: Tobacco, alcohol, body mass index, physical activity,
and the risk of head and neck cancer in the prostate, lung,
colorectal, and ovarian (PLCO) cohort. Head Neck. 35:914–922. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lin YS, Jen YM, Wang BB, Lee JC and Kang
BH: Epidemiology of oral cavity cancer in taiwan with emphasis on
the role of betel nut chewing. ORL J Otorhinolaryngol Relat Spec.
67:230–236. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Smith EM, Rubenstein LM, Haugen TH,
Pawlita M and Turek LP: Complex etiology underlies risk and
survival in head and neck cancer human papillomavirus, tobacco, and
alcohol: A case for multifactor disease. J Oncol. 2012:5718622012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Park MR, Kim SG, Cho IA, Oh D, Kang KR,
Lee SY, Moon SM, Cho SS, Yoon G, Kim CS, et al: Licochalcone-A
induces intrinsic and extrinsic apoptosis via ERK1/2 and p38
phosphorylation-mediated TRAIL expression in head and neck squamous
carcinoma FaDu cells. Food Chem Toxicol. 77:34–43. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chi AC, Day TA and Neville BW: Oral cavity
and oropharyngeal squamous cell carcinoma-an update. CA Cancer J
Clin. 65:401–421. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Epstein JB, Thariat J, Bensadoun RJ,
Barasch A, Murphy BA, Kolnick L, Popplewell L and Maghami E: Oral
complications of cancer and cancer therapy: From cancer treatment
to survivorship. CA Cancer J Clin. 62:400–422. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fesik SW: Promoting apoptosis as a
strategy for cancer drug discovery. Nat Rev Cancer. 5:876–885.
2005. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shen H, Zeng G, Sun B, Cai X, Bi L, Tang G
and Yang Y: A polysaccharide from Glycyrrhiza inflata
Licorice inhibits proliferation of human oral cancer cells by
inducing apoptosis via mitochondrial pathway. Tumour Biol.
36:4825–4831. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lee CK, Park KK, Lim SS, Park JH and Chung
WY: Effects of the licorice extract against tumor growth and
cisplatin-induced toxicity in a mouse xenograft model of colon
cancer. Biol Pharm Bull. 30:2191–2195. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fu Y, Hsieh TC, Guo J, Kunicki J, Lee MY,
Darzynkiewicz Z and Wu JM: Licochalcone-A, a novel flavonoid
isolated from licorice root (Glycyrrhiza glabra), causes G2
and late-G1 arrests in androgen-independent PC-3 prostate cancer
cells. Biochem Biophys Res Commun. 322:263–270. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jo EH, Kim SH, Ra JC, Kim SR, Cho SD, Jung
JW, Yang SR, Park JS, Hwang JW, Aruoma OI, et al: Chemopreventive
properties of the ethanol extract of Chinese licorice
(Glycyrrhiza uralensis) root: Induction of apoptosis and G1
cell cycle arrest in MCF-7 human breast cancer cells. Cancer Lett.
230:239–247. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cho JJ, Chae JI, Yoon G, Kim KH, Cho JH,
Cho SS, Cho YS and Shim JH: Licochalcone A, a natural chalconoid
isolated from Glycyrrhiza inflata root, induces apoptosis
via Sp1 and Sp1 regulatory proteins in oral squamous cell
carcinoma. Int J Oncol. 45:667–674. 2014.PubMed/NCBI
|
|
16
|
Kinghorn AD, Pan L, Fletcher JN and Chai
H: The relevance of higher plants in lead compound discovery
programs. J Nat Prod. 74:1539–1555. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wittschier N, Faller G and Hensel A:
Aqueous extracts and polysaccharides from liquorice roots
(Glycyrrhiza glabra L.) inhibit adhesion of Helicobacter
pylori to human gastric mucosa. J Ethnopharmacol. 125:218–223.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lee SK, Park KK, Park JH, Lim SS and Chung
WY: The inhibitory effect of roasted licorice extract on human
metastatic breast cancer cell-induced bone destruction. Phytother
Res. 27:1776–1783. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tamir S, Eizenberg M, Somjen D, Stern N,
Shelach R, Kaye A and Vaya J: Estrogenic and antiproliferative
properties of glabridin from licorice in human breast cancer cells.
Cancer Res. 60:5704–5709. 2000.PubMed/NCBI
|
|
20
|
Yoon G, Jung YD and Cheon SH: Cytotoxic
allyl retrochalcone from the roots of Glycyrrhiza inflata.
Chem Pharm Bull (Tokyo). 53:694–695. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lee HN, Cho HJ, Lim DY, Kang YH, Lee KW
and Park JH: Mechanisms by which licochalcone e exhibits potent
anti-inflammatory properties: Studies with phorbol ester-treated
mouse skin and lipopolysaccharide-stimulated murine macrophages.
Int J Mol Sci. 14:10926–10943. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhou T, Deng X and Qiu J: Antimicrobial
activity of licochalcone E against Staphylococcus aureus and its
impact on the production of staphylococcal alpha-toxin. J Microbiol
Biotechnol. 22:800–805. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kim SS, Lim J, Bang Y, Gal J, Lee SU, Cho
YC, Yoon G, Kang BY, Cheon SH and Choi HJ: Licochalcone E activates
Nrf2/antioxidant response element signaling pathway in both
neuronal and microglial cells: Therapeutic relevance to
neurodegenerative disease. J Nutr Biochem. 23:1314–1323. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Park HG, Bak EJ, Woo GH, Kim JM, Quan Z,
Kim JM, Yoon HK, Cheon SH, Yoon G, Yoo YJ, et al: Licochalcone E
has an antidiabetic effect. J Nutr Biochem. 23:759–767. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kwon SJ, Park SY, Kwon GT, Lee KW, Kang
YH, Choi MS, Yun JW, Jeon JH, Jun JG and Park JH: Licochalcone E
present in licorice suppresses lung metastasis in the 4T1 mammary
orthotopic cancer model. Cancer Prev Res (Phila). 6:603–613. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
International head and neck cancer
epidemiology consortium: Update no. 19. Head Neck. 37:1401–1402.
2015. View Article : Google Scholar
|
|
27
|
Howren MB, Christensen AJ, Karnell LH and
Funk GF: Psychological factors associated with head and neck cancer
treatment and survivorship: Evidence and opportunities for
behavioral medicine. J Consult Clin Psychol. 81:299–317. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ohnishi S and Takeda H: Herbal medicines
for the treatment of cancer chemotherapy-induced side effects.
Front Pharmacol. 6:142015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lee KH: Research and future trends in the
pharmaceutical development of medicinal herbs from Chinese
medicine. Public Health Nutr. 3:515–522. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Peng F, Du Q, Peng C, Wang N, Tang H, Xie
X, Shen J and Chen J: A review: The pharmacology of
isoliquiritigenin. Phytother Res. 29:969–977. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Song NR, Kim JE, Park JS, Kim JR, Kang H,
Lee E, Kang YG, Son JE, Seo SG, Heo YS and Lee KW: Licochalcone A,
a polyphenol present in licorice, suppresses UV-induced COX-2
expression by targeting PI3K, MEK1, and B-Raf. Int J Mol Sci.
16:4453–4470. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kuwajima H, Taneda Y, Chen WZ, Kawanishi
T, Hori K, Taniyama T, Kobayashi M, Ren J and Kitagawa I: Variation
of chemical constituents in processed licorice roots: Quantitative
determination of saponin and flavonoid constituents in bark removed
and roasted licorice roots. Yakugaku Zasshi. 119:945–955. 1999.(In
Japanese). View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Stewart PM and Prescott SM: Can licorice
lick colon cancer? J Clin Invest. 119:760–763. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hu C, Liu H, Du J, Mo B, Qi H, Wang X, Ye
S and Li Z: Estrogenic activities of extracts of Chinese licorice
(Glycyrrhiza uralensis) root in MCF-7 breast cancer cells. J
Steroid Biochem Mol Biol. 113:209–216. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jo EH, Hong HD, Ahn NC, Jung JW, Yang SR,
Park JS, Kim SH, Lee YS and Kang KS: Modulations of the Bcl-2/Bax
family were involved in the chemopreventive effects of licorice
root (Glycyrrhiza uralensis Fisch) in MCF-7 human breast
cancer cell. J Agric Food Chem. 52:1715–1719. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zeng G, Shen H, Yang Y, Cai X and Xun W:
Licochalcone A as a potent antitumor agent suppresses growth of
human oral cancer SCC-25 cells in vitro via caspase-3 dependent
pathways. Tumour Biol. 35:6549–6555. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yuan X, Li T, Xiao E, Zhao H, Li Y, Fu S,
Gan L and Wang Z, Zheng Q and Wang Z: Licochalcone B inhibits
growth of bladder cancer cells by arresting cell cycle progression
and inducing apoptosis. Food Chem Toxicol. 65:242–251. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yuan X, Li D, Zhao H, Jiang J, Wang P, Ma
X, Sun X and Zheng Q: Licochalcone A-induced human bladder cancer
T24 cells apoptosis triggered by mitochondria dysfunction and
endoplasmic reticulum stress. Biomed Res Int. 2013:4742722013.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Huang HC, Tsai LL, Tsai JP, Hsieh SC, Yang
SF, Hsueh JT and Hsieh YH: Licochalcone A inhibits the migration
and invasion of human lung cancer cells via inactivation of the Akt
signaling pathway with downregulation of MMP-1/−3 expression.
Tumour Biol. 35:12139–12149. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Xiao XY, Hao M, Yang XY, Ba Q, Li M, Ni
SJ, Wang LS and Du X: Licochalcone A inhibits growth of gastric
cancer cells by arresting cell cycle progression and inducing
apoptosis. Cancer Lett. 302:69–75. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yo YT, Shieh GS, Hsu KF, Wu CL and Shiau
AL: Licorice and licochalcone-A induce autophagy in LNCaP prostate
cancer cells by suppression of Bcl-2 expression and the mTOR
pathway. J Agric Food Chem. 57:8266–8273. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Chang HJ, Yoon G, Park JS, Kim MH, Baek
MK, Kim NH, Shin BA, Ahn BW, Cheon SH and Jung YD: Induction of
apoptosis by the licochalcone E in endothelial cells via modulation
of NF-kappaB and Bcl-2 Family. Biol Pharm Bull. 30:2290–2293. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Oberhammer FA, Hochegger K, Fröschl G,
Tiefenbacher R and Pavelka M: Chromatin condensation during
apoptosis is accompanied by degradation of lamin A+B, without
enhanced activation of cdc2 kinase. J Cell Biol. 126:827–837. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Weissmann C: Fas ligand and Fas: a death
factor and its receptor. Jpn J Cancer Res. 85:inside front cover.
1994.PubMed/NCBI
|
|
45
|
Li Y, Wang H, Wang Z, Makhija S, Buchsbaum
D, LoBuglio A, Kimberly R and Zhou T: Inducible resistance of tumor
cells to tumor necrosis factor-related apoptosis-inducing ligand
receptor 2-mediated apoptosis by generation of a blockade at the
death domain function. Cancer Res. 66:8520–8528. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ikner A and Ashkenazi A: TWEAK induces
apoptosis through a death-signaling complex comprising
receptor-interacting protein 1 (RIP1), Fas-associated death domain
(FADD) and caspase-8. J Biol Chem. 286:21546–21554. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Li H, Zhu H, Xu CJ and Yuan J: Cleavage of
BID by caspase 8 mediates the mitochondrial damage in the Fas
pathway of apoptosis. Cell. 94:491–501. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Fischer B, Coelho D, Dufour P, Bergerat
JP, Denis JM, Gueulette J and Bischoff P: Caspase 8-mediated
cleavage of the pro-apoptotic BCL-2 family member BID in
p53-dependent apoptosis. Biochem Biophys Res Commun. 306:516–522.
2003. View Article : Google Scholar : PubMed/NCBI
|