Introduction
Gastric cancer (GC) is a type of malignant digestive
tract tumor and is the second most common cause of
cancer-associated mortality worldwide (1). It is estimated that there are 100,0000
new cases of GC and >700,000 GC-associated mortalities worldwide
each year (2). The pathogenesis of GC
is multifactorial, and genetic and epigenetic alterations of
oncogenes, tumor suppressor genes and growth factors have been
implicated in the development of GC (3). Treatment of GC predominantly involves
surgery, chemotherapy and radiotherapy. Despite recent advances in
the treatment of GC, >50% of all patients with advanced stage GC
succumb to recurrence or metastasis, and eventually death, even
after a subtotal gastrectomy (4).
However, currently, there exists no effective method to predict and
prevent the metastasis of GC. Therefore, understanding the
molecular mechanisms underlying tumor metastasis are important to
further improve the survival of patients with GC.
microRNAs (miRs), which are a class of small
(~22-nucleotide) non-coding RNA molecules, are widely expressed in
numerous organisms (5). miRs regulate
the expression of their downstream target genes by base pairing
with the 3′-untranslated region (UTR) of mRNA, leading to mRNA
cleavage or translation repression (6). Previous studies have verified that miRs
are aberrantly expressed in various types of human cancers
(7–9).
Increasingly, evidence has suggested that these dysregulated miRs
are critical in the proliferation, apoptosis, migration and
invasion of tumor cells (10). To
date, a number of dysregulated miRs have been observed in GC, and
have been shown to participate in GC cell proliferation, apoptosis,
migration and invasion, as well as in sensitivity to chemotherapy
and radiotherapy by regulating different tumor-related target genes
(11–14). Therefore, further investigation of the
function of miRs will provide insight into the mechanisms of GC
development and identify therapeutic targets.
miR-429 has been reported to be downregulated in GC;
however, whether miR-429 has a role in the metastasis of GC has yet
to be investigated. The present study aimed to elucidate the effect
of miR-429 on GC motility and to investigate its underlying
mechanisms.
Materials and methods
Cell culture
The human GC cell lines, SGC-7901 and AGS, were
purchased from the Shanghai Institute of Biochemistry and Cell
Biology (Shanghai, China). The cell lines were cultured in
RPMI-1640 medium supplemented with 10% fetal bovine serum (Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) in an atmosphere
of 5% CO2 at 37°C.
Cell transfection
Mature miR-429 mimics, negative control (NC) miR
mimics and the luciferase reporter plasmid were designed and
synthesized by Shanghai GenePharma Co., Ltd. (Shanghai, China).
Cell transfection and co-transfection were performed using
Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.),
according to the manufacturer's protocol.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from GC cells using TRIzol
reagent (Invitrogen; Thermo Fisher Scientific, Inc.), according to
the manufacturer's protocol. Briefly, RNA (500 ng) was reverse
transcribed into cDNA using a reverse transcription kit (Tiangen
Biotech Co., Ltd., Beijing, China). qPCR was performed on the ABI
7300 thermal cycler (Applied Biosystems; Thermo Fisher Scientific,
Inc.) using SYBR PrimeScript miRNA RT-PCR kit. Every sample was
analyzed three times. U6 was used as an internal control.
Cell migration and invasion
assays
In vitro migration and invasion assays were
performed using Transwell plates (BD Biosciences, Franklin Lakes,
NJ, USA) with 8-µm pores. After transfection with miR-429 or NC,
the cells (1×104 cells) in 20 µl RPMI-1640 medium were
added to the upper chamber of the Transwell plates. RPMI-1640
medium containing 20% FBS was added to the upper chamber as a
chemoattractant. After 12-h incubation, cells on the upper surface
were removed using cotton wool and the cells attached to the bottom
were fixed with methanol and stained with 0.5% crystal violet. For
the invasion assays, cells (1×104 cells) in 20 µl
RPMI-1640 medium were added to the upper chamber pre-coated with
Matrigel (BD Biosciences). After 24-h incubation, cells on the
upper surface were removed using cotton wool and the cells attached
to the bottom were fixed with methanol and stained with 0.5%
crystal violet. Images were captured and the cells were counted
using a photomicroscope (Olympus Corporation, Tokyo, Japan).
Western blot analysis
Primary antibodies used in this study included
rabbit anti-human monoclonal specificity protein 1 (Sp1; dilution,
1:1,000; catalog no., 9389; Cell Signaling Technology, Inc.,
Beverly, MA, USA) and rabbit anti-human monoclonal β-actin
(dilution, 1:1,000; catalog no., 8457; Cell Signaling Technology,
Inc.). GC cells were lysed using radioimmunoprecipitation assay
lysis buffer (Beyotime Institute of Biotechnology, Haimen, China)
supplemented with a protease inhibitor cocktail at 72 h following
transfection. Equal amounts of protein were separated by 10%
SDS-PAGE and transferred onto polyvinylidene fluoride membranes
(EMD Millipore, Billerica, MA, USA). Subsequently, the membranes
were blocked with 5% skimmed in Tris-buffered saline containing
0.1% Tween-20 at room temperature for 1 h, and then incubated with
the primary antibodies overnight at 4°C. Goat anti-rabbit
horseradish peroxidase-conjugated secondary antibody (dilution,
1:200; catalog no., 7074; Cell Signaling Technology, Inc.) was used
to detect the primary antibodies. Finally, the bands were
visualized using enhanced chemiluminescence reagents and images
were captured using the FluorChem imaging system (ProteinSimple,
San Jose, CA, USA). AlphaEase FC software (version 4.0.1;
ProteinSimple, San Jose, CA, USA) was used to analyze the western
blotting results.
Luciferase assay
Sp1 was identified as a target of miR-429 using
TargetScan (http://www.targetscan.org/vert_71/). To determine
whether Sp1 is a direct target of miR-429, luciferase assays were
performed. Luciferase reporter plasmids, including pGL3 Sp1 3′UTR
wild type (WT) and pGL3 Sp1 3′UTR mutant type (Mut), were
synthesized and confirmed by GenePharma Co., Ltd. GC cells were
seeded into 24-well plates at a density of 40–50% confluence and
co-transfected with miR-429 or NC and the luciferase reporter
plasmid using Lipofectamine 2000, according to the manufacturer's
protocol. After a 48-h incubation, the cells were harvested and
analyzed for luciferase activity using the Dual-Luciferase Reporter
Assay System (Promega Corporation, Manheim, Germany). Each assay
was replicated three times.
Statistical analysis
Data are presented as the mean ± standard deviation,
and compared using Stata 10.0 software (StataCorp LP, College
Station, TX, USA). Double-tailed P-values <0.05 were considered
to be statistically significant.
Results
Expression of miR-429 in GC cell lines
prior to and following transfection with miR-429
RT-qPCR was conducted to assess the transfection
efficiency following transfection of SGC-7901 and AGS cells with
miR-429. As shown in Fig. 1, the
basal expression of miR-429 in GC cells was at the detection limit.
Following transfection of GC cells with miR-429, the expression
level of miR-429 was markedly increased until ~144 h later, as the
level of miR-429 declined gradually over time.
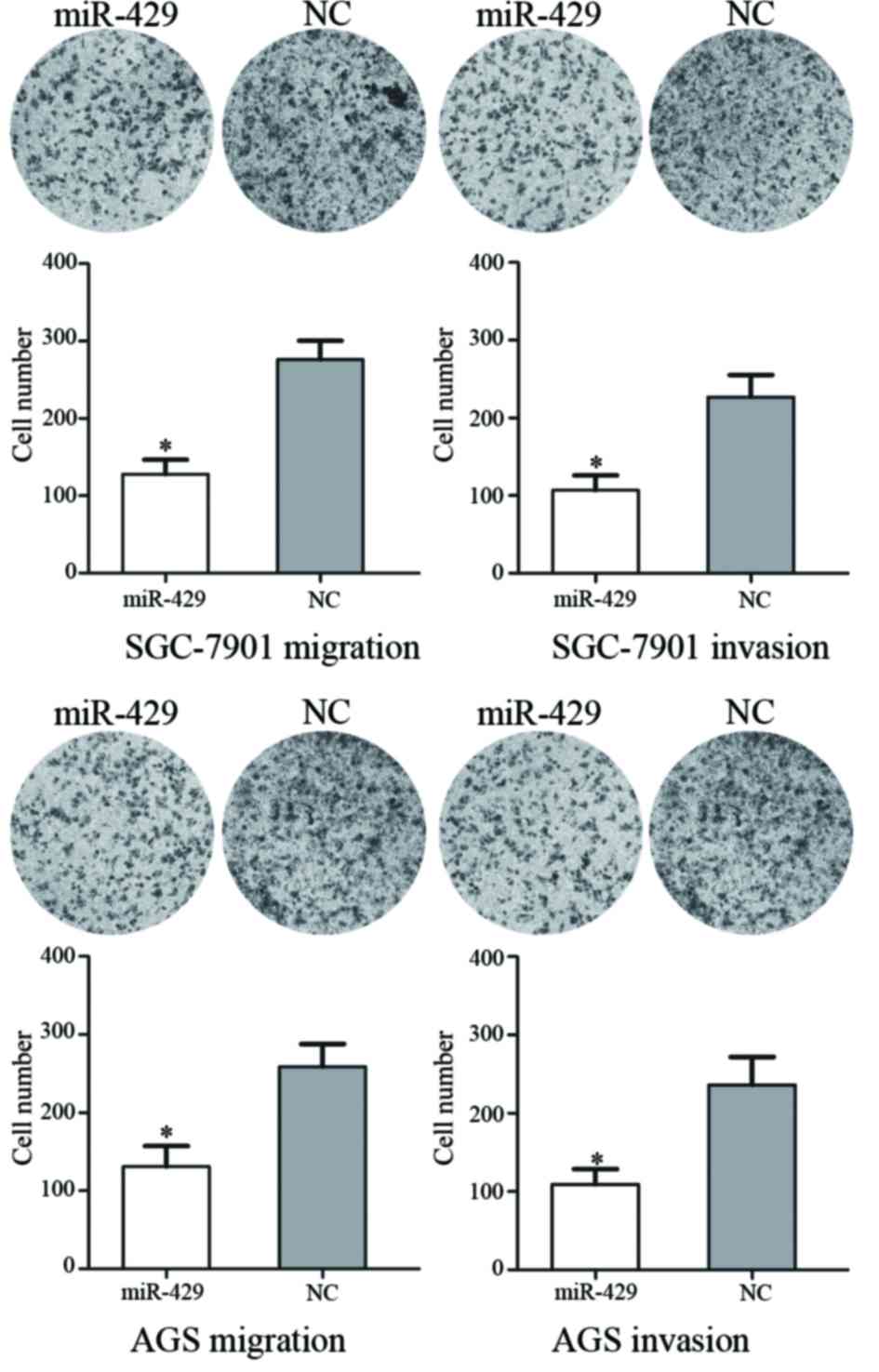
miR-429 suppresses GC cell migration
and invasion
Transwell assays were used to assess the effects of
miR-429 on GC SGC-7901 and AGS cell migration and invasion
(Fig. 2). In the migration assay, it
was demonstrated that miR-429 transfection resulted in a
53.69±5.42% decrease in SGC-7901 cells and a 48.27±6.23% decrease
in AGS cells. In the invasion assay, it was observed that miR-429
transfection led to a 56.54±5.68% decrease in SGC-7901 cells and a
52.81±7.39% decrease in AGS cells. These results suggest that
miR-429 inhibits the motility of GC SGC-7901 and AGS cells.
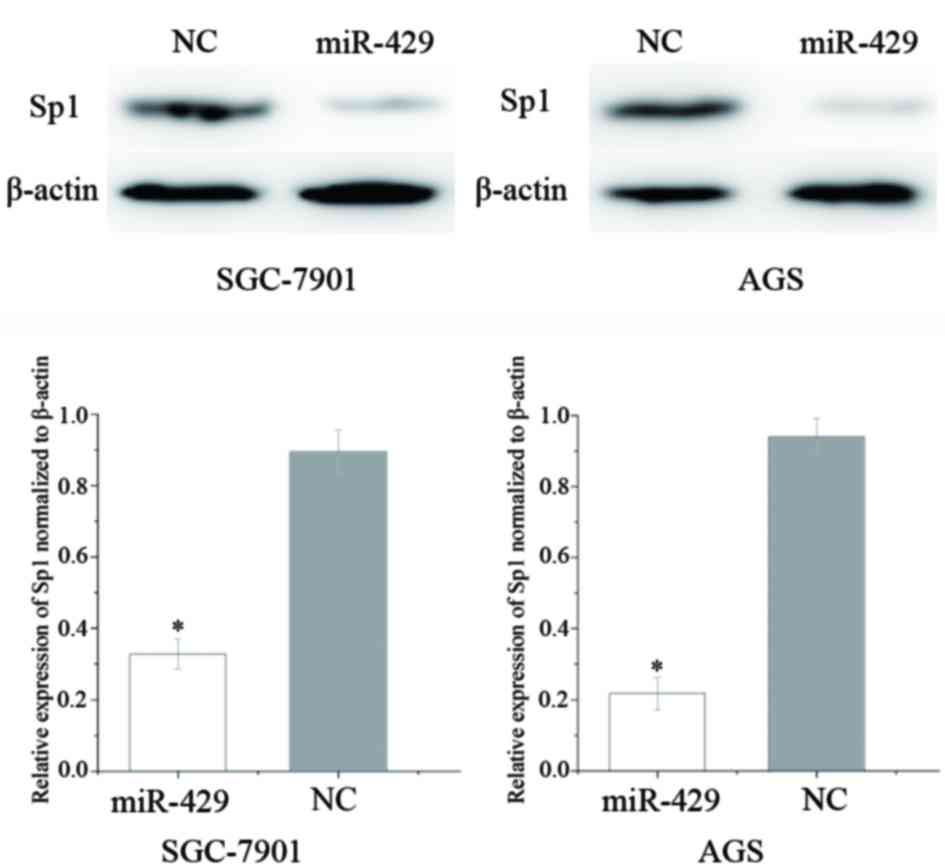
Sp1 is downregulated following
transfection of GC cells with miR-429
Western blot analysis was performed to investigate
the effect of increased expression levels of miR-429 on Sp1
expression in GC cells. As shown in Fig.
3, Sp1 was significantly downregulated in GC SGC-7901 (P=0.021)
and AGS (P=0.014) cell lines following overexpression of miR-429.
These results suggest that miR-429 reduces the protein expression
level of Sp1 in GC cells.
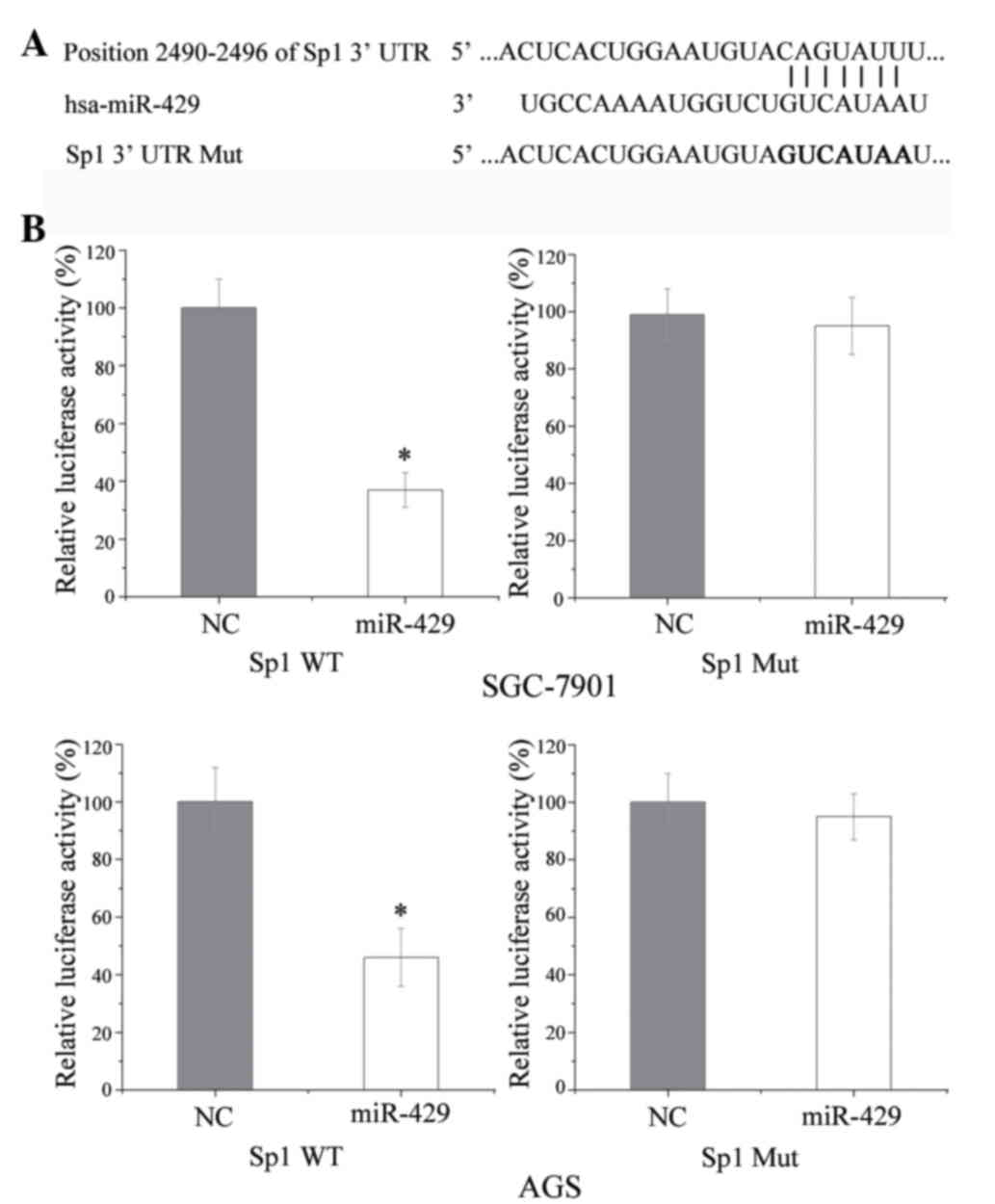
Sp1 is a direct target of miR-429 in
GC
Sp1 was identified as a target of miR-429 using
TargetScan. As shown in Fig. 4A, Sp1
mRNA contained a miR-429 7-nucleotide seed match at position
2,490–2,496 of the Sp1 3′-UTR. To verify whether miR-429 directly
targets Sp1, a luciferase activity assay was performed. As shown in
Fig. 4B, miR-429 significantly
inhibited the luciferase activity of the vector carrying the
wild-type but not the mutant Sp1 3′-UTR in GC SGC-7901 and AGS
cells. These results suggest that Sp1 may be a direct target of
miR-429 in vitro.
Discussion
The miR-200 family, which is located on chromosome 1
and has been implicated in numerous cancers, can be divided into
two clusters: The first cluster consists of miR-200a, miR-200b and
miR-429, and the second cluster comprises miR-200c and miR-141
(15). Previous studies have
demonstrated that miR-429 is dysregulated in various cancers,
including non-small cell lung cancer (16), Ehrlich ascites tumor cells (17), renal cell carcinoma (18), colorectal carcinoma, nasopharyngeal
carcinoma (19) and endometrial
endometrioid carcinomas (20). In
addition, the expression level of miR-429 was upregulated in
bladder cancer and ovarian carcinoma (21,22), and
its upregulation in patients with serous ovarian carcinoma was
correlated with a poor prognosis (22). Therefore, miR-429 is likely involved
in carcinogenesis and cancer progression, and may have different
roles depending on the type of cancer.
In osteosarcoma, overexpression of miR-429 inhibited
cell proliferation and enhanced cell apoptosis. Furthermore,
miR-429 was shown to exert tumor-suppressing effects in
osteosarcoma by binding to the 3′-UTR of zinc finger E-box binding
homeobox 1 (ZEB1) (23). In
esophageal carcinoma, miR-429 was shown to be downregulated and its
expression was significantly associated with the occurrence of
lymph node metastases. Upregulation of miR-429 inhibited cell
migration and enhanced cell apoptosis in esophageal carcinoma cell
lines by directly targeting B-cell lymphoma-2 and Sp1 (24). In breast cancer, downregulation of
miR-429 was shown to prevent the bone metastasis of cancer cells.
Conversely, ectopic expression of miR-429 markedly suppressed
breast cancer cell invasion through downregulation of ZEB1 and
V-Crk avian sarcoma virus CT10 oncogene homolog-like (25). Together, these findings suggested that
miR-429 may used for the development of novel therapeutic
strategies for cancer.
miR-429 was downregulated in human GC tissues and
the level of miR-429 was associated with lymph node metastasis.
Upregulation of miR-429 inhibited the proliferation of tumor cells
and their attachment to fibronectin and laminin in a dose-dependent
manner (26). However, no previous
study has investigated the functional role of miR-429 in GC cell
migration and invasion. In the present study, miR-429 was shown to
inhibit cell migration and invasion by directly targeting Sp1.
These findings suggested that Sp1 should be investigated as a
target therapy to block GC from becoming invasive.
Sp1 is a sequence-specific DNA-binding protein that
was the first transcription factor to be cloned from mammalian
cells in 1983 (27). The expression
level of Sp1 has been shown to be upregulated in numerous human
cancers, including GC (28), breast
cancer (29), hepatocellular
carcinomas (30), thyroid cancer
(31), colorectal cancer (32), pancreatic cancer (33) and lung cancer (34). Increasingly, evidence has suggested
that Sp1 may be involved in a variety of cellular processes,
including cell growth, survival, differentiation, tumor development
and tumor progression (35–38). miRs, which regulate the expression of
their target genes by base pairing with seed sequences in the
3′-UTR of mRNAs, have been shown to regulate the expression of Sp1
(39,40). In a previous study, miR-22 was
reported to be downregulated in GC and inhibited cell
proliferation, migration and invasion by targeting Sp1 (41). The results of the present study
suggested that miR-429 suppresses GC cell migration and invasion by
directly targeting Sp1. Therefore, miR-429 could be investigated
for its value in the early detection of tumor recurrence and as a
potential target of therapy to prevent GC from becoming
invasive.
The metastasis of GC is a key step in tumor
progression and is and indicator of a poor prognosis (42). Metastasis involves a series of
sequential events, including detachment, migration, invasion,
extravasation, angiogenesis, survival in the circulatory system and
extravasation (43,44). Several miRs have been reported to have
an important role in tumor metastasis (45–47).
Furthermore, since miRs regulate multiple target genes
simultaneously, miR-based therapy is expected to be more efficient
than the traditional single target therapy (48,49). In
the present study, miR-429 was shown to be an important regulator
in tumor cell migration and invasion, which emphasized an essential
role of miR-429 in regulating GC metastasis.
In conclusion, the present study demonstrated that
upregulation of miR-429 in GC cell lines inhibited GC cell
metastasis in vitro. In addition, Sp1 was shown to be
negatively regulated by miR-429 and to contain a binding site for
miR-429 in the 3′-UTR of its mRNA. This newly identified
association between miR-429 and Sp1 may potentially provide a novel
therapeutic strategy for GC.
References
|
1
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Otani K, Li X, Arakawa T, Chan FK and Yu
J: Epigenetic-mediated tumor suppressor genes as diagnostic or
prognostic biomarkers in gastric cancer. Expert Rev Mol Diagn.
13:445–455. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kim SJ, Wang YG, Lee HW, Kang HG, La SH,
Choi IJ, Irimura T, Ro JY, Bresalier RS and Chun KH: Up-regulation
of neogenin-1 increases cell proliferation and motility in gastric
cancer. Oncotarget. 5:3386–3398. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shenouda SK and Alahari SK: MicroRNA
function in cancer: Oncogene or a tumor suppressor? Cancer
Metastasis Rev. 28:369–378. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Xu L, Li Y, Yan D, He J and Liu D:
MicroRNA-183 inhibits gastric cancer proliferation and invasion via
directly targeting Bmi-1. Oncol Lett. 8:2345–2351. 2014.PubMed/NCBI
|
|
7
|
Sun YC, Wang J, Guo CC, Sai K, Wang J,
Chen FR, Yang QY, Chen YS, Wang J, To TS, et al: MiR-181b
sensitizes glioma cells to teniposide by targeting MDM2. BMC
Cancer. 14:6112014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen C, Zhao Z, Liu Y and Mu D:
microRNA-99a is downregulated and promotes proliferation, migration
and invasion in non-small cell lung cancer A549 and H1299 cells.
Oncol Lett. 9:1128–1134. 2015.PubMed/NCBI
|
|
9
|
Zhang W, Liu K, Liu S, Ji B, Wang Y and
Liu Y: MicroRNA-133a functions as a tumor suppressor by targeting
IGF-1R in hepatocellular carcinoma. Tumour Biol. 36:9779–9788.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lu J, Getz G, Miska EA, Alvarez-Saavedra
E, Lamb J, Peck D, Sweet-Cordero A, Ebert BL, Mak RH, Ferrando AA,
et al: MicroRNA expression profiles classify human cancers. Nature.
435:834–838. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yang TS, Yang XH, Wang XD, Wang YL, Zhou B
and Song ZS: MiR-214 regulate gastric cancer cell proliferation,
migration and invasion by targeting PTEN. Cancer Cell Int.
13:682013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang F, Li T, Zhang B, Li H, Wu Q, Yang L,
Nie Y, Wu K, Shi Y and Fan D: MicroRNA-19a/b regulates multidrug
resistance in human gastric cancer cells by targeting PTEN. Biochem
Biophys Res Commun. 434:688–694. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hsu KW, Wang AM, Ping YH, Huang KH, Huang
TT, Lee HC, Lo SS, Chi CW and Yeh TS: Downregulation of tumor
suppressor MBP-1 by microRNA-363 in gastric carcinogenesis.
Carcinogenesis. 35:208–217. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Song JH and Meltzer SJ: MicroRNAs in
pathogenesis, diagnosis and treatment of gastroesophageal cancers.
Gastroenterology. 143:35–47 e32. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tang J, Li L, Huang W, Sui C, Yang Y, Lin
X, Hou G, Chen X, Fu J, Yuan S, et al: MiR-429 increases the
metastatic capability of HCC via regulating classic Wnt pathway
rather than epithelial-mesenchymal transition. Cancer Lett.
364:33–43. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhu W, He J, Chen D, Zhang B, Xu L, Ma H,
Liu X, Zhang Y and Le H: Expression of miR-29c, miR-93 and miR-429
as potential biomarkers for detection of early stage non-small lung
cancer. PLoS One. 9:e877802014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Said NA and Williams ED: Growth factors in
induction of epithelial-mesenchymal transition and metastasis.
Cells Tissues Organs. 193:85–97. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sun Y, Shen S, Liu X, Tang H, Wang Z, Yu
Z, Li X and Wu M: MiR-429 inhibits cells growth and invasion and
regulates EMT-related marker genes by targeting Onecut2 in
colorectal carcinoma. Mol Cell Biochem. 390:19–30. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bartis D, Mise N, Mahida RY, Eickelberg O
and Thickett DR: Epithelial-mesenchymal transition in lung
development and disease: Does it exist and is it important? Thorax.
69:760–765. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Miyazono K: Transforming growth
factor-beta signaling in epithelial-mesenchymal transition and
progression of cancer. Proc Jpn Acad Ser B Phys Biol Sci.
85:314–323. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xie P, Xu F, Cheng W, Gao J, Zhang Z, Ge
J, Wei Z, Xu X and Liu Y: Infiltration related miRNAs in bladder
urothelial carcinoma. J Huazhong Univ Sci Technolog Med Sci.
32:576–580. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Leskela S, Leandro-Garcia LJ, Mendiola M,
Barriuso J, Inglada-Pérez L, Muñoz I, Martínez-Delgado B, Redondo
A, de Santiago J, Robledo M, et al: The miR-200 family controls
beta-tubulin III expression and is associated with paclitaxel-based
treatment response and progression-free survival in ovarian cancer
patients. Endocr Relat Cancer. 18:85–95. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu X, Liu Y, Wu S, Shi X, Li L, Zhao J
and Xu H: Tumor-suppressing effects of miR-429 on human
osteosarcoma. Cell Biochem Biophys. 70:215–224. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang Y, Li M, Zang W, Ma Y, Wang N, Li P,
Wang T and Zhao G: MiR-429 up-regulation induces apoptosis and
suppresses invasion by targeting Bcl-2 and SP-1 in esophageal
carcinoma. Cell Oncol (Dordr). 36:385–394. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ye ZB, Ma G, Zhao YH, Xiao Y, Zhan Y, Jing
C, Gao K, Liu ZH and Yu SJ: miR-429 inhibits migration and invasion
of breast cancer cells in vitro. Int J Oncol. 46:531–538.
2015.PubMed/NCBI
|
|
26
|
Sun T, Wang C, Xing J and Wu D: miR-429
modulates the expression of c-myc in human gastric carcinoma cells.
Eur J Cancer. 47:2552–2559. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dynan WS and Tjian R: The
promoter-specific transcription factor Sp1 binds to upstream
sequences in the SV40 early promoter. Cell. 35:79–87. 1983.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xu Y, Zhao F, Wang Z, Song Y, Luo Y, Zhang
X, Jiang L, Sun Z, Miao Z and Xu H: MicroRNA-335 acts as a
metastasis suppressor in gastric cancer by targeting Bcl-w and
specificity protein 1. Oncogene. 31:1398–1407. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yue L, Li L, Liu F, Hu N, Zhang W, Bai X,
Li Y, Zhang Y, Fu L, Zhang X and Ye L: The oncoprotein HBXIP
activates transcriptional coregulatory protein LMO4 via Sp1 to
promote proliferation of breast cancer cells. Carcinogenesis.
34:927–935. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yin P, Zhao C, Li Z, Mei C, Yao W, Liu Y,
Li N, Qi J, Wang L, Shi Y, et al: Sp1 is involved in regulation of
cystathionine gamma-lyase gene expression and biological function
by PI3K/Akt pathway in human hepatocellular carcinoma cell lines.
Cell Signal. 24:1229–1240. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bonofiglio D, Qi H, Gabriele S, Catalano
S, Aquila S, Belmonte M and Andò S: Peroxisome
proliferator-activated receptor gamma inhibits follicular and
anaplastic thyroid carcinoma cells growth by upregulating
p21Cip1/WAF1 gene in a Sp1-dependent manner. Endocr Relat Cancer.
15:545–557. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Pathi S, Jutooru I, Chadalapaka G, Nair V,
Lee SO and Safe S: Aspirin inhibits colon cancer cell and tumor
growth and downregulates specificity protein (Sp) transcription
factors. PLoS One. 7:e482082012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Huang C and Xie K: Crosstalk of Sp1 and
Stat3 signaling in pancreatic cancer pathogenesis. Cytokine Growth
Factor Rev. 23:25–35. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang YT, Chuang JY, Shen MR, Yang WB,
Chang WC and Hung JJ: Sumoylation of specificity protein 1 augments
its degradation by changing the localization and increasing the
specificity protein 1 proteolytic process. J Mol Biol. 380:869–885.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Black AR, Black JD and Azizkhan-Clifford
J: Sp1 and krüppel-like factor family of transcription factors in
cell growth regulation and cancer. J Cell Physiol. 188:143–160.
2001. View
Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li L, He S, Sun JM and Davie JR: Gene
regulation by Sp1 and Sp3. Biochem Cell Biol. 82:460–471. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Safe S and Abdelrahim M: Sp transcription
factor family and its role in cancer. Eur J Cancer. 41:2438–2448.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang L, Guan X, Zhang J, Jia Z, Wei D, Li
Q, Yao J and Xie K: Targeted inhibition of Sp1-mediated
transcription for antiangiogenic therapy of metastatic human
gastric cancer in orthotopic nude mouse models. Int J Oncol.
33:161–167. 2008.PubMed/NCBI
|
|
39
|
Sun L, Liang J, Wang Q, Li Z, Du Y and Xu
X: MicroRNA-137 suppresses tongue squamous carcinoma cell
proliferation, migration and invasion. Cell Prolif. 49:628–635.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Liu X, Duan H, Zhou S, Liu Z, Wu D, Zhao
T, Xu S, Yang L and Li D: microRNA-199a-3p functions as tumor
suppressor by regulating glucose metabolism in testicular germ cell
tumors. Mol Med Rep. 14:2311–2320. 2016.PubMed/NCBI
|
|
41
|
Guo MM, Hu LH, Wang YQ, Chen P, Huang JG,
Lu N, He JH and Liao CG: miR-22 is down-regulated in gastric cancer
and its overexpression inhibits cell migration and invasion via
targeting transcription factor Sp1. Med Oncol. 30:5422013.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Oue N, Aung PP, Mitani Y, Kuniyasu H,
Nakayama H and Yasui W: Genes involved in invasion and metastasis
of gastric cancer identified by array-based hybridization and
serial analysis of gene expression. Oncology. 69 Suppl 1:17–22.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Gupta GP and Massague J: Cancer
metastasis: Building a framework. Cell. 127:679–695. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Klein CA: Cancer. The metastasis cascade.
Science. 321:1785–1787. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhang W, Liu J, Qiu J, Fu X, Tang Q, Yang
F, Zhao Z and Wang H: MicroRNA-382 inhibits prostate cancer cell
proliferation and metastasis through targeting COUP-TFII. Oncol
Rep. 36:3707–3715. 2016.PubMed/NCBI
|
|
46
|
Zhou Q, Zhu Y, Wei X, Zhou J, Chang L, Sui
H, Han Y, Piao D, Sha R and Bai Y: MiR-590-5p inhibits colorectal
cancer angiogenesis and metastasis by regulating nuclear factor
90/vascular endothelial growth factor A axis. Cell Death Dis.
7:e24132016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Cui Z and Hu Y: MicroRNA-124 suppresses
Slug-mediated lung cancer metastasis. Eur Rev Med Pharmacol Sci.
20:3802–3811. 2016.PubMed/NCBI
|
|
48
|
Shah MY, Ferrajoli A, Sood AK,
Lopez-Berestein G and Calin GA: microRNA Therapeutics in Cancer -
An Emerging Concept. EBioMedicine. 12:34–42. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Naidu S, Magee P and Garofalo M:
MiRNA-based therapeutic intervention of cancer. J Hematol Oncol.
8:682015. View Article : Google Scholar : PubMed/NCBI
|