Introduction
Prostate cancer is the fourth leading cause of
cancer-associated mortality and the second most prevalent cancer
worldwide (1). In developed
countries, prostate cancer is the most commonly diagnosed cancer in
men and affects >17% of men worldwide (1). In the US, prostate cancer is the most
common malignancy and the second leading cause of cancer-associated
mortality (2). Globally, a high
incidence of prostate cancer is associated with excessive
consumption of Ω-6 polyunsaturated fatty acids (PUFAs), commonly
found in red and organ meats, refined vegetable oils and cooked
processed meat (3–5). Conversely, evidence suggests that an Ω-3
PUFA-rich diet is inversely associated with prostate cancer
development (6–9).
Both Ω-6 and Ω-3 PUFAs are essential fatty acids
(10), and while Ω-3 PUFAs have
protective effects, Ω-6 PUFAs may serve a role in cancer
development (11–14). It has been demonstrated that dietary
intake of long-chain Ω-3 PUFAs reduces the incidence of several
types of cancer and alleviates cancer-associated complications
(15,16). Clinically, long-term intake of dietary
or supplemental docosahexaenoic acid (DHA) and eicosapentaenoic
acid (EPA) (both Ω-3 PUFAs) is associated with a decreased risk of
prostate cancer development (6–9), increased
sensitivity to chemotherapy treatment (14) and decreased risk of advanced stage
disease, metastases and cancer-associated mortality (8,17,18). Additionally, dietary Ω-3 PUFAs enhance
hormone ablation therapy in androgen-dependent prostate cancer
(19) and attenuate prostate cancer
growth in primary prostate cancer development (20) and xenograft models (19). Therefore, Ω-3 PUFAs may be important
in the prevention and treatment of prostate cancer.
The EPA-rich Saprolegnia diclina gene, sdd17,
encodes an Ω-3 fatty acid desaturase that converts exogenous
arachidonic acid (AA), an Ω-6 PUFA, into EPA (21). It has been reported that sdd17 from
EPA-rich fungus is expressed at high levels and increases Ω-3 fatty
acid concentrations in mammalian cells (22). In the present study, prostate cancer
cells were infected with a lentivirus carrying the sdd17 gene and
the underlying mechanisms of Ω-3 PUFAs on prostate cell
proliferation were evaluated. The potential positive outcomes of
the present study may benefit patients with prostate cancer.
Materials and methods
Reagents
All cell culture reagents were purchased from
Incoterm Fisher Scientific, Inc. (Waltham, MA, USA). AA, EPA,
propidium iodide (PI) and PUFA standards were obtained from
Sigma-Aldrich; Merck Millipore (Darmstadt, Germany). Water-soluble
tetrazolium (WST) was acquired from Dojindo Molecular Technologies,
Inc. (Kumamoto, Japan). Anti-E2F transcription factor 1 antibodies
(#sc-251; dilution, 1:1,000) were obtained from Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA) and anti-β-actin antibodies
(#ab8226; dilution, 1:2,000) were purchased from Abcam (Cambridge,
UK).
Cell culture and proliferation
assays
The human prostate cancer cell line VCaP was
obtained from the American Type Culture Collection (Manassas, VA,
USA). VCaP cells were cultured at 37°C and 5% CO2 in
Dulbecco's modified Eagle's medium supplemented with 10% fetal
bovine serum (Invitrogen; Thermo Fisher Scientific, Inc.), 100
IU/ml penicillin and 100 µg/ml streptomycin. Cell proliferation
assays were performed in 96-well plates. Cells (5×103
per well) were incubated for 24 h with different concentrations of
PUFAs (10–50 µM), stained with WST at 37°C for 1 h and quantified
at 450 nm (ELX800; BioTek Instruments, Inc., Winooski, VT,
USA).
Overexpression of msdd17 by lentiviral
transfection
The sdd17 gene was cloned from S. diclina
based on its nucleotide sequence (GenBank accession no. AY373823).
The codons of sdd17 cDNA were optimized for efficient translation
in mammalian cells, resulting in the msdd17 gene. The msdd17 cDNA
was subsequently inserted into the PLJM1 lentivirus vector
(Addgene, Inc., Cambridge, MA, USA); pMD2.G and psPAX2 plasmids
were co-transfected with PLJM1-msdd17 (vehicle plasmid) into 293T
cells (American Type Culture Collection, Manassas, VA, USA) using
X-tremeGENE™ HP DNA transfection reagent (Roche Diagnostics,
Indianapolis, IN, USA). In order to generate a stable
msdd17-overexpressing cell line, lentivirus-containing supernatant
was harvested 48 h post-transfection and used to infect VCaP
cells.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis of msdd17 gene
expression levels
Total RNA was extracted using TRIzol®
reagent, and was subsequently treated with DNase I and reverse
transcribed using the PrimeScript™ RT reagent kit (Takara Bio,
Inc., Shiga, Japan) following the manufacturer protocol. qPCR was
performed using an Applied Biosystems StepOnePlus™ Real-Time PCR
system (Applied Biosystems; Thermo Fisher Scientific, Inc.).
FastStart Universal SYBR Green Master (Rox) was obtained from Roche
Diagnostics (#04913914001). The qPCR conditions consisted of 1
cycle at 50°C for 2 min, followed by 1 cycle at 95°C for 30 sec,
and 40 cycles at 95°C for 15 sec, 58°C for 30 sec and 72°C for 30
sec. The experiments were repeated six times. The results were
normalized according to the expression levels of β-actin RNA.
Results were expressed using the 2−ΔΔCq method (23). The primer sequences were as follows:
msdd17, forward, 5′-GTACACAAACCAAGCTCCGC-3′ and reverse,
5′-CCATCCTGACCCCATTCGAG-3′; and β-actin, forward,
5′-GCTCTGGCTCCTAGCACCAT-3′ and reverse,
5′-GGGCCGGACTCATCGTACT-3′.
Gas chromatography (GC) analysis
Lipid extraction from VCaP cells was performed
following a previously described protocol (24). Gas chromatography was performed on an
Agilent 7890A Gas Chromatograph (Agilent Technologies, Inc., Santa
Clara, CA, USA). Compounds were identified by comparing their
retention times with those of PUFA standards.
Cell cycle analysis
Following incubation overnight in Dulbecco's
modified Eagle's medium (Invitrogen), VCaP cells were harvested and
rinsed with PBS twice. Prior to cell cycle analysis, cells were
fixed in 70% pre-cold ethanol at 4°C overnight, and prior to flow
cytometry, cells were washed twice with PBS and then resuspended
with PBS containing 10 mg/ml PI (Sigma-Aldrich; Merck Millipore)
and incubated for 15 min in the dark at room temperature. DNA
content was analyzed using a BD FACStar flow cytometer and the
percentages of different cell cycle phases were determined using a
ModFit LT software version 4.0 (BD Biosciences, San Jose, CA,
USA).
Western blotting
VCaP cells were treated with PUFA agents at 37°C for
various times as indicated in the experiments. The cells were
collected into lysis buffer [50 mM Tris-HCl (pH 8.0), 150 mM NaCl,
0.5% sodium deoxycholate, 1% Nonidet P-40, 0.1% SDS, 100 g/ml PMSF
and aprotinin] and placed on ice for 30 min. Cell lysates were
sonicated on ice for at least 30 sec and then cleared by
centrifugation at 12,000 × g for 30 min at 4°C. Equal amounts of
total protein (40 µg) were separated by SDS-PAGE and transferred
onto a nitrocellulose membrane. The membranes were probed with the
appropriate antibodies at 4°C overnight with gentle agitation.
Immunoreactivity was detected by ECL and quantified using ImageLab
version 4.0 analysis software (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA).
Statistical analysis
Data are presented as the mean ± standard error of
at least three independent experiments. All statistical analyses
were performed using GraphPad Prism version 5.01 (GraphPad
Software, Inc., La Jolla, CA, USA) and included either two-tailed
Student's t-test or one-way analysis of variance followed by
Dunnett's test for comparing the means of two or multiple groups,
respectively. P<0.05 was considered to indicate a statistically
significant difference.
Results
Effect of Ω-3 PUFAs on VCaP cell
growth
To assess the effects of Ω-3 PUFAs on prostate
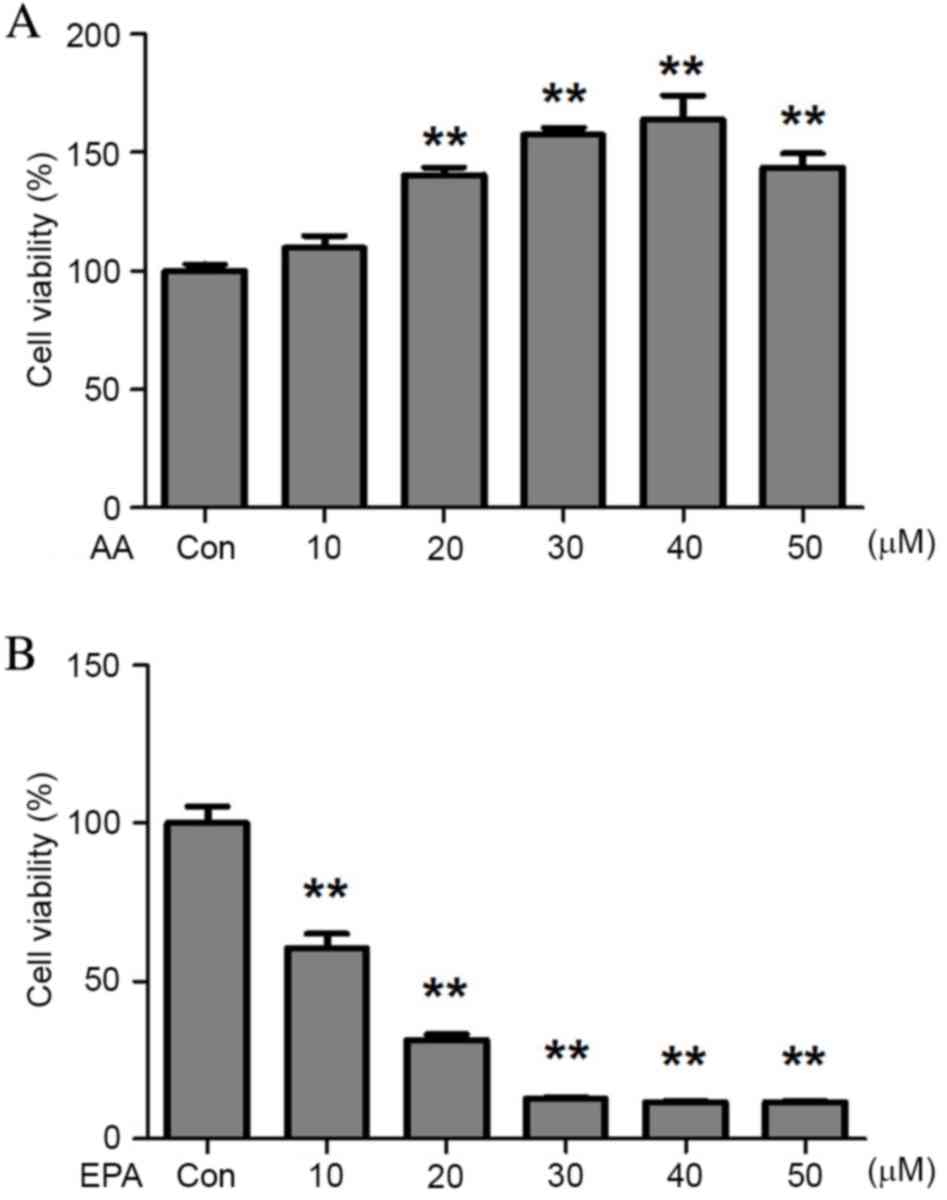
cancer cells, VCaP cells were exposed to either AA or EPA. The
results demonstrated that AA stimulated VCaP cell proliferation
(Fig. 1A), which was consistent with
previous observations in breast (24)
and endometrial (25,26) cancer cell lines. Conversely, EPA
effectively inhibited prostate cancer cell growth in vitro.
At the highest concentration (50 µM), EPA caused an 8-fold
reduction in VCaP cell viability (Fig.
1B), demonstrating that it inhibited VCaP cell viability in a
dose-dependent manner.
Effect of msdd17 on VCaP cell
growth
To assess the effect of msdd17 on prostate cancer
cells, VCaP cells were infected with a lentivirus carrying the
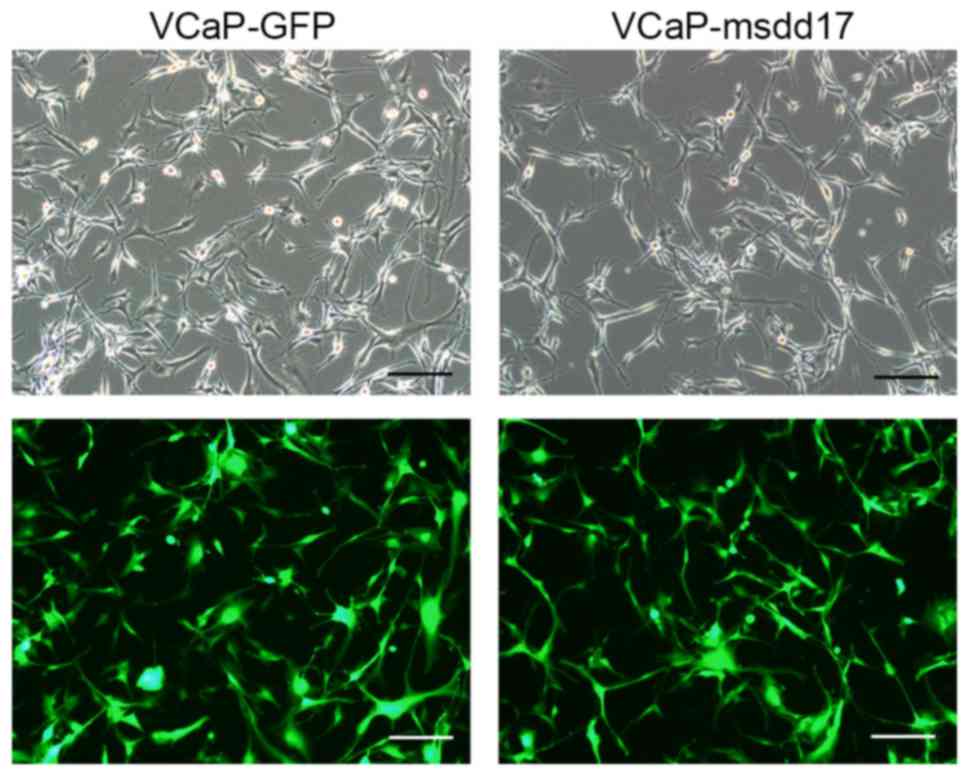
msdd17 gene (VCaP-msdd17 cells). A green fluorescent protein
(GFP)-expressing line, VCaP-GFP, was generated as a control.
The lentivirus carrying the msdd17 gene was used to
infect VCaP cells and the co-expression of GFP allowed the
identification of the cells that expressed the msdd17 gene.
Following infection with the lentivirus, the VCaP cells exhibited
bright fluorescence indicating a high expression level of the
transgene (Fig. 2). qPCR was
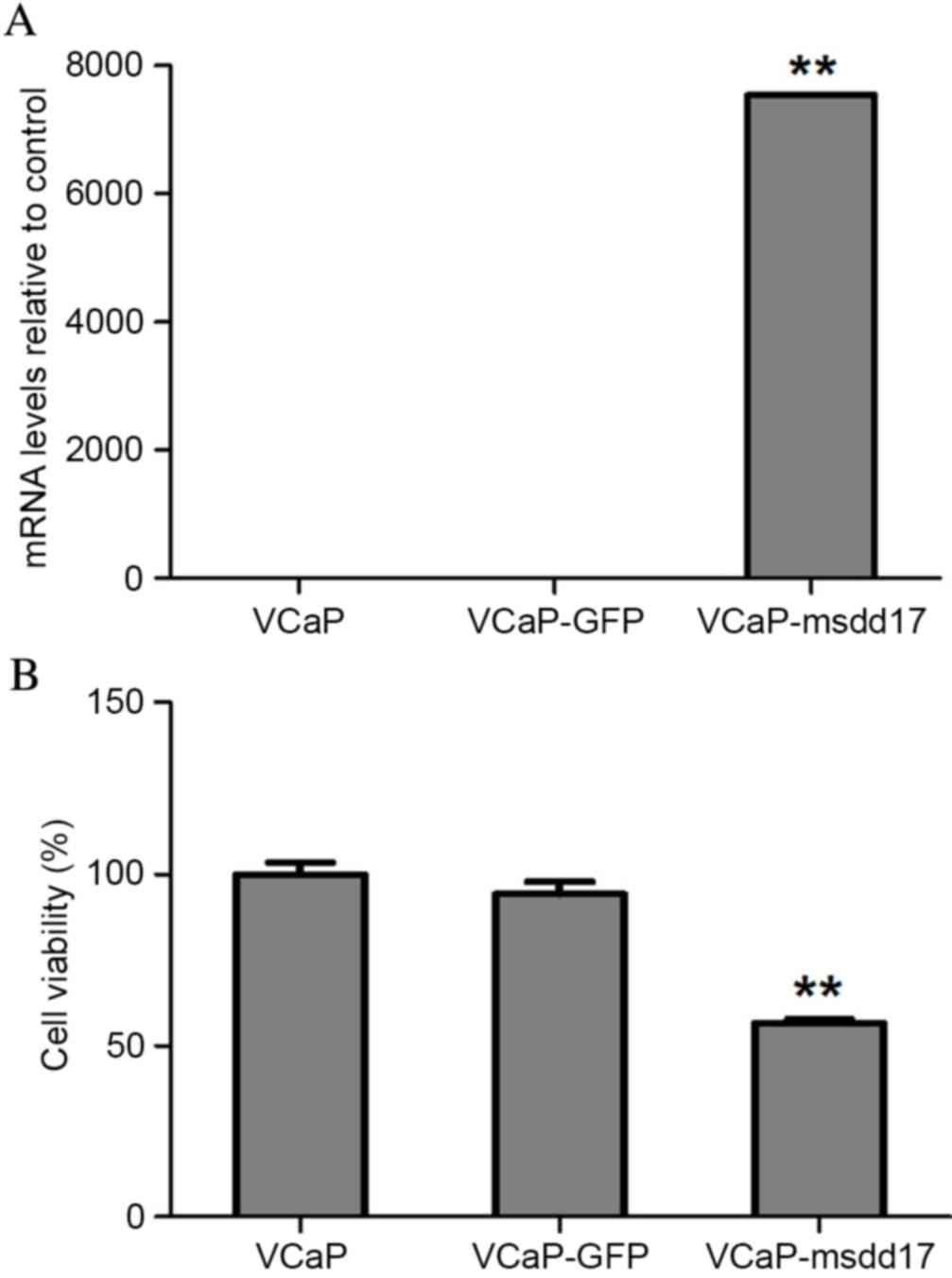
performed to analyze msdd17 gene expression levels. The results
indicated significantly increased msdd17 expression levels in the
VCaP-msdd17 cells compared with those of the control cells
(Fig. 3A), demonstrating that the
msdd17 gene may be highly expressed in transfected VCaP cells,
which lack the gene naturally.
Msdd17 gene transfection resulted in the conversion
of Ω-6 PUFAs into Ω-3 PUFAs in the VCaP cells (Table I). GC analyses demonstrated that 26%
of AA was converted into EPA (Table
I). Consistent with this observation, msdd17 gene expression
significantly suppressed VCaP cell viability (Fig. 3B). Taken together, these results
indicated that endogenous EPA, mediated by msdd17 gene expression,
directly inhibited prostate cancer cell growth.
 | Table I.Ω-6 and Ω-3 PUFA levels in VCaP-msdd17
cells compared with controls. |
Table I.
Ω-6 and Ω-3 PUFA levels in VCaP-msdd17
cells compared with controls.
| PUFAs, % | VCaP-GFP | VCaP-msdd17 |
|---|
| LA (C18:2, Ω-6) | 1.21±0.06 | 1.23±0.30 |
| AA (C20:4, Ω-6) | 6.21±0.12 |
4.57±0.07a |
| ALA (C18:3
Ω-3) | 0.16±0.00 | 0.15±0.02 |
| EPA (C20:5,
Ω-3) | 1.20±0.02 |
1.64±0.02a |
| DPA (C22:5,
Ω-3) | 2.97±0.12 |
4.63±0.05a |
| DHA (C22:6,
Ω-3) | 6.67±0.55 | 7.15±0.09 |
Effect of msdd17 on VCaP cell
cycle
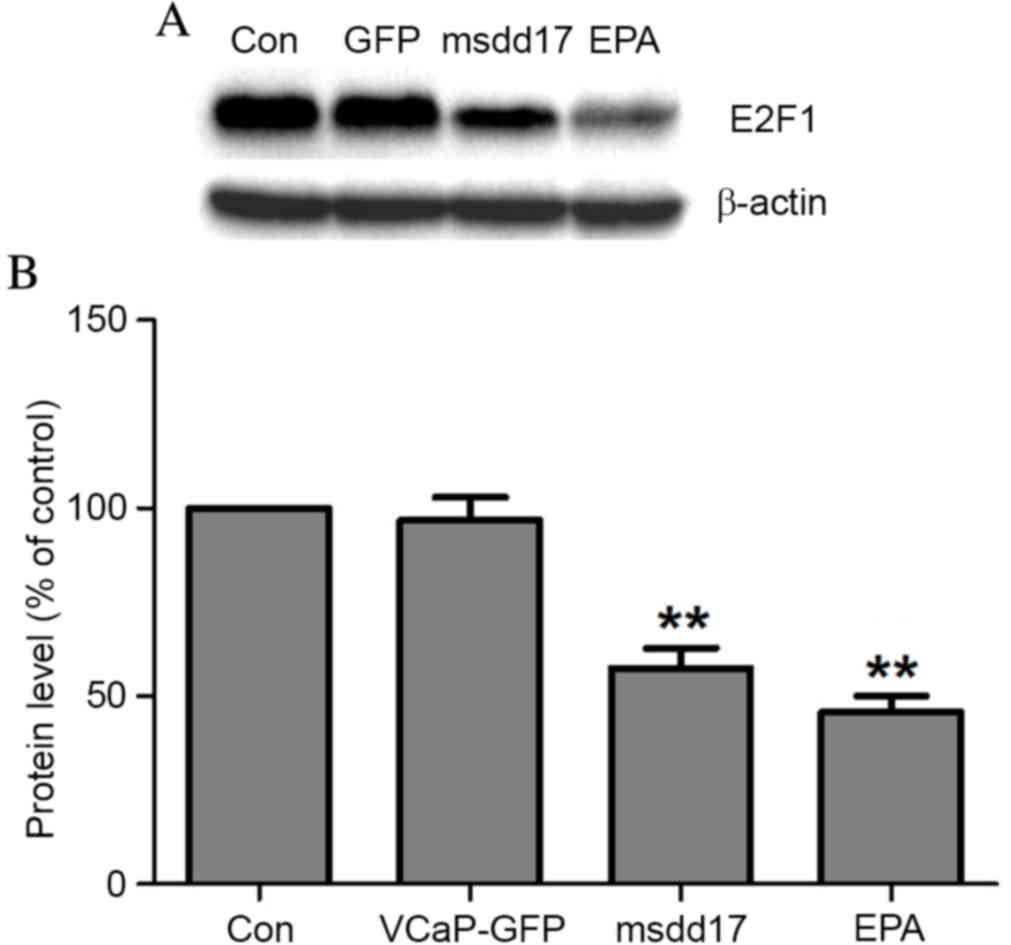
Flow cytometry analyses demonstrated that msdd17
gene expression resulted in G2 arrest in the cell cycle of VCaP
cells (Table II). To assess the
mechanism by which Ω-3 PUFAs suppress cell proliferation through
cell cycle arrest, the expression of E2F1 was evaluated following
EPA treatment. Western blot analysis demonstrated that EPA
treatment significantly decreased E2F1 expression and that the
expression of msdd17 mimicked the effect of EPA on E2F1 regulation
(Fig. 4).
 | Table II.Flow cytometry analyses of cell cycle
in VCaP cells. |
Table II.
Flow cytometry analyses of cell cycle
in VCaP cells.
|
| G1/G0, % | S, % | G2/M, % |
|---|
| VCaP-GFP | 80.96±1.02 | 10.12±0.90 | 8.92±0.31 |
| VCaP-msdd17 | 81.22±3.35 |
3.40±1.83a |
15.38±1.53a |
| EPA | 78.98±2.62 |
3.81±1.18a |
17.21±1.47a |
Discussion
It is well known that Ω-3 PUFAs have
tumor-suppressing effects. The msdd17 gene, which encodes an Ω-3
fatty acid desaturase, converts AA into EPA (21,22).
However, little is known regarding the function of the msdd17 gene
in tumor cells in vitro. Therefore, the present study aimed
to investigate the effect and underlying mechanisms of the msdd17
gene on prostate cancer. The msdd17 gene was transfected directly
into VCaP cells and the inhibitory effects of the gene on prostate
cancer cell proliferation was confirmed. Further experiments
demonstrated that msdd17 gene expression induced G2 cell cycle
arrest in prostate cancer cells and that E2F1 may be associated
with this process. To the best of our knowledge, these results
demonstrate, for the first time, that msdd17 gene transfection into
prostate cancer cells may be used as a novel therapeutic strategy
to treat prostate cancer.
Mounting evidence has linked the dietary consumption
of Ω-3 PUFAs with the prevention or attenuation of several types of
cancer, including breast (27),
endometrial (28) and prostate
(6–9)
cancer. In the present study, VCaP cells were exposed to either AA
or EPA, and it was demonstrated that while EPA inhibited prostate
cancer cell proliferation in a dose-dependent manner, AA stimulated
cell proliferation. GC analyses indicated an association between
msdd17 gene expression and the successful conversion of AA into
EPA. Additionally, cell proliferation assays demonstrated that
msdd17 gene expression inhibited the proliferation of prostate
cancer cells. Therefore, expression of the msdd17 gene may suppress
tumorigenesis by simultaneously increasing endogenous EPA levels
and decreasing endogenous AA levels.
E2F1 is a transcription factor involved in the
pRb/E2F1 pathway and in cell cycle regulation (29), and it enhances glycolysis by
suppressing Sirt6 transcription in prostate cancer cells (30). Previous studies have reported that
G2/M cell cycle arrest is associated with the downregulation of
E2F1 (31–33). In the present study, cell cycle
analyses indicated that EPA and msdd17 gene expression inhibited
prostate cancer cell proliferation by inducing prostate cancer G2
cell cycle arrest. As E2F1 serves a critical role in cellular
proliferation, differentiation and apoptosis (34,35), the
present study investigated whether E2F1 was involved in msdd17 gene
expression-induced cell proliferation arrest in prostate cancer
cells. The results demonstrated that msdd17 gene expression and
exogenous EPA treatment significantly decreased E2F1
expression.
In conclusion, to the best of our knowledge, the
present study is the first to evaluate the function of the msdd17
gene in tumor cells in vitro. The msdd17 gene inhibited
prostate cancer cell proliferation by regulating the prostate
cancer cell cycle. Therefore, stimulating the conversion of AA into
EPA may be an effective therapeutic approach to treat prostate
cancer.
Acknowledgements
The present study was supported by grants from the
National Basic Research Program of China (973 Program; grant no.
2013CB945202), the National Natural Science Foundation of China
(grant nos. 81630021 and 81170780 to A.Z., 81372798 to F.L. and
81200570 to L.X.), the Ph.D. Programs Foundation of Ministry of
Education of China (grant no. 20113234110005), the Scientific
Support Program of Jiangsu Province (grant no. BE2012756), the
Natural Science Foundation of Jiangsu Province of China (grant nos.
BK20130059 and 2011766), the Natural Science Foundation of Nanjing
Medical University (grant no. 2016NJMUZD027 to J.P.), the Young
Medical Talents of Jiangsu Province (grant no. QNRC2016663 to
J.P.), and the High-level Innovative Talents Reward from Jiangsu
Province (to F.L.).
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Apte SA, Cavazos DA, Whelan KA and
Degraffenried LA: A low dietary ratio of omega-6 to omega-3 Fatty
acids may delay progression of prostate cancer. Nutr Cancer.
65:556–562. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rodriguez C, McCullough ML, Mondul AM,
Jacobs EJ, Chao A, Patel AV, Thun MJ and Calle EE: Meat consumption
among Black and White men and risk of prostate cancer in the cancer
prevention study II nutrition cohort. Cancer Epidemiol Biomarkers
Prev. 15:211–216. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Walker M, Aronson KJ, King W, Wilson JW,
Fan W, Heaton JP, MacNeily A, Nickel JC and Morales A: Dietary
patterns and risk of prostate cancer in Ontario, Canada. Int J
Cancer. 116:592–598. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hedelin M, Chang ET, Wiklund F, Bellocco
R, Klint A, Adolfsson J, Shahedi K, Xu J, Adami HO, Grönberg H and
Bälter KA: Association of frequent consumption of fatty fish with
prostate cancer risk is modified by COX-2 polymorphism. Int J
Cancer. 120:398–405. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Norrish AE, Skeaff CM, Arribas GL, Sharpe
SJ and Jackson RT: Prostate cancer risk and consumption of fish
oils: A dietary biomarker-based case-control study. Br J Cancer.
81:1238–1242. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Leitzmann MF, Stampfer MJ, Michaud DS,
Augustsson K, Colditz GC, Willett WC and Giovannucci EL: Dietary
intake of n-3 and n-6 fatty acids and the risk of prostate cancer.
Am J Clin Nutr. 80:204–216. 2004.PubMed/NCBI
|
|
9
|
Pham TM, Fujino Y, Kubo T, Ide R, Tokui N,
Mizoue T, Ogimoto I, Matsuda S and Yoshimura T: Fish intake and the
risk of fatal prostate cancer: Findings from a cohort study in
Japan. Public Health Nutr. 12:609–613. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Simopoulos AP: The importance of the
omega-6/omega-3 fatty acid ratio in cardiovascular disease and
other chronic diseases. Exp Biol Med (Maywood). 233:674–688. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
de Lorgeril M and Salen P: New insights
into the health effects of dietary saturated and omega-6 and
omega-3 polyunsaturated fatty acids. BMC Med. 10:502012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Berquin IM, Edwards IJ and Chen YQ:
Multi-targeted therapy of cancer by omega-3 fatty acids. Cancer
Lett. 269:363–377. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Patel MI, Kurek C and Dong Q: The
arachidonic acid pathway and its role in prostate cancer
development and progression. J Urol. 179:1668–1675. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hajjaji N and Bougnoux P: Selective
sensitization of tumors to chemotherapy by marine-derived lipids: A
review. Cancer Treat Rev. 39:473–488. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Larsson SC, Kumlin M, Ingelman-Sundberg M
and Wolk A: Dietary long-chain n-3 fatty acids for the prevention
of cancer: A review of potential mechanisms. Am J Clin Nutr.
79:935–945. 2004.PubMed/NCBI
|
|
16
|
Azrad M, Turgeon C and Demark-Wahnefried
W: Current evidence linking polyunsaturated Fatty acids with cancer
risk and progression. Front Oncol. 3:2242013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fradet V, Cheng I, Casey G and Witte JS:
Dietary omega-3 fatty acids, cyclooxygenase-2 genetic variation,
and aggressive prostate cancer risk. Clin Cancer Res. 15:2559–2566.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pelser C, Mondul AM, Hollenbeck AR and
Park Y: Dietary fat, fatty acids, and risk of prostate cancer in
the NIH-AARP diet and health study. Cancer Epidemiol Biomarkers
Prev. 22:697–707. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
McEntee MF, Ziegler C, Reel D, Tomer K,
Shoieb A, Ray M, Li X, Neilsen N, Lih FB, O'Rourke D and Whelan J:
Dietary n-3 polyunsaturated fatty acids enhance hormone ablation
therapy in androgen-dependent prostate cancer. Am J Pathol.
173:229–241. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Berquin IM, Min Y, Wu R, Wu J, Perry D,
Cline JM, Thomas MJ, Thornburg T, Kulik G, Smith A, et al:
Modulation of prostate cancer genetic risk by omega-3 and omega-6
fatty acids. J Clin Invest. 117:1866–1875. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pereira SL, Huang YS, Bobik EG, Kinney AJ,
Stecca KL, Packer JC and Mukerji P: A novel omega3-fatty acid
desaturase involved in the biosynthesis of eicosapentaenoic acid.
Biochem J. 378:665–671. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen Y, Zhang M and Gou K: SDD17
desaturase can convert arachidonic acid to eicosapentaenoic acid in
mammalian cells. Biochem Biophys Res Commun. 394:158–162. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen Z, Zhang Y, Jia C, Wang Y, Lai P,
Zhou X, Song Q, Lin J, Ren Z, Gao Q, et al: mTORC1/2 targeted by
n-3 polyunsaturated fatty acids in the prevention of mammary
tumorigenesis and tumor progression. Oncogene. 33:4548–4557. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pan J, Cheng L, Bi X, Zhang X, Liu S, Bai
X, Li F and Zhao AZ: Elevation of ω-3 polyunsaturated fatty acids
attenuates PTEN-deficiency induced endometrial cancer development
through regulation of COX-2 and PGE2 Production. Sci Rep.
5:149582015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zheng H, Tang H, Liu M, He M, Lai P, Dong
H, Lin J, Jia C, Zhong M, Dai Y, et al: Inhibition of endometrial
cancer by n-3 polyunsaturated fatty acids in preclinical models.
Cancer Prev Res (Phila). 7:824–834. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gago-Dominguez M, Yuan JM, Sun CL, Lee HP
and Yu MC: Opposing effects of dietary n-3 and n-6 fatty acids on
mammary carcinogenesis: The Singapore Chinese Health Study. Br J
Cancer. 89:1686–1692. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Arem H, Neuhouser ML, Irwin ML, Cartmel B,
Lu L, Risch H, Mayne ST and Yu H: Omega-3 and omega-6 fatty acid
intakes and endometrial cancer risk in a population-based
case-control study. Eur J Nutr. 52:1251–1260. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen HZ, Tsai SY and Leone G: Emerging
roles of E2Fs in cancer: An exit from cell cycle control. Nat Rev
Cancer. 9:785–797. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wu M, Seto E and Zhang J: E2F1 enhances
glycolysis through suppressing Sirt6 transcription in cancer cells.
Oncotarget. 6:11252–11263. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Maudet C, Mano M, Sunkavalli U, Sharan M,
Giacca M, Förstner KU and Eulalio A: Functional high-throughput
screening identifies the miR-15 microRNA family as cellular
restriction factors for Salmonella infection. Nat Commun.
5:47182014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jaganathan A, Chaurasia P, Xiao GQ,
Philizaire M, Lv X, Yao S, Burnstein KL, Liu DP, Levine AC and
Mujtaba S: Coactivator MYST1 regulates nuclear factor-κB and
androgen receptor functions during proliferation of prostate cancer
cells. Mol Endocrinol. 28:872–885. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang S, Li W, Xue Z, Lu Y, Narsinh K, Fan
W, Li X, Bu Q, Wang F, Liang J, et al: Molecular imaging of p53
signal pathway in lung cancer cell cycle arrest induced by
cisplatin. Mol Carcinog. 52:900–907. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lee KY, Lee JW, Nam HJ, Shim JH, Song Y
and Kang KW: PI3-kinase/p38 kinase-dependent E2F1 activation is
critical for Pin1 induction in tamoxifen-resistant breast cancer
cells. Mol Cells. 32:107–111. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gomez-Gutierrez JG, Garcia-Garcia A, Hao
H, Rao XM, de Montes Oca-Luna R, Zhou HS and McMasters KM:
Adenovirus-mediated expression of truncated E2F-1 suppresses tumor
growth in vitro and in vivo. Cancer. 116:4420–4423. 2010.
View Article : Google Scholar : PubMed/NCBI
|