Introduction
The global incidence and mortality rates of gastric
cancer (GC) are amongst the highest for all malignant tumor types
(1). Risk factors that may increase
an individual's chance of developing GC include Helicobacter
pylori infection, a diet high in salty/smoked food and low in
fruit/vegetables, tobacco smoking and genetic susceptibility
(2). Additionally, the incidence rate
of GC is ~2 times higher in males compared with females,
independently of known gender-specific variables (3). Therefore, it has been proposed that
steroid hormone production influences the risk of developing GC
(4,5).
Furthermore, numerous studies have suggested a protective role of
17β-estradiol (E2) in gastric carcinogenesis (6–12).
Although the majority of E2 is produced in the ovaries, it is also
synthesized locally in peripheral tissues in males and females
(13).
There are two routes involved in the local synthesis
of E2, the sulfatase and aromatase signaling pathways (13). The sulfatase signaling pathway
involves the desulfation of dehydroepiandrosterone sulfate (DHEA-S)
and estrone sulfate (E1-S) to DHEA and E1, respectively, by steroid
sulfatase (STS). Subsequently, E1 is reduced to E2 by
17β-hydroxysteroid dehydrogenases (HSD17Bs; types 1, 5 and 7). In
addition, DHEA is converted to androstenedione (adione) by
hydroxy-delta-5-steroid dehydrogenase 3 beta- and steroid
delta-isomerase 1 (HSD3B1). In the aromatase signaling pathway,
adione and testosterone are converted into E1 and E2, respectively,
by aromatase (CYP19A1) (14). In
recent studies, the mRNA and protein expression of enzymes
belonging to the HSD17B family, including HSD17B1, 2 and 5 was
demonstrated in healthy and tumoral gastric mucosa (15–17). In
addition, it was revealed that the HGC-27 and EPG 85–257 GC cell
lines were able to synthesize E2 in vitro (15).
In the present study, the mRNA levels of certain
genes that participate in the synthesis of E2 through the sulfatase
and aromatase signaling pathways, including STS, HSD3B1, CYP19A1
and HSD17B7, were investigated in primary tumoral and healthy
adjacent gastric mucosa samples from patients with GC. Furthermore,
considering the fact that the cellular functions of steroid
hormones are mediated through binding to their receptors and that
the abnormal expression of genes encoding nuclear estrogen
receptors α (ESR1) and β (ESR2), and androgen receptor (AR) have
been demonstrated in GC (18,19), the mRNA expression of coactivators and
corepressors of steroid hormone receptors were also determined in
the tissue samples. The following coactivators and corepressors
were investigated: Proline, glutamate and leucine rich protein 1
(PELP1); CREB binding protein (CREBBP); nuclear receptor
coactivator 1 (NCOA1); nuclear receptor corepressor 1 (NCOR1); and
nuclear receptor subfamily 2, group F, member 1 (NR2F1).
Additionally, the association between the mRNA expression of the
genes investigated and the clinicopathological features of patients
with GC was investigated.
Materials and methods
Patients and tissue specimens
Primary tumoral gastric mucosa specimens were
collected between December 2012 and September 2015 from 60 patients
with a mean age of 67.2 years old who underwent a total gastrectomy
at the First Department of Surgical Oncology and General Surgery at
the Greater Poland Cancer Centre or the Department of General and
Endocrine Surgery and Gastroenterological Oncology, Heliodor
Święcicki Clinical Hospital at the Poznań University of Medical
Sciences (Poznań, Poland). The clinicopathological characteristics
of the patients are presented in Table
I; however, for certain patients not all the information was
available. In addition, healthy gastric mucosa tissue samples
located ≥10 cm away from the tumoral lesions was obtained from each
patient. Specimens were snap-frozen in liquid nitrogen and stored
at −80°C until required for RNA isolation. An experienced
pathologist performed histopathological assessments of the tissue
samples (Table I). The present study
was approved by the Ethics Committee of Poznań University of
Medical Sciences. Written informed consent was obtained from all
patients.
 | Table I.Available clinicopathological
characteristics of patients with gastric cancer. |
Table I.
Available clinicopathological
characteristics of patients with gastric cancer.
| Clinicopathological
characteristic | No. of
patients |
|---|
| Gender
(male/female) | 36/24 |
| GC
localization |
|
Multisite | 31 |
|
Cardia | 10 |
|
Trunk | 6 |
|
Fundus | 1 |
| Lesser
curvature | 5 |
|
Pylorus | 3 |
| Histological
type |
|
Diffuse | 19 |
|
Intestinal | 23 |
|
Undetermined | 12 |
| Tumor stage |
| T1 | 2 |
| T2 | 9 |
| T3 | 32 |
| T4 | 15 |
| Lymph node
metastasis stage |
| N0 | 17 |
| N1 | 8 |
| N2 | 14 |
| N3 | 19 |
| Metastasis
stage |
| M0 | 45 |
| M1 | 3 |
| Histological
grading |
| G1 | 1 |
| G2 | 17 |
| G3 | 38 |
Reverse transcription-quantitative
polymerase chain reaction analysis
Total RNA from patient tissue samples was isolated
according to Chomczynski and Sacchi's single-step method (20), which involves homogenization. The
concentration and integrity of the RNA isolated was assessed using
a Nano-100 Micro Spectrophotometer (Hangzhou Allsheng Instruments
Co., Ltd., Hangzhou, China) and non-denaturing electrophoresis on a
1.5% agarose gel, respectively. RNA samples were treated with DNase
I and reverse-transcribed into cDNA using a SuperScript®
III First-Strand Synthesis system (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) according to the manufacturer's protocol, using 1
µg of RNA as a template.
The target cDNA was quantified by the relative
quantification method using a calibrator, as is described in the
Relative Quantification Manual (Roche Diagnostics GmbH, Mannheim,
Germany) (21). The calibrator was
prepared as a cDNA mix from all of the patient samples and its
successive dilutions were used to create a standard curve. qPCR
reactions were performed using a LightCycler® 480
Real-Time PCR system (Roche Diagnostics GmbH, Mannheim, Germany).
Each qPCR reaction contained 1 µl of total cDNA solution obtained
in reverse transcription, 9 µl LightCycler 480 SYBR Green I Master
mix (Roche Diagnostics GmbH) and 0.1 µM of the corresponding primer
pair (Table II). Primers for ESR1,
ESR2 and HSD17B7 were purchased from Sigma-Aldrich (Merck
Millipore, Darmstadt, Germany) and the β2-microglobulin (B2M)
primers were previously designed by Hashimoto et al
(22). The primers for CYP19A1 were
designed using the Universal ProbeLibrary website (Roche
Diagnostics GmbH). All other primers were designed using OLIGO
Primer Analysis Software (version 5.0; Molecular Biology Insights,
Inc., Colorado Springs, CO, USA). qPCR thermocycling conditions
consisted of a pre-denaturation step at 95°C for 7 min followed by
42 PCR cycles specific for each primer. Reaction conditions for
each primer pair are detailed in Table
II. The analyzed transcript levels were expressed as the ratio
between the amount of target transcript in a sample and target
transcript in the calibrator. The quantity of analyzed transcripts
in each sample was standardized by the geometric mean of B2M,
β-glucuronidase and porphobilinogen deaminase, and presented as the
decimal logarithm.
 | Table II.Primer sequences and qPCR
conditions. |
Table II.
Primer sequences and qPCR
conditions.
| Gene | Sequence
(5′-3′) | Exon number | Product size
(bp) | qPCR thermocycling
conditions (denaturation; annealing; elongation) |
|---|
| STS |
GCCAGAAGATTGATGAGCCCAC |
ENSE00001203198 | 174 | 95°C/8 sec; |
|
|
AGGCGTTGCAGTAATGGAAGAG |
ENSE00001136339 |
| 62°C/8 sec; |
|
|
|
|
| 72°C/8 sec |
| HSD3B1 |
GATGTCTTCGGTGTCACTCA |
ENSE00001722296 | 129 | 95°C/8 sec; |
|
|
GGCTACCTCTATGCTACTG |
ENSE00001846945 |
| 57°C/8 sec; |
|
|
|
|
| 72°C/8 sec |
| CYP19A1 |
CAAACCCAATGAATTTACTCT |
ENSE00003683464 | 111 | 95°C/6 sec; |
|
|
ACCATGGCGATGTACTTTCC |
ENSE00001524808 |
| 58°C/6 sec; |
|
|
|
|
| 72°C/6 sec |
| AR |
GATCCTTCACCAATGTCAACT |
ENSE00001282597 | 109 | 95°C/10 sec; |
|
|
CTCATTCGGACACACTGGCT |
ENSE00001165458 |
| 60°C/10 sec; |
|
|
|
|
| 72°C/10 sec |
| PELP1 |
GAGCATTCAGCAGGTGTTAC |
ENSE00003644127 | 132 | 95°C/10 sec; |
|
|
AGGTGGTTCATGGAGATGTC |
ENSE00003476735 |
| 60°C/10 sec; |
|
|
|
|
| 72°C/10 sec |
| CREBBP |
TCTTCCATTGCCACCCACCT |
ENSE00003665970 | 142 | 95°C/10 sec; |
|
|
CTGTCTTCAGTTGCTTGTTTG |
ENSE00003538086 |
| 60°C/10 sec; |
|
|
|
|
| 72°C/10 sec |
| NCOA1 |
GCTGGTATCCTTCCTTAGTG |
ENSE00000808889 | 136 | 95°C/10 sec; |
|
|
TGGCGTTGCTTGTTGTGGTG |
ENSE00000808890 |
| 60°C/10 sec; |
|
|
|
|
| 72°C/10 sec |
| NCOR1 |
GCGTTATGATCAGCTCATGG |
ENSE00003681554 | 202 | 95°C/10 sec; |
|
|
ACTCCTAGCAATGGTGGCTG |
ENSE00003668751 |
| 62°C/10 sec; |
|
|
|
|
| 72°C/10 sec |
| NR2F1 |
CGCATCTTCCAGGAGCAGGT |
ENSE00001249995 | 226 | 95°C/6 sec; |
|
|
GCAGTCGCAGCAGCAGTTTG |
ENSE00001250004 |
| 60°C/6 sec; |
|
|
|
|
| 72°C/6 sec |
| GUSB |
CGCCGACTTCTCTGACAAC |
ENSE00001799401 | 174 | 95°C/10 sec; |
|
|
ATCACCTCCCGTTCGTACC |
ENSE00003687473 |
| 60°C/10 sec; |
|
|
|
|
| 72°C/10 sec |
| PBGD |
GCCAAGGACCAGGACATC |
ENSE00003460195 | 160 | 95°C/10 sec; |
|
|
TCAGGTACAGTTGCCCATC |
ENSE00003609229/ |
| 60°C/10 sec; |
|
|
|
ENSE00003610664 |
| 72°C/10 sec |
| B2M |
CACCCCCACTGAAAAAGATG |
ENSE00003659794 | 106 | 95°C/10 sec; |
|
|
CCTCCATGATGCTGCTTACA |
ENSE00003459883/ |
| 60°C/10 sec; |
|
|
|
ENSE00002538889 |
| 72°C/10 sec |
Statistical analysis
Normality of data distribution was assessed using
the Shapiro-Wilk test, followed by a Student's t-test (two-tailed)
or Mann-Whitney U test to determine significant differences between
mean values. P<0.01 was considered to indicate a statistically
significant difference. STATISTICA software (version 10; StatSoft,
Inc., Tulsa, OK, USA) was used to perform all statistical
analyses.
Results
mRNA expression of STS, HSD3B1,
CYP19A1, HSD17B7, ESR1, ESR2, AR, PELP1, CREBBP, NCOA1, NCOR1 and
NR2F1 in primary tumoral and healthy gastric mucosa of patients
with GC
RT-qPCR was used to measure the mRNA expression of
genes encoding steroidogenic enzymes (STS, CYP19A1, HSD3B1,
HSD17B7), steroid hormone receptors (ESR1, ESR2, AR) and
coregulators of steroid hormone receptors (PELP1, CREBBP, NCOA1,
NCOR1, NR2F1), in primary tumoral and adjacent healthy mucosa
tissue samples from 60 patients with GC (Fig. 1). Expression of STS and HSD17B7 mRNA
was detected in all tissue samples examined, whereas expression of
HSD3B1 mRNA was absent in one healthy tissue sample and CYP19A1 was
not detected in seven healthy specimens (Fig. 1A). Furthermore, five cancerous tissue
samples demonstrated no expression of HSD3B1 mRNA. There was no
difference in the mRNA level of HSD17B7 in primary tumoral and
adjacent healthy mucosa (P=0.2; Fig.
1A). However, significantly lower mRNA levels of STS
(P<0.00001) and HSD3B1 (P=0.0051) were identified in tumoral
tissue samples compared with healthy tissue samples. Tumoral tissue
samples revealed a significantly increased expression of CYP19A1
mRNA compared with control samples (P<0.00001; Fig. 1A). However, despite the significant
differences observed, HSD3B1 and CYP19A1 mRNA levels were
maintained at low levels in primary tumoral and healthy gastric
mucosa compared with STS and HSD17B7 mRNA (data not shown).
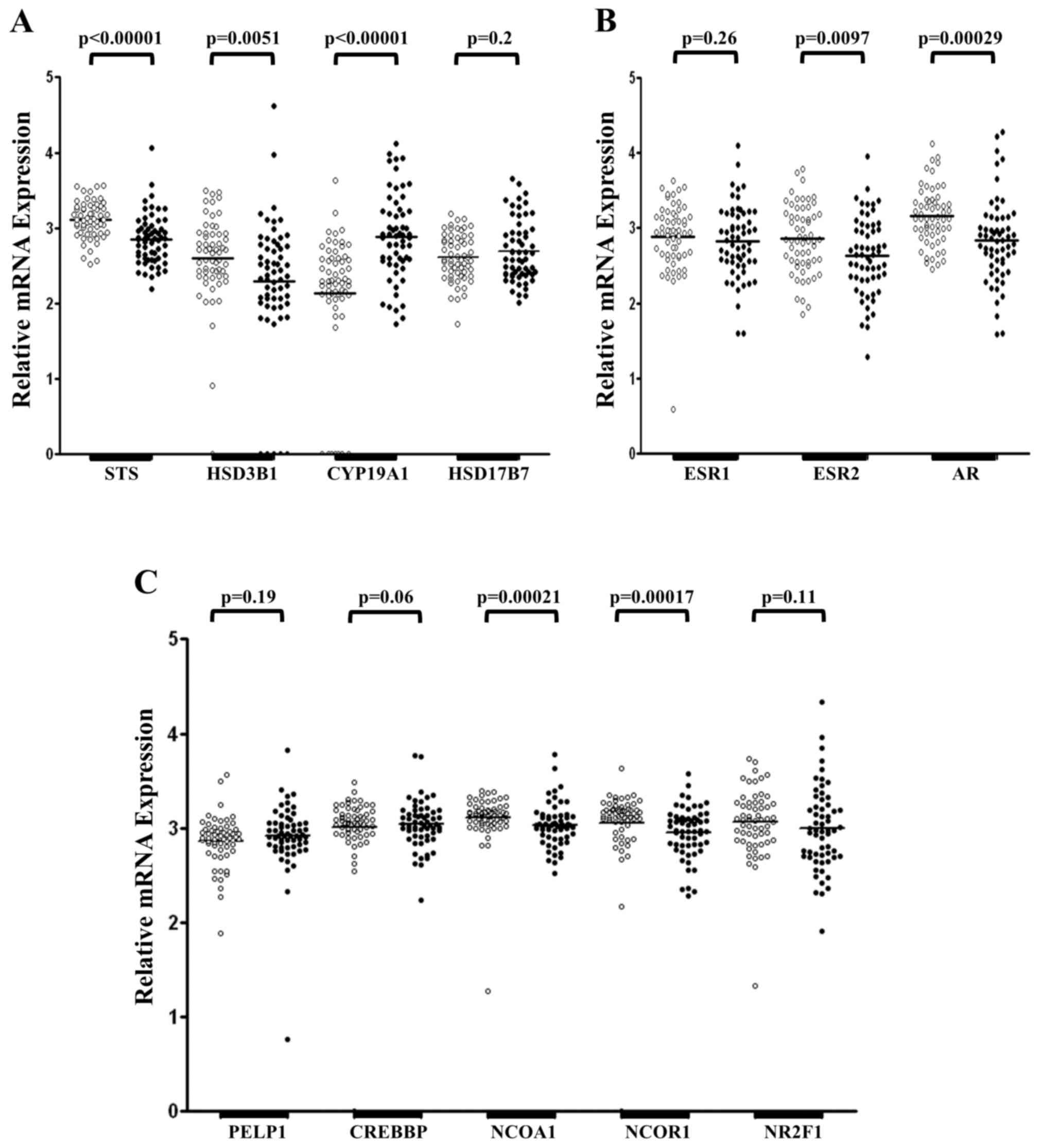 | Figure 1.Relative expression of STS, HSD3B1,
CYP19A1, HSD17B7, ESR1, ESR2, AR, PELP1, CREBBP, NCOA1, NCOR1 and
NR2F1 mRNA in healthy and primary tumoral gastric tissue samples.
(A) Expression of genes encoding steroidogenic enzymes. (B)
Expression levels of genes encoding steroid hormone receptors. (C)
Expression of genes encoding coregulators of steroid hormone
receptors. White dots represent healthy tissue and black dots
represent primary tumoral tissue from patients with gastric cancer,
which were analyzed through reverse transcription-quantitative
polymerase chain reaction analysis and normalized to expression
levels of β2-microglobulin, β-glucuronidase and porphobilinogen
deaminase. The quantity of analyzed genes is presented as the
decimal logarithm that represents the ratio between the amount of
target gene in a sample and the target gene in the calibrator. STS,
steroid sulfatase; HSD3B1, hydroxy-delta-5-steroid dehydrogenase 3
beta- and steroid delta-isomerase 1; CYP19A1, aromatase; HSD17B7,
17β-hydroxysteroid dehydrogenase type 7; ESR1, estrogen receptor α;
ESR2, estrogen receptor β; AR, androgen receptor; PELP1, proline,
glutamate and leucine rich protein 1; CREBBP, CREB binding protein;
NCOA1, nuclear receptor coactivator 1; NCOR1, nuclear receptor
corepressor 1; NR2F1, nuclear receptor subfamily 2, group F, member
1. |
mRNA of the investigated steroid hormone receptors
(Fig. 1B) and their coregulators
(Fig. 1C) was detected in all 60
pairs of gastric tumor and adjacent healthy control samples. No
significant difference was observed in the expression of ESR1 mRNA
between primary tumoral and adjacent healthy mucosa (P=0.26;
Fig. 1B). Furthermore, significantly
decreased levels of ESR2 (P=0.0097) and AR (P=0.00029) mRNA were
detected in GC specimens compared with adjacent healthy controls
(Fig. 1B). Amongst the steroid
hormone receptors investigated, the expression levels of ESR2 and
AR mRNA were the highest, with expression of ESR1 mRNA low in
tumoral and adjacent healthy gastric tissue samples (data not
shown). Amongst the coregulators of steroid hormones receptors
examined, the expression of NCOA1 (P=0.00021) and NCOR1 (P=0.00017)
mRNA was significantly reduced in tumoral mucosa compared with
adjacent healthy mucosa (Fig. 1C). In
addition, no significant differences were observed in the
expression of PELP1 (P=0.19), CREBBP (P=0.06) and NR2F1 (P=0.11)
mRNA between tumoral and healthy tissue samples (Fig. 1C).
Analysis of the clinicopathological
characteristics of patients with GC and mRNA levels of STS, HSD3B1,
CYP19, ESR2, AR, NCOA1 and NCOR1
Decreased expression of STS, HSD3B1, ESR2, AR, NCOA1
and NCOR1 mRNA, and increased expression of CYP19A1 mRNA in tumoral
tissue samples compared with healthy controls, were associated with
certain clinicopathological features of patients with GC (Table III). Expression of STS
(P<0.00001), ESR2 (P=0.0024), AR (P=0.0001), NCOA1 (P=0.00028)
and NCOR1 (P=0.00067) mRNA were significantly lower in tumoral
tissue samples compared with the control in patients >60 years.
Furthermore, males had significantly lower levels of AR (P=0.0018),
NCOA1 (P=0.00051) and NCOR1 (P=0.000095) mRNA in tumoral tissue
samples compared with control tissue samples, whereas in females
the mRNA level of HSD3B1 was significantly lower in tumoral
compared with control tissue samples (P=0.004).
 | Table III.Association between the expression of
STS, HSD3B1, CYP19, ESR2, AR, NCOA1 and NCOR1 mRNA in tumoral and
healthy gastric tissue samples and the clinicopathological
characteristics of patients with GC. |
Table III.
Association between the expression of
STS, HSD3B1, CYP19, ESR2, AR, NCOA1 and NCOR1 mRNA in tumoral and
healthy gastric tissue samples and the clinicopathological
characteristics of patients with GC.
|
| Gene analyzed
(P-value, GC vs. control tissue) |
|---|
|
|
|
|---|
| Clinicopathological
characteristic | ↓STSa |
↓HSD3B1b |
↑CYP19A1b | ↓ESR2a | ↓ARa | ↓NCOA1b | ↓NCOR1b |
|---|
| All patients | <0.00001 | 0.0051 | <0.00001 | 0.0097 | 0.00029 | 0.00021 | 0.00017 |
| Age (years
old) |
|
≤60 | 0.9 | 0.2 | 0.00074 | 0.89 | 0.35 | 0.27 | 0.22 |
|
>60 | <0.00001 | 0.02 | 0.000012 | 0.0024 | 0.0001 | 0.00028 | 0.00067 |
| Gender |
|
Male | 0.0014 | 0.28 | 0.000072 | 0.08 | 0.0018 | 0.00051 | 0.000095 |
|
Female | 0.000069 | 0.004 | 0.00023 | 0.056 | 0.04 | 0.11 | 0.48 |
| GC
localization |
|
Multisite | 0.013 | 0.0034 | 0.00001 | 0.04 | 0.026 | 0.12 | 0.11 |
|
Cardia | 0.0012 | 0.57 | 0.054 | 0.55 | 0.0012 | 0.001 | 0.0073 |
|
Body | – | – | – | – | – | – | – |
|
Fundus | – | – | – | – | – | – | – |
| Lesser
curvature | – | – | – | – | – | – | – |
|
Pylorus | – | – | – | – | – | – | – |
| Histological
type |
|
Diffuse | 0.034 | 0.034 | 0.0018 | 0.73 | 0.69 | 0.36 | 0.038 |
|
Intestinal | 0.037 | 0.022 | 0.0074 | 0.022 | 0.000046 | 0.0007 | 0.019 |
|
Indeterminate | 0.0032 | 0.14 | 0.0009 | 0.047 | 0.47 | 0.71 | 0.98 |
| Tumor stage |
| T1 | – | – | – | – | – | – | – |
| T2 | 0.57 | 0.96 | 0.11 | 0.79 | 0.19 | 0.093 | 0.077 |
| T3 | 0.00018 | 0.0062 | 0.00001 | 0.022 | 0.027 | 0.016 | 0.038 |
| T4 | 0.014 | 0.11 | 0.089 | 0.14 | 0.0086 | 0.046 | 0.074 |
| Lymph node
metastasis stage |
| N0 | 0.17 | 0.11 | 0.0024 | 0.29 | 0.16 | 0.039 | 0.027 |
| N1 | 0.18 | 0.27 | 0.052 | 0.76 | 0.035 | 0.014 | 0.024 |
| N2 | 0.019 | 0.49 | 0.041 | 0.22 | 0.38 | 0.48 | 0.53 |
| N3 | 0.000089 | 0.06 | 0.001 | 0.045 | 0.048 | 0.062 | 0.21 |
| Metastasis
stage |
| M0 | 0.0021 | 0.003 | <0.00001 | 0.014 | 0.00064 | 0.0028 | 0.0018 |
| M1 | – | – | – | – | – | – | – |
| Histological
grading |
| G1 | – | – | – | – | – | – | – |
| G2 | 0.07 | 0.07 | 0.036 | 0.24 | 0.00062 | 0.0027 | 0.042 |
| G3 | 0.000054 | 0.0012 | <0.00001 | 0.027 | 0.044 | 0.021 | 0.0049 |
The multisite localization of GC was demonstrated to
be associated with a significantly lower level of HSD3B1 mRNA
(P=0.0034) and tumors located in the cardia region had
significantly lower STS (P=0.0012), AR (P=0.0012), NCOA1 (P=0.001)
and NCOR1 (P=0.0073) mRNA, compared with healthy mucosa. However,
only 10 patients with tumor localization in the cardia region were
included in the analysis. Patients with the intestinal type of GC
had significantly lower mRNA levels of AR (P=0.000046) and NCOA1
(P=0.0007) in tumoral tissue samples compared with the control
mucosa. In addition, significantly lower levels of STS mRNA were
identified in tumoral tissue compared with adjacent healthy tissue
in patients with indeterminate GC (P=0.0032). Additionally,
cancerous tissue samples of a T3 grade expressed significantly
lower levels of STS (P=0.00018) and HSD3B1 (P=0.0062) mRNA compared
with healthy tissue samples. A significantly lower level of AR mRNA
was also identified in tumoral tissue graded as T4 compared with
the control (P=0.0086). Expression of STS mRNA was significantly
decreased in the tumoral tissue samples of patients with N3 lymph
node metastases compared with the controls samples
(P=0.000089).
The majority of patients were diagnosed with an M0
metastasis stage, thus no associations between the mRNA levels of
the analyzed genes and metastasis grade were investigated.
Significantly lower levels of AR (P=0.00062) and NCOA1 (P=0.0027)
mRNA were identified in the tumoral tissue of patients with G2
stage GC compared with adjacent healthy tissue. In addition, STS
(P=0.000054) and HSD3B1 (P=0.0012) mRNA expression was
significantly lower in G3 histological grade tumors compared with
healthy tissue samples. However, the expression of CYP19A1 mRNA was
significantly increased in tumoral tissue samples compared with the
control in patients with multisite localization of GC (P=0.00001),
a T3 tumor stage (P=0.00001), N0 (P=0.0024) or N3 (P=0.001) lymph
node metastasis grades and G3 histological grade tumors
(P<0.00001). The expression of CYP19A1 mRNA in primary tumoral
tissue samples compared with healthy adjacent mucosa samples was
significantly increased in all patients studied, regardless of age,
gender, and histological type and localization of the tumor.
Discussion
In the present study, the mRNA levels of specific
genes involved in the synthesis of E2, in addition to genes
encoding steroid hormone receptors and their coregulators, were
investigated in primary tumoral and adjacent healthy gastric mucosa
samples obtained from patients with GC. The presence of mRNA was
detected for all genes analyzed in the majority of gastric
specimens examined. Furthermore, it was identified that the
expression of STS, HSD3B1, ESR2, AR, NCOA1 and NCOR1 mRNA was
significantly decreased, whereas, the level of CYP19A1 mRNA was
significantly increased, in cancerous gastric tissue compared with
the control samples. To the best of our knowledge, no previous
studies have investigated the expression of STS, HSD3B1 and NCOA1
mRNA in patients with GC, although their association with other
tumor types has been examined.
Previous studies have identified lower levels of STS
mRNA in primary colorectal adenocarcinoma compared with adjacent
healthy colon mucosa (23). This is
consistent with the results of the present study, which identified
significantly decreased levels of STS mRNA in tumoral compared with
healthy gastric tissue. As E1 and DHEA are typically found in their
inactive sulfated form in the blood and have to undergo
STS-mediated activation prior to entering the steroidogenesis
pathway (24,25), downregulation of STS in a particular
tissue may suppress the synthesis of estrogens and androgens
(26). By contrast, higher levels of
E2 have been identified in cancerous tissue compared with healthy
tissue in patients with breast cancer (26,27). In
addition, numerous studies have reported an increase in the
activity and expression of STS in breast cancer (28–33).
Therefore, it has been suggested that upregulation of STS may be
associated with higher concentrations of intratumoral E2 (28,30,32).
Desulfated DHEA that is converted into adione,
typically by HSD3B1, may serve as a substrate of E2 synthesis
through the aromatase signaling pathway (25). However, low expression of HSD3B1 mRNA
was observed in tumoral and healthy gastric tissue samples, with
expression lower in the tumoral mucosa compared with the adjacent
healthy tissue. Conversely to the results of the present study,
increased expression of HSD3B1 mRNA has been identified in benign
prostatic hyperplasia compared with healthy adjacent prostate
tissue samples (34), in addition to
castration-resistant metastases compared with primary prostate
tumors (35). HSD3B1 activity is
essential for the production of adione, which is subsequently used
as a substrate for the synthesis of testosterone, an important
hormone that regulates the proliferation of prostate cells
(36). Furthermore, adione and
testosterone can be directly converted to E1 and E2, respectively,
by CYP19A1 (25). In the current
study, expression of CYP19A1 mRNA was demonstrated in the majority
of healthy gastric tissue samples and all tumoral gastric tissue
samples. A previous study identified the presence of CYP19A1 in
23/30 GC tissue samples; however, all healthy gastric mucosa
specimens tested negative for this enzyme (37). The presence of CYP19A1 mRNA and
protein has been revealed in healthy and tumoral gastric tissue
samples, although no significant difference in the level of CYP19A1
mRNA was demonstrated between healthy and tumoral gastric tissue
samples (38). These findings oppose
the results of the present study; however, this may have been due
to the fact that the previous study analyzed a total of five cases
(38).
Numerous animal studies have demonstrated that
parietal cells are capable of converting circulating androgens into
estrogens, whilst simultaneously expressing CYP19A1 (39–44).
Furthermore, the synthesis of E2 through the aromatization of
exogenous testosterone has been demonstrated in various GC cell
lines (38). Considering these
findings and the numerous evidence for the protective role of E2
against GC (6–12), the increased level of CYP19A1 mRNA in
tumoral tissue samples compared with the control group observed in
the present study is difficult to explain. However, it was observed
that the expression of CYP19A1 mRNA in cancerous and healthy
tissues was maintained at a low level, indicating that the role
CYP19A1 serves in estrogen synthesis in gastric tissue may be
limited. Additionally, it has been demonstrated that E2 may inhibit
CYP19A1 and STS activity in breast cancer cells (45,46),
suggesting that the increased mRNA expression of CYP19A1 in GC
could be due to lower intracellular concentrations of E2.
Cellular responses to steroid hormones are
facilitated by steroid hormones binding to their receptors, such as
ESR1, ESR2 and AR (47). Studies that
investigated the association between these receptors and GC have
produced inconsistent results (18,19,48). ESR1
has been suggested to mediate the cancer-promoting effects of E2 in
breast (49,50), colon (51), prostate (52) and gastric (53–57) cells,
whereas binding of E2 to ESR2 could inhibit cell proliferation in
tumors of these tissues (51,53,55,57–61).
In the current study, no significant difference in the expression
of ESR1 mRNA was identified between healthy and tumoral gastric
tissue samples; however, the expression of ESR2 mRNA was
significantly lower in cancerous mucosa compared with the control.
Thus, the results of the present study support the hypothesis that
reduced ESR2 expression is associated with the development of GC.
Conversely, Matsuyama et al (62) suggested that the role served by ESR2
in GC may differ depending on the subtype. Furthermore, Guo et
al (63) demonstrated that
certain splicing variants of ESR2 mRNA (ESR2-1, −2 and −5) are
differentially expressed in GC and healthy tissue samples.
Additionally, higher levels of ESR2-5 mRNA were detected in GC
compared with healthy tissue samples, and were associated with
tumor-node-metastasis (TNM) staging, while decreased mRNA levels of
ESR2-1 in GC did not correlate with any clinicopathological
characteristics. In the current study, patients who were >60
years old had significantly lower levels of ESR2 mRNA in tumoral
compared with healthy gastric tissue samples; however, all ESR2
splicing variants were analyzed simultaneously. A previous study
suggested that ESR1 may exhibit antiproliferative activity, and
reduce the motility and invasion of GC cells (64). Furthermore, ESR1 has been associated
with an early TNM stage in GC (18).
Thus, further studies investigating the role of ESRs in the
etiology of GC are warranted.
In addition to investigating ESRs, the expression of
AR mRNA was investigated in the current study. Previous studies
have proposed that AR expression is an unfavorable factor in GC
(19,65,66). In
the present study, it was demonstrated that expression of AR mRNA
was significantly decreased in GC tissue compared with controls.
Similarly, decreased mRNA expression of AR in GC tissue samples has
been reported by Gan et al (18). Low expression of AR and ESR1 mRNA was
observed in cancerous and wild-type gastric mucosa, whereas the
ESR2 mRNA was predominantly expressed in the both of these tissues.
However, the expression of AR and ESR2 mRNA in GC tissue samples
and matched controls was the highest amongst the analyzed steroid
hormone receptors in the present study. The STS substrates E1-S and
DHEA-S have been demonstrated to induce transactivation of ESRs and
AR in a concentration-dependent manner in the MVLN invasive ductal
carcinoma and CHO-K1 ovarian cell lines, respectively (67). The results of the current study
suggest that DHEA-S is hydrolyzed by STS prior to AR activation,
whereas E1-S may be active prior to STS-mediated hydrolysis. Thus,
the decreased expression of AR mRNA in GC may have been due to
reduced androgen synthesis caused by STS downregulation. Notably,
decreased expression of HSD3B1 mRNA, which is essential for
androgen synthesis from DHEA, was detected in the present
study.
The activity of nuclear steroid hormone receptors is
regulated by various coactivators and corepressors (68). In the current study, NCOR1 and NCOA1
were demonstrated to be downregulated in GC tissue samples compared
with healthy controls. Furthermore, mRNA and protein expression of
NCOR1 has been identified to be downregulated in gastrointestinal
stromal tumors, where it has been proposed to serve as a tumor
suppressor through the SMAD signaling pathway (69). In contrast to these findings, another
study identified increased mRNA expression of NCOR1 in malignant
endometrial tissue samples compared with healthy tissue samples
(70). Furthermore, high mRNA
expression of NCOR1 has been associated with the improved prognosis
of patients with breast cancer (71),
whereas a loss of nuclear NCOR1 has been revealed to cause
increased expression of cancer-associated genes and be
significantly associated with the progression of invasive malignant
melanoma (72). Upregulation of NCOR1
mRNA and protein expression, induced by progestins, has been
associated with the suppression of estrogen-induced growth in T47D
breast cancer cells (73).
In the present study, it was demonstrated that
expression of NCOA1 mRNA was reduced in tumoral compared with
healthy gastric tissue samples. Similarly, downregulation of NCOA1
mRNA has been identified in cancerous bladder urothelium samples
(74). Additionally, high mRNA levels
of NCOA1 have been observed in healthy breast tissue samples,
intermediate levels in tumoral tissue samples and low levels in
breast cancer cell lines (75).
However, numerous studies have identified an association between
increased expression of NCOA1 and enhanced angiogenesis, cell
proliferation and survival, disease recurrence, higher tumor grade
and poor prognosis in breast cancer (76–81).
Notably, upregulation of NCOA1 has been observed in patients with
breast cancer treated with aromatase inhibitors (82). Thus, the decreased levels of NCOA1
mRNA in GC tissue identified in the present study may have been due
to upregulation of CYP19A1 mRNA in tumoral gastric mucosa. Tai
et al (83) demonstrated that
overexpression of NCOA1 enhanced the E2-induced growth of MCF-7
breast cancer cells. Increased expression of NCOA1 has also been
associated with the DHEA-mediated activation of AR in prostate
cancer (84). Therefore, the
decreased expression of AR and NCOA1 mRNA that were observed in
cancerous gastric tissue samples in the present study may have been
the result of limited desulfation of DHEA-S due to the
downregulation of STS.
In conclusion, the presence of STS, HSD3B1, CYP19A1,
HSD17B7, ESR1, ESR2, AR, PELP1, CREBBP, NCOA1, NCOR1 and NR2F1 mRNA
was demonstrated in the majority of tumoral and adjacent healthy
gastric mucosa tissue samples. Furthermore, significantly decreased
mRNA levels of STS and HSD3B1, in addition to significantly
increased expression levels of CYP19A1 mRNA, in tumoral gastric
tissue samples compared with matched controls were identified.
However, compared with STS, the expression of HSD3B1 and CYP19A1
mRNA was low in the majority of the examined tissue samples,
indicating that their role in gastric carcinogenesis is limited.
Additionally, tumoral gastric tissue samples exhibited decreased
levels of NCOA1 and NCOR1 mRNA compared with adjacent healthy
controls, suggesting that deregulated expression of these
coregulators may serve a role in gastric carcinogenesis. A
limitation of the current study was the use of homogenized tissue
samples, and thus the possibility that the samples included
non-cancerous cells, such as fibroblasts, endothelial cells, smooth
muscle cells or blood cells, could not be excluded. Furthermore,
mRNA abundance does not always correlate with protein expression.
Therefore, further studies on the expression levels of the genes
involved in the steroidogenesis pathway and their role in gastric
carcinogenesis are warranted.
Acknowledgements
The present study was supported by the Poznań
University of Medical Sciences (grant nos. 502-14-01124182-09811
and 502-01-01118170-05274).
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Karimi P, Islami F, Anandasabapathy S,
Freedman ND and Kamangar F: Gastric cancer: Descriptive
epidemiology, risk factors, screening and prevention. Cancer
Epidemiol Biomarkers Prev. 23:700–713. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sipponen P and Correa P: Delayed rise in
incidence of gastric cancer in females results in unique sex ratio
(M/F) pattern: Etiologic hypothesis. Gastric Cancer. 5:213–219.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hogan AM, Collins D, Baird AW and Winter
DC: Estrogen and gastrointestinal malignancy. Mol Cell Endocrinol.
307:19–24. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Camargo MC, Goto Y, Zabaleta J, Morgan DR,
Correa P and Rabkin CS: Sex hormones, hormonal interventions, and
gastric cancer risk: A meta-analysis. Cancer Epidemiol Biomarkers
Prev. 21:20–38. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lindblad M, Ye W, Rubio C and Lagergren J:
Estrogen and risk of gastric cancer: A protective effect in a
nationwide cohort study of patients with prostate cancer in Sweden.
Cancer Epidemiol Biomarkers Prev. 13:2203–2207. 2004.PubMed/NCBI
|
|
7
|
Nylander-Koski O, Kiviluoto T,
Puolakkainen P, Kivilaakso E and Mustonen H: The effect of nitric
oxide, growth factors, and estrogen on gastric cell migration. J
Surg Res. 143:230–237. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ohtani M, García A, Rogers AB, Ge Z,
Taylor NS, Xu S, Watanabe K, Marini RP, Whary MT, Wang TC and Fox
JG: Protective role of 17 beta-estradiol against the development of
Helicobacter pylori-induced gastric cancer in INS-GAS mice.
Carcinogenesis. 28:2597–2604. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chandanos E and Lagergren J: Oestrogen and
the enigmatic male predominance of gastric cancer. Eur J Cancer.
44:2397–2403. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Duell EJ, Travier N, Lujan-Barroso L,
Boutron-Ruault MC, Clavel-Chapelon F, Palli D, Krogh V, Mattiello
A, Tumino R, Sacerdote C, et al: Menstrual and reproductive
factors, exogenous hormone, use and gastric cancer risk in a cohort
of women from the European Prospective Investigation Into Cancer
and Nutrition. Am J Epidemiol. 172:1384–1393. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ohtani M, Ge Z, García A, Rogers AB,
Muthupalani S, Taylor NS, Xu S, Watanabe K, Feng Y, Marini RP, et
al: 17 β-estradiol suppresses Helicobacter pylori-induced
gastric pathology in male hypergastrinemic INS-GAS mice.
Carcinogenesis. 32:1244–1250. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sheh A, Ge Z, Parry NM, Muthupalani S,
Rager JE, Raczynski AR, Mobley MW, McCabe AF, Fry RC, Wang TC and
Fox JG: 17β-estradiol and tamoxifen prevent gastric cancer by
modulating leukocyte recruitment and oncogenic pathways in
Helicobacter pylori-infected INS-GAS male mice. Cancer Prev
Res (Phila). 4:1426–1435. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Simpson ER: Sources of estrogen and their
importance. J Steroid Biochem Mol Biol. 86:225–230. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Labrie F: All sex steroids are made
intracellularly in peripheral tissue by the mechanisms of
intracrinology after menopause. J Steroid Biochem Mol Biol.
145:133–138. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Frycz BA, Murawa D, Wysocki-Borejsza M,
Marciniak R, Murawa P, Drews M and Jagodziński PP: Expression of
17β-hydroxysteroid dehydrogenase type 1 in gastric cancer. Biomed
Pharmacother. 67:651–657. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Frycz BA, Murawa D, Borejsza-Wysocki M,
Marciniak R, Murawa P, Drews M and Jagodziński PP: Expression of
17β-hydroxysteroid dehydrogenase type 2 is associated with some
clinicopathological features in gastric cancer. Biomed
Pharmacother. 70:24–27. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Frycz BA, Murawa D, Borejsza-Wysocki M,
Wichtowski M, Spychała A, Marciniak R, Murawa P, Drews M and
Jagodziński PP: Transcript level of AKR1C3 is down-regulated in
gastric cancer. Biochem Cell Biol. 94:138–146. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gan L, He J, Zhang X, Zhang YJ, Yu GZ,
Chen Y, Pan J, Wang JJ and Wang X: Expression profile and
prognostic role of sex hormone receptors in gastric cancer. BMC
Cancer. 12:5662012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tian Y, Wan H, Lin Y, Xie X, Li Z and Tan
G: Androgen receptor may be responsible for gender disparity in
gastric cancer. Med Hypotheses. 80:672–674. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chomczynski P and Sacchi N: The
single-step method of RNA isolation by acid guanidinium
thiocyanate-phenol-chloroform extraction: Twenty-something years
on. Nat Protoc. 1:581–585. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tellmann G: The E-Method: A highly
accurate technique for gene-expression analysis. Nature methods.
3:i–ii. 2006. View
Article : Google Scholar
|
|
22
|
Hashimoto T, Bazmi HH, Mirnics K, Wu Q,
Sampson AR and Lewis DA: Conserved regional patterns of
GABA-related transcript expression in the neocortex of subjects
with schizophrenia. Am J Psychiatry. 165:479–489. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rawłuszko AA, Antoniucci M, Horbacka K,
Lianeri M, Krokowicz P and Jagodziński PP: Reduced expression of
steroid sulfatase in primary colorectal cancer. Biomed
Pharmacother. 67:577–582. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ruder HJ, Loriaux L and Lipsett MB:
Estrone sulfate: Production rate and metabolism in man. J Clin
Invest. 51:1020–1033. 1972. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Labrie F: Extragonadal synthesis of sex
steroids: Intracrinology. Ann Endocrinol (Paris). 64:95–107.
2003.PubMed/NCBI
|
|
26
|
Secky L, Svoboda M, Klameth L, Bajna E,
Hamilton G, Zeillinger R, Jäger W and Thalhammer T: The sulfatase
pathway for estrogen formation: Targets for the treatment and
diagnosis of hormone-associated tumors. J Drug Deliv.
2013:9576052013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chetrite GS, Cortes-Prieto J, Philippe JC,
Wright F and Pasqualini JR: Comparison of estrogen concentrations,
estrone sulfatase and aromatase activities in normal, and in
cancerous, human breast tissue samples. J Steroid Biochem Mol Biol.
72:23–27. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Utsumi T, Yoshimura N, Takeuchi S, Maruta
M, Maeda K and Harada N: Elevated steroid sulfatase expression in
breast cancers. J Steroid Biochem Mol Biol. 73:141–145. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Miyoshi Y, Ando A, Hasegawa S, Ishitobi M,
Taguchi T, Tamaki Y and Noguchi S: High expression of steroid
sulfatase mRNA predicts poor prognosis in patients with estrogen
receptor-positive breast cancer. Clin Cancer Res. 9:2288–2293.
2003.PubMed/NCBI
|
|
30
|
Suzuki T, Miki Y, Nakata T, Shiotsu Y,
Akinaga S, Inoue K, Ishida T, Kimura M, Moriya T and Sasano H:
Steroid sulfatase and estrogen sulfotransferase in normal human
tissue and breast carcinoma. J Steroid Biochem Mol Biol.
86:449–454. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Poisson Paré D, Song D, Luu-The V, Han B,
Li S, Liu G, Labrie F and Pelletier G: Expression of estrogen
sulfotransferase 1E1 and steroid sulfatase in breast cancer: A
immunohistochemical study. Breast Cancer (Auckl). 3:9–21.
2009.PubMed/NCBI
|
|
32
|
Suzuki M, Ishida H, Shiotsu Y, Nakata T,
Akinaga S, Takashima S, Utsumi T, Saeki T and Harada N: Expression
level of enzymes related to in situ estrogen synthesis and
clinicopathological parameters in breast cancer patients. J Steroid
Biochem Mol Biol. 113:195–201. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hanamura T, Niwa T, Gohno T, Kurosumi M,
Takei H, Yamaguchi Y, Ito K and Hayashi S: Possible role of the
aromatase-independent steroid metabolism pathways in hormone
responsive primary breast cancers. Breast Cancer Res Treat.
143:69–80. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Khvostova EP, Otpuschennikov AA,
Pustylnyak VO and Gulyaeva LF: Gene expression of androgen
metabolising enzymes in benign and malignant prostatic tissues.
Horm Metab Res. 47:119–124. 2015.PubMed/NCBI
|
|
35
|
Montgomery RB, Mostaghel EA, Vessella R,
Hess DL, Kalhorn TF, Higano CS, True LD and Nelson PS: Maintenance
of intratumoral androgens in metastatic prostate cancer: A
mechanism for castration-resistant tumor growth. Cancer Res.
68:4447–4454. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chang KH, Ercole CE and Sharifi N:
Androgen metabolism in prostate cancer: From molecular mechanisms
to clinical consequences. Br J Cancer. 111:1249–1254. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Saitoh Y, Sasano H, Naganuma H, Ohtani H,
Sasano N, Ohuchi A and Matsuno S: De novo expression of aromatase
in gastric carcinoma. Light and electron microscopic
immunohistochemical and immunoblot study. Pathol Res Pract.
188:53–60. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Izawa M, Inoue M, Osaki M, Ito H, Harada
T, Terakawa N and Ikeguchi M: Cytochrome P450 aromatase gene
(CYP19) expression in gastric cancer. Gastric Cancer. 11:103–110.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ueyama T, Shirasawa N, Numazawa M, Yamada
K, Shelangouski M, Ito T and Tsuruo Y: Gastric parietal cells:
Potent endocrine role in secreting estrogen as a possible regulator
of gastro-hepatic axis. Endocrinology. 143:3162–3170. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ueyama T, Shirasawa N, Ito T and Tsuruo Y:
Estrogen-producing steroidogenic pathways in parietal cells of the
rat gastric mucosa. Life Sci. 74:2327–2337. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kobayashi H, Yoshida S, Sun YJ, Shirasawa
N and Naito A: 17β-Estradiol in the systemic circulation derives
mainly from the parietal cells in cholestatic female rats. J
Endocrinol Invest. 39:389–400. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ozawa M, Takahashi K, Akazawa KH,
Takashima T, Nagata H, Doi H, Hosoya T, Wada Y, Cui Y, Kataoka Y
and Watanabe Y: PET of aromatase in gastric parietal cells using
11C-vorozole. J Nucl Med. 52:1964–1969. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kobayashi H, Yoshida S, Sun YJ, Shirasawa
N and Naito A: Postnatal development of gastric aromatase and
portal venous estradiol-17β levels in male rats. J Endocrinol.
218:117–124. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kobayashi H, Yoshida S, Sun YJ, Shirasawa
N and Naito A: Gastric estradiol-17β (E2) and liver ERα correlate
with serum E2 in the cholestatic male rat. J Endocrinol. 219:39–49.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Pasqualini JR and Chetrite G: Paradoxical
effect of estradiol: It can block its own bioformation in human
breast cancer cells. J Steroid Biochem Mol Biol. 78:21–24. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Pasqualini JR and Chetrite GS: Estradiol
as an anti-aromatase agent in human breast cancer cells. J Steroid
Biochem Mol Biol. 98:12–17. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Levin ER: Extranuclear steroid receptors
are essential for steroid hormone actions. Annu Rev Med.
66:271–280. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Rahman Ur MS and Cao J: Estrogen receptors
in gastric cancer: Advances and perspectives. World J
Gastroenterol. 22:2475–2482. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Hayashi SI, Eguchi H, Tanimoto K, Yoshida
T, Omoto Y, Inoue A, Yoshida N and Yamaguchi Y: The expression and
function of estrogen receptor alpha and beta in human breast cancer
and its clinical application. Endocr Relat Cancer. 10:193–202.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Shanle EK and Xu W: Selectively targeting
estrogen receptors for cancer treatment. Adv Drug Deliv Rev.
62:1265–1276. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Xie LQ, Yu JP and Luo HS: Expression of
estrogen receptor beta in human colorectal cancer. World J
Gastroenterol. 10:214–217. 2004.PubMed/NCBI
|
|
52
|
Risbridger GP, Davis ID, Birrell SN and
Tilley WD: Breast and prostate cancer: More similar than different.
Nat Rev Cancer. 10:205–212. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Chandanos E, Rubio CA, Lindblad M, Jia C,
Tsolakis AV, Warner M, Gustafsson JA and Lagergren J: Endogenous
estrogen exposure in relation to distribution of histological type
and estrogen receptors in gastric adenocarcinoma. Gastric Cancer.
11:168–174. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Kameda C, Nakamura M, Tanaka H, Yamasaki
A, Kubo M, Tanaka M, Onishi H and Katano M: Oestrogen
receptor-alpha contributes to the regulation of the hedgehog
signalling pathway in ERalpha-positive gastric cancer. Br J Cancer.
102:738–747. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Xu CY, Guo JL, Jiang ZN, Xie SD, Shen JG,
Shen JY and Wang LB: Prognostic role of estrogen receptor alpha and
estrogen receptor beta in gastric cancer. Ann Surg Oncol.
17:2503–2509. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Deng H, Huang X, Fan J, Wang L, Xia Q,
Yang X, Wang Z and Liu L: A variant of estrogen receptor-alpha,
ER-alpha36 is expressed in human gastric cancer and is highly
correlated with lymph node metastasis. Oncol Rep. 24:171–176.
2010.PubMed/NCBI
|
|
57
|
Liu S, He L, Wang Z and Wen G: Expression
of sex hormone receptor in gastric cancer with synchronous ovarian
metastasis and its significance. Zhonghua Yi Xue Za Zhi.
94:1861–1865. 2014.(In Chinese). PubMed/NCBI
|
|
58
|
Järvinen TA, Pelto-Huikko M, Holli K and
Isola J: Estrogen receptor beta is coexpressed with ERalpha and PR
and associated with nodal status, grade, and proliferation rate in
breast cancer. Am J Pathol. 156:29–35. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Zhu X, Leav I, Leung YK, Wu M, Liu Q, Gao
Y, McNeal JE and Ho SM: Dynamic regulation of estrogen
receptor-beta expression by DNA methylation during prostate cancer
development and metastasis. Am J Pathol. 164:2003–2012. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Wang M, Pan JY, Song GR, Chen HB, An LJ
and Qu SX: Altered expression of estrogen receptor alpha and beta
in advanced gastric adenocarcinoma: Correlation with prothymosin
alpha and clinicopathological parameters. Eur J Surg Oncol.
33:195–201. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Ryu WS, Kim JH, Jang YJ, Park SS, Um JW,
Park SH, Kim SJ, Mok YJ and Kim CS: Expression of estrogen
receptors in gastric cancer and their clinical significance. J Surg
Oncol. 106:456–461. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Matsuyama S, Ohkura Y, Eguchi H, Kobayashi
Y, Akagi K, Uchida K, Nakachi K, Gustafsson JA and Hayashi S:
Estrogen receptor beta is expressed in human stomach
adenocarcinoma. J Cancer Res Clin Oncol. 128:319–324. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Guo JL, Xu CY, Jiang ZN, Dong MJ, Xie SD,
Shen JG, Cao J and Wang LB: Estrogen receptor beta variants mRNA
expressions in gastric cancer tissue samples and association with
clinicopathologic parameters. Hepatogastroenterology. 57:1584–1588.
2010.PubMed/NCBI
|
|
64
|
Zhou J, Teng R, Xu C, Wang Q, Guo J, Xu C,
Li Z, Xie S, Shen J and Wang L: Overexpression of ERα inhibits
proliferation and invasion of MKN28 gastric cancer cells by
suppressing β-catenin. Oncol Rep. 30:1622–1630. 2013.PubMed/NCBI
|
|
65
|
Kominea A, Konstantinopoulos PA, Kapranos
N, Vandoros G, Gkermpesi M, Andricopoulos P, Artelaris S, Savva S,
Varakis I, Sotiropoulou-Bonikou G and Papavassiliou AG: Androgen
receptor (AR) expression is an independent unfavorable prognostic
factor in gastric cancer. J Cancer Res Clin Oncol. 130:253–258.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Zhang BG, Du T, Zang MD, Chang Q, Fan ZY,
Li JF, Yu BQ, Su LP, Li C, Yan C, et al: Androgen receptor promotes
gastric cancer cell migration and invasion via AKT-phosphorylation
dependent upregulation of matrix metalloproteinase 9. Oncotarget.
5:10584–10595. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Bjerregaard-Olesen C, Ghisari M, Kjeldsen
LS, Wielsøe M and Bonefeld-Jørgensen EC: Estrone sulfate and
dehydroepiandrosterone sulfate: Transactivation of the estrogen and
androgen receptor. Steroids. 105:50–58. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
McKenna NJ, Lanz RB and O'Malley BW:
Nuclear receptor coregulators: Cellular and molecular biology.
Endocr Rev. 20:321–344. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Wang W, Song XW, Bu XM, Zhang N and Zhao
CH: PDCD2 and NCoR1 as putative tumor suppressors in gastric
gastrointestinal stromal tumors. Cell Oncol (Dordr). 39:129–137.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Kershah SM, Desouki MM, Koterba KL and
Rowan BG: Expression of estrogen receptor coregulators in normal
and malignant human endometrium. Gynecol Oncol. 92:304–313. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Zhang Z, Yamashita H, Toyama T, Sugiura H,
Ando Y, Mita K, Hamaguchi M, Hara Y, Kobayashi S and Iwase H: NCOR1
mRNA is an independent prognostic factor for breast cancer. Cancer
Lett. 237:123–129. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Gallardo F, Padrón A, Garcia-Carbonell R,
Rius C, González-Perez A, Arumí-Uria M, Iglesias M, Nonell L,
Bellosillo B, Segura S, et al: Cytoplasmic accumulation of NCoR in
malignant melanoma: Consequences of altered gene repression and
prognostic significance. Oncotarget. 6:9284–9294. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Kashima H, Horiuchi A, Uchikawa J,
Miyamoto T, Suzuki A, Ashida T, Konishi I and Shiozawa T:
Up-regulation of nuclear receptor corepressor (NCoR) in
progestin-induced growth suppression of endometrial hyperplasia and
carcinoma. Anticancer Res. 29:1023–1029. 2009.PubMed/NCBI
|
|
74
|
Boorjian SA, Heemers HV, Frank I, Farmer
SA, Schmidt LJ, Sebo TJ and Tindall DJ: Expression and significance
of androgen receptor coactivators in urothelial carcinoma of the
bladder. Endocr Relat Cancer. 16:123–137. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Berns EM, van Staveren IL, Klijn JG and
Foekens JA: Predictive value of SRC-1 for tamoxifen response of
recurrent breast cancer. Breast Cancer Res Treat. 48:87–92. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Fleming FJ, Myers E, Kelly G, Crotty TB,
McDermott EW, O'Higgins NJ, Hill AD and Young LS: Expression of
SRC-1, AIB1, and PEA3 in HER2 mediated endocrine resistant breast
cancer; a predictive role for SRC-1. J Clin Pathol. 57:1069–1074.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Wang S, Yuan Y, Liao L, Kuang SQ, Tien JC,
O'Malley BW and Xu J: Disruption of the SRC-1 gene in mice
suppresses breast cancer metastasis without affecting primary tumor
formation. Proc Natl Acad Sci USA. 106:151–156. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Qin L, Chen X, Wu Y, Feng Z, He T, Wang L,
Liao L and Xu J: Steroid receptor coactivator-1 upregulates
integrin α5 expression to promote breast cancer cell
adhesion and migration. Cancer Res. 71:1742–1751. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Xu Y, Hu B, Qin L, Zhao L, Wang Q, Wang Q,
Xu Y and Jiang J: SRC-1 and Twist1 expression positively correlates
with a poor prognosis in human breast cancer. Int J Biol Sci.
10:396–403. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Qin L, Wu YL, Toneff MJ, Li D, Liao L, Gao
X, Bane FT, Tien JC, Xu Y, Feng Z, et al: NCOA1 Directly Targets
M-CSF1 Expression to Promote Breast Cancer Metastasis. Cancer Res.
74:3477–3488. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Qin L, Xu Y, Xu Y, Ma G, Liao L, Wu Y, Li
Y, Wang X, Wang X, Jiang J, et al: NCOA1 promotes angiogenesis in
breast tumors by simultaneously enhancing HIF1α- and AP-1-mediated
VEGFa transcription. Oncotarget. 6:23890–23904. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Flågeng MH, Moi LL, Dixon JM, Geisler J,
Lien EA, Miller WR, Lønning PE and Mellgren G: Nuclear receptor
co-activators and HER-2/neu are upregulated in breast cancer
patients during neo-adjuvant treatment with aromatase inhibitors.
Br J Cancer. 101:1253–1260. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Tai H, Kubota N and Kato S: Involvement of
nuclear receptor coactivator SRC-1 in estrogen-dependent cell
growth of MCF-7 cells. Biochem Biophys Res Commun. 267:311–316.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Culig Z, Comuzzi B, Steiner H, Bartsch G
and Hobisch A: Expression and function of androgen receptor
coactivators in prostate cancer. J Steroid Biochem Mol Biol.
92:265–271. 2004. View Article : Google Scholar : PubMed/NCBI
|















