Introduction
Glioma is a type of tumor that originates from glial
cells of the neuroderm (1). At
present, postoperative radiotherapy is the standard treatment for
high-grade glioma. Randomized controlled trials have demonstrated
that postoperative radiotherapy prolongs the median survival time
of patients from 3–6 months to 9–12 months (1). For glioma, intensity-modulated radiation
therapy (IMRT) has been revealed to provide a more conformal dose
distribution compared with conventional radiotherapy, with improved
sparing of adjacent tissues (2–4).
Volumetric modulated arc therapy (VMAT) is a novel
form of IMRT optimization that regulates the radiation dose with
enhanced degrees of freedom, by continuously modulating the
multi-leaf collimator (MLC) field shape, gantry rotation speed and
dose rate. VMAT enables for additional flexibility in dose delivery
and could further improve dose conformity and sparing of vital
tissues. Compared with IMRT, the potential advantages of VMAT
include a large reduction in treatment time and a concomitant
reduction in the number of monitor units (MUs) required to deliver
a given fraction size (5–9). It has been demonstrated that the removal
of the flattening filter results in changes to the dose rate
(10–13). Clinically, the MUs and dose rate
increase with the use of flattening filter-free (FFF) beams and the
treatment delivery time is reduced compared with IMRT (10–13).
A total of 21 patients with tumors located within
the frontal lobe area (11 patients) and temporal lobe area (10
patients), who had been treated for tumor with radiotherapy at The
First Affiliated Hospital of Zhengzhou University (Zhengzhou,
China) between 2013 and 2014 were retrospectively selected. All
patients had a pathological diagnosis of glioma. For each patient,
four treatment plans, including a 6X-dynamic (d) IMRT, 6FFF-dIMRT,
6X-VMAT and 6FFF-VMAT plan were generated. The dose prescription
was set to 60 Gy delivered over 30 fractions. The dose
distributions for the planning target volume, organs at risk (OARs)
and normal tissue were compared. The MUs were also evaluated. The
dose distribution of target (Dmax, Dmin, Dmean, dose conformity and
heterogeneity index), OARs (Dmax) and normal tissue (Dmean, V20Gy,
V10Gy and V5Gy) were compared among the four plans.
Materials and methods
Patients
A total of 21 patients with glioma were selected
from The First Affiliated Hospital of Zhengzhou University between
September 2013 and August 2014 to be included in the present study.
According to the World Health Organization classification, the
clinical stages were as follows: Astrocytoma (stage II, n=5);
oligodendroglioma (stage II, n=5); anaplastic astrocytoma (stage
III, n=3); anaplastic oligodendroglioma (stage III, n=3); and
glioblastoma (stage IV, n=5) (8). The
exclusion criteria included any patient with abnormal function of
heart, lung, liver and kidney (Karnofsky performance status
<80). The median age was 43 years (range, 33–76 years), with 14
males and 7 males. For all patients, the location of glioma was in
the frontal lobe area (11 patients) or temporal lobe area (10
patients).
For frontal lobe glioma, the largest beam field of
planning target volume (PTV) was 11.5×13.5 cm2 (range,
8×6.5–11.5×13.5 cm2) and median PTV was 134.4
cm3 (range, 59.5–364.5 cm3). For temporal
lobe glioma, the largest beam field of PTV was 11.5×9
cm2 (range, 6.5×7-11.5×9 cm2) and the median
PTV was 109.7 cm3 (range, 43.6–194.2
cm3).
Definition and contour of targets
The target volume was delineated according to the
no. 50 and 62 reports of the International Commission on Radiation
Units and Measurements (8). For
low-grade glioma, the gross tumor volume (GTV) was defined as the
abnormal signal intensity area of T2-weighted-fluid-attenuated
inversion recovery on a magnetic resonance imaging (MRI) scan,
while a margin of 2.0 cm was added to the GTV to produce the
clinical target volume (CTV). For high-grade glioma, the GTV was
defined as the residual tumor and/or cavity of T1 on the MRI scan,
and the CTV was defined as the GTV plus a margin of 3.0 cm. The CTV
was expanded by 5 mm to produce the PTV.
Treatment planning
The treatment plans were generated using
Eclipse™ 3D-TPS software (version 10; Varian Medical
Systems, Palo Alto, CA, USA). dIMRT and VMAT plans were produced
using 6-MV photons, and the dose prescription was set to 60 Gy in
30 fractions. The dose constraints to the OARs were determined
using a Radiation Therapy Oncology Group protocol (6). The dIMRT plans consisted of six coplanar
fields at gantry angles of 220°. The VMAT plans consisted of a
single arc, starting at a gantry angle of 179° and rotating
counter-clockwise through 358° to stop at a gantry angle of 181°,
and another arc in the opposite direction. The two plans adopted
the same approach during optimization. The upper limits of the dose
rate for the 6X and FFF beams were 600 and 1,200 MU/min,
respectively.
Dosimetric comparison
The dose volume histogram included the Dmax, Dmean
and Dmin of CTV; and the Dmax, Dmean and Dmin of PTV. Conformity
index was calculated as follows:
(PTVref/VPTV) ×
(PTVref/Vref) (14). Heterogeneity index (HI) was calculated
as follows: D5/D95. To quantify the dose
distribution on OARs and normal tissue (NT) at different dose
levels, the percentage volume of the OARs and NT receiving a dose
of 20, 10 and 5 Gy (V20, V10 and V5, respectively) were evaluated
and compared.
Statistical analysis
Statistical significance was evaluated using a
two-tailed Student's t-test. P<0.05 was considered to indicate a
statistically significant difference. Analyses were performed using
SPSS software (version 19.0; SPSS, Inc., Chicago, IL, USA).
Results
Frontal lobe glioma PTV coverage
In the same model, the Dmax and Dmean of CTV and PTV
in dIMRT were increased compared with that in the VMAT plans
(Table I). In the 6X, the Dmax and
Dmean of CTV was 2.27 and 0.49%, respectively, and of PTV was 2.62
and 0.64%, respectively. In a model of 6FFF, the corresponding
values were 1.58, 0.54, 1.75 and 0.59%, respectively. In the
flattening filter (FF), the HI was more improved compared with the
VMAT plans.
 | Table I.Comparison of dosimetric parameters of
PTV, CTV, HI and CI. |
Table I.
Comparison of dosimetric parameters of
PTV, CTV, HI and CI.
| Parameter | 6X-dIMRT | 6FFF-dIMRT | 6X-VMAT | 6FFF-VMAT | P-value |
|---|
| CTV, cGy |
| Dmax | 6,474.44±30.17 | 6,442.74±20.91 | 6,330.69±16.18 | 6,344.22±19.85 | <0.001 |
|
Dmean | 6,152.70±11.82 | 6,164.70±10.85 | 6,122.73±7.68 | 6,131.68±8.20 | 0.001a |
| Dmin | 5,782.30±119.66 | 5,854.56±92.31 | 5,920.35±18.60 | 5,900.39±15.05 | 0.006a |
| PTV, cGy |
| Dmax | 6,532.77±34.95 | 6,506.39±30.62 | 6,365.63±19.35 | 6,394.85±20.01 | <0.001 |
|
Dmean | 6,150.95±9.85 | 6,154.30±8.93 | 6,111.22±6.00 | 6,118.94±6.07 |
<0.001a |
| Dmin | 5,020.95±252.45 | 5,089.82±191.71 | 5,515.19±48.07 | 5,458.32±47.62 | 0.218 |
| HI | 1.051±0.0029 | 1.050±0.0025 | 1.032±0.0014 | 1.067±0.0326 |
<0.001a |
| CI | 0.916±0.0048 | 0.922±0.0024 | 0.921±0.0028 | 0.925±0.0048 | 0.335 |
Traditionally, the FF in the X-ray beam path of a
linear accelerator produces an almost uniform fluence over a
collimated field. Based on these functions, the removal of the FF
results in an increase in the dose rate and a decrease in radiation
leak. Thus, in the FFF model, the dose distribution to a single
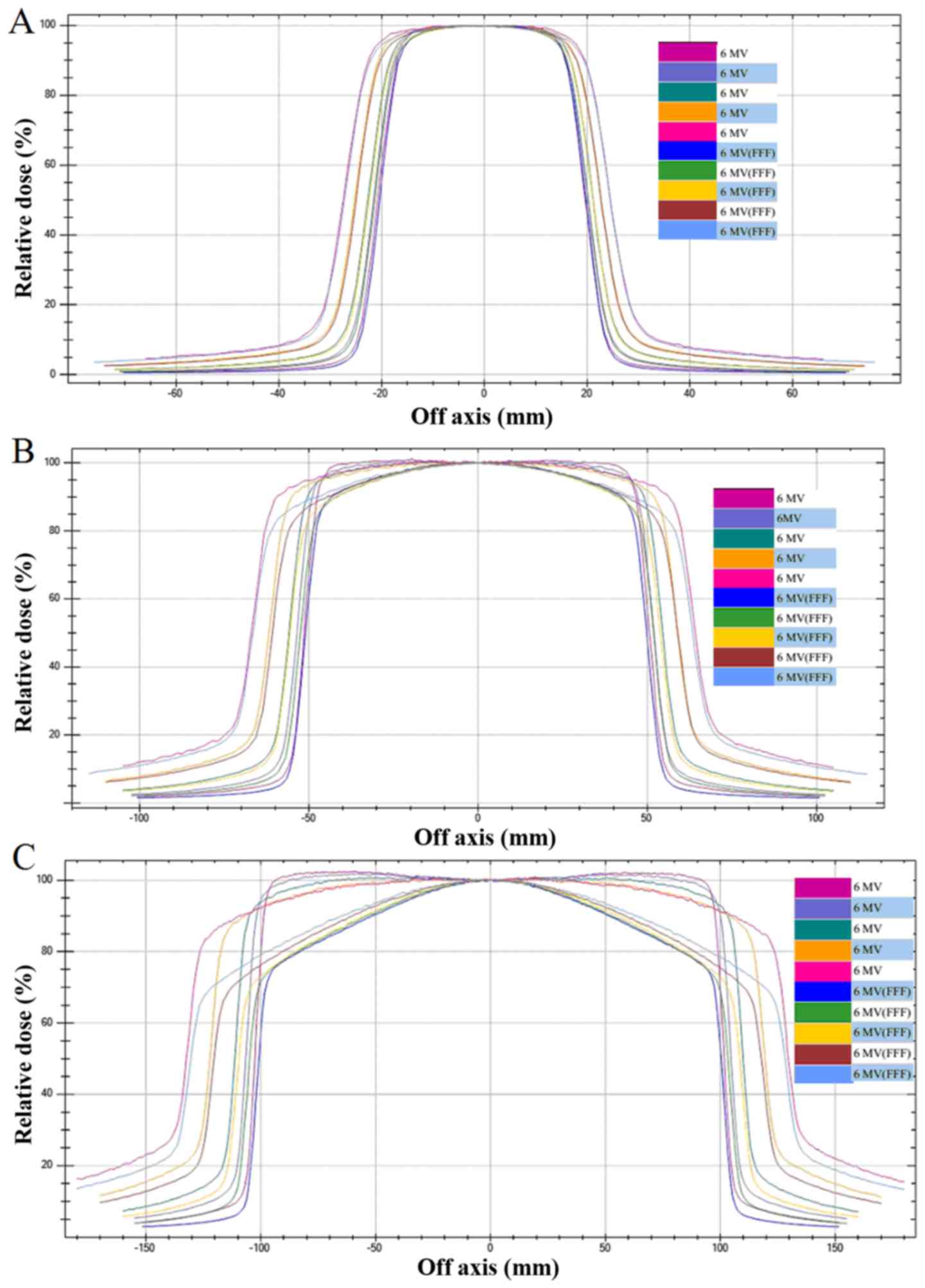
field will be different from that of an FF beam (Fig. 1). The off axis response to dose
distribution in the control group was similar in the 6X and FFF
model (Fig. 1A), while the off axis
responses in the experimental groups were significantly larger in
the 6X model compared with the FFF model (Fig. 1B and C).
Integral dose to the OARs and NT
Compared with dIMRT, the VMAT plan significantly
decreased the mean Dmax of the brainstem to 36% in the 6X
(P=0.008), while the same index was 30% in the 6FFF model
(P=0.045). In 6FFF-dIMRT, the mean Dmax decreased to 21% in the
ipsilateral eye lens and 17% in the contralateral eye lens.
Compared with 6X-VMAT, 6FFF-VMAT reduced the mean Dmax of the
ipsilateral and contralateral eye lens to 16 and 10%, respectively
(Table II).
 | Table II.Comparison of dosimetric parameters of
organs at risk and normal tissue. |
Table II.
Comparison of dosimetric parameters of
organs at risk and normal tissue.
| Parameter | 6X-dIMRT | 6FFF-dIMRT | 6X-VMAT | 6FFF-VMAT | P-value |
|---|
| Brainstem Dmax,
cGy | 1,838.13±508.77 | 1,711.94±463.59 | 1,174.82±311.91 | 1,188.24±323.59 | 0.001 |
| Pituitary Dmax,
cGy | 820.50±353.48 | 843.42±376.29 | 961.63±475.67 | 957.14±480.44 | 0.162a |
| Lens-ips Dmax,
cGy | 276.03±67.43 | 218.31±52.58 | 245.62±58.40 | 214.97±54.61 |
<0.001a |
| Lens-cont Dmax,
cGy | 237.39±58.12 | 197.24±48.84 | 212.46±46.97 | 194.10±44.36 | 0.021 |
| ON-ips Dmax, cGy | 1,046.32±333.81 | 1,071.75±364.82 | 1,709.34±655.80 | 1,761.39±667.07 | 0.840a |
| ON-cont Dmax,
cGy | 648.60±217.87 | 640.32±229.76 | 926.66±358.32 | 884.28±351.54 | 0.782a |
| Chiasma Dmax,
cGy | 1,219.61±538.25 | 1,209.86±533.38 | 1,421.86±694.97 | 1,418.78±693.35 | 0.106a |
| TL-cont Dmax,
cGy | 1,719.79±454.25 | 1,697.98±444.88 | 1,706.99±478.26 | 1,486.35±472.11 | 0.203 |
| Brain |
| Dmean, cGy | 788.88±102.17 | 780.53±102.7 | 772.62±104.14 | 771.04±106.71 | 0.003a |
| V20Gy, cm3 | 673.85±84.12 | 667.25±83.56 | 625.99±90.76 | 621.50±92.94 | <0.001 |
| V10Gy, cm3 | 1,048.81±118.52 | 1,024.69±118.65 | 1,076.32±124.07 | 1,064.28±126.42 | <0.001 |
| V5Gy, cm3 | 1,503.2±105.09 | 1,480.34±108.02 | 1,490.86±107.5 | 1,477.09±108.59 | 0.171 |
| MU | 543.9±29.78 | 560.4±23.35 | 414.7±18.29 | 455.6±16.77 | 0.002a |
Regarding the Dmean of NT, the 6X-dIMRT was
increased compared with 6FFF-dIMRT, and the 6X-dIMRT was
significantly increased compared with 6X-VMAT (P=0.013). No
significant differences were identified in other groups. For the
mean of low-dose of volume in NTs (V20Gy and V10Gy), the 6X-dIMRT
was increased compared with 6X-VMAT, and compared with 6FFF-VMAT,
the 6FFF-dIMRT was significantly increased (P<0.05). Among the
four groups, there were significant differences in the mean of MUs.
Compared with 6X-dIMRT, the group of 6X-VMAT decreased the mean of
MUs from 543.9±29.78 to 414.7±18.29. The number of MUs for
6FFF-VMAT decreased by 19% compared with for 6FFF-dIMRT (Table III).
 | Table III.Comparison of dosimetric parameters of
PTV, CTV, HI and CI. |
Table III.
Comparison of dosimetric parameters of
PTV, CTV, HI and CI.
| Parameter | 6X-dIMRT | 6FFF-dIMRT | 6X-VMAT | 6FFF-VMAT | P-value |
|---|
| CTV, cGy |
| Dmax | 6,451.92±17.98 | 6,433.79±22.18 | 6,389.89±28.34 | 6,418.67±26.01 | 0.248 |
|
Dmean | 6,164.94±9.62 | 6,177.13±10.35 | 6,054.71±93.71 | 6,163.13±9.85 | 0.138a |
|
Dmin |
5,760.52±118.36 |
5,784.79±115.23 | 5,797.90±78.30 | 5,785.88±75.23 | 0.664a |
| PTV, cGy |
|
Dmax | 6,489.08±19.67 | 6,456.15±19.80 | 6,417.40±22.14 | 6,439.05±24.58 | 0.131 |
|
Dmean | 6,155.03±8.01 | 6,160.96±8.73 | 6,131.84±6.45 | 6,145.86±8.24 | 0.052 |
|
Dmin |
5,066.49±118.40 |
5,113.98±100.47 | 5,333.67±88.79 | 5,316.28±95.28 | <0.001 |
| HI | 1.05±0.0023 | 1.050±0.0027 | 1.075±0.0376 | 1.041±0.0023 | 0.004a |
| CI | 0.912±0.0105 | 0.918±0.0091 | 0.904±0.0067 | 0.905±0.0057 | 0.136 |
Temporal lobe glioma
PTV coverage
Under two different models, the mean of PTV's Dmin
in dIMRT was reduced compared with the VMAT plan. The average value
was 5,066.49±118.4 cGy and 5,333.67±88.79 cGy in 6X, while
5,113.98±100.47 cGy and 5,316.28±95.28 cGy in 6FFF. Regarding the
HI, the 6FFF-dIMRT was increased compared with 6FFF-VMAT. The mean
value was 1.050±0.0027 and 1.041±0.0023. No statistical differences
were identified in other parameters.
Integral dose to the OARs and NT
In comparison to dIMRT, 6X-VMAT significantly
increased the average value of Dmax and Dmean of the pituitary
gland by 21% (P=0.012), while in the 6FFF-VMAT, the value increased
by 19% (P=0.030). No statistical differences were identified in the
other groups.
Regarding the bilateral lens, the mean of Dmax was
significantly reduced in 6FFF-dIMRT compared with 6X (P<0.05).
There was a reduction in the mean of Dmax of the ipsilateral lens
in 6X-dIMRT (304.93±47.99 cGy) compared with 6FFF-dIMRT
(283.45±43.96 cGy), with a simultaneous decrease in the value of
the contralateral lens from 265.30±48.68 to 258.32±47.72 cGy.
The data revealed that the mean Dmax of the
ipsilateral optic nerve was significantly reduced in 6FFF-dIMRT
compared with that of 6FFF-VMAT (P<0.05). All values for the
chiasma were compared in pairs, and the mean of Dmax of dIMRT was
identified to be significantly decreased compared with VMAT in 6X
and 6FFF (P<0.05). While compared with dIMRT, the plan of
6X-VMAT reduced the value of Dmax on the contralateral temporal
lobe by 19%, and in the 6FFF model by 20%.
In the healthy brain tissue, the Dmean in 6X-dIMRT
was increased compared with 6FFF-dIMRT, and the 6X-dIMRT was
significantly increased compared with 6X-VMAT (P<0.001). No
other statistical differences were identified. For the low-dose
volume of NTs, the mean of V20Gy was increased in 6X-dIMRT compared
with 6X-VMAT, and 6FFF-dIMRT was significantly increased compared
with 6FFF-VMAT (P<0.05). For V10Gy in dIMRT, the model of 6FFF
reduced the mean value from 1,013.02±114.88 to 977.86±109.01
compared with 6X. While in VMAT the 6FFF model reduced the mean
value from 1,044.68±117.59 to 981.25±110.29.
Compared with 6X-dIMRT, the 6X-VMAT reduced the mean
value of MUs from 981.25±110.29 to 450.0±24.93. The mean of MUs in
6FFF-VMAT was 469.2±22.56, which was reduced by ~20% compared with
in 6FFF-dIMRT (583.8±28.66) (Table
IV).
 | Table IV.Comparison of dosimetric parameters
of organs at risk and normal tissue. |
Table IV.
Comparison of dosimetric parameters
of organs at risk and normal tissue.
| Parameter | 6X-dIMRT | 6FFF-dIMRT | 6X-VMAT | 6FFF-VMAT | P-value |
|---|
| Brainstem Dmax,
cGy |
2,619.63±510.16 |
2,617.22±514.88 |
2,333.51±489.88 |
2,370.47±502.15 | 0.484a |
| Pituitary Dmax,
cGy |
1,605.23±413.22 |
1,667.76±451.34 |
1,950.39±502.59 |
1,976.34±505.30 | 0.001 |
| Lens-ips Dmax,
cGy | 304.93±47.99 | 283.45±43.96 | 314.69±46.79 | 307.36±46.05 | 0.024a |
| Lens-cont Dmax,
cGy | 265.30±48.68 | 258.32±47.72 | 290.01±47.46 | 300.42±52.40 | 0.039a |
| ON-ips Dmax,
cGy |
1,223.89±361.25 |
1,225.89±359.12 |
1,805.86±536.71 |
1,835.06±488.52 | 0.024a |
| ON-cont Dmax,
cGy | 627.95±122.62 | 643.67±130.22 | 870.37±191.30 | 826.98±165.04 | 0.068 |
| Chiasma Dmax,
cGy |
1,601.55±471.82 |
1,594.55±472.96 |
2,112.10±596.47 |
2,129.90±585.43 | 0.042a |
| TL-cont Dmax,
cGy |
1,708.14±385.60 |
1,688.19±387.35 |
1,386.26±289.71 |
1,346.53±289.44 | 0.003 |
| Brain |
| Dmean, cGy | 581.15±60.41 | 580.92±60.90 | 525.80±59.51 | 513.82±57.63 | <0.001 |
| V20Gy, cm3 |
1,013.02±114.88 | 977.86±109.01 |
1,044.68±117.59 | 981.25±110.29 | 0.012a |
| V10Gy, cm3 |
1,524.98±163.77 |
1,494.73±160.67 |
1,507.08±166.81 |
1,487.40±165.50 | 0.141 |
| V5Gy, cm3 | 562.7±26.24 | 583.8±28.66 | 450.0±24.93 | 469.2±22.56 | <0.001 |
| MU | 581.15±60.41 | 580.92±60.90 | 525.80±59.51 | 513.82±57.63 | <0.001 |
Discussion
Previous studies have suggested that the clinical
application of FFF in prostate cancer or nasopharynx carcinoma
improves the protection of the rectum and bladder (15,16).
Considering the decreased dose to OARs and NT, FFF has an advantage
over FF. The mean MUs were greater in the 6FFF model compared with
6X, which may be due to the softness of the rays. If the dose of
the rays to deeper tissue is reduced, the MUs should be increased
to reach the same depth.
During the process of VMAT the parameters, including
the dose rate, the gantry rotation speed and the site of MLC change
dynamically. Two types of products are currently in clinical use,
Varian RapidArc and Elekta VMAT. As the application develops, the
VMAT plan may become equal or superior to IMRT and tomotherapy.
Compared with IMRT, the VMAT plan may increase the scattering of
NTs, reduce the MUs and reduce the treatment duration (17,18).
In the current study, for frontal lobe glioma, the
Dmax and Dmean of PTV in VMAT were increased compared with dIMRT,
but no significant differences were identified in the OARs and NTs.
For temporal lobe glioma, the protection of OARs, including
pituitary gland, optic nerve and chiasma were more improved in the
dIMRT plan compared with VMAT. The reason for this is primarily due
to the spatial association between the location of the glioma and
OARs. In an identical ray model, the differences in dose
distribution to OARs between the two plans were not demonstrated to
be statistically significant, as frontal lobe glioma is far from
the lens and optic nerve. However, temporal lobe glioma is close to
the OARs, and in certain patients, the tumor had invaded the edge
of chiasma. The rotatory speed of the machine is constant at 4.8°/s
in the process of VMAT; the dose of adjacent field shape may be
overlaid because of the speed of MLC, the positioning accuracy and
the leakage ray, and as a result the Dmax of the pituitary gland
and chiasma in VAMT was increased compared with dIMRT.
In the past few years, the VMAT plan has been
gradually applied in clinical treatment. The FFF model provides a
broad range of dose rates, and will be useful in the optimization
of VMAT. However, simultaneously, the specialty of high dose rate
in the FFF model complicates the regulation of the quality. In the
future, as the speed of MLC increases, the high dose rate of FFF
will be taken full advantage of. The VMAT plan should be
reconsidered as the treatment duration may be reduced with use of
the FFF beam or another technical innovation.
References
|
1
|
Walker MD, Green SB, Byar DP, Alexander E
Jr, Batzdorf U, Brooks WH, Hunt WE, MacCarty CS, Mahaley MS Jr,
Mealey J Jr, et al: Randomized comparison of radiotherapy and
nitrosoureas for the malignant glioma after surgery. N Eng J Med.
303:1323–1329. 1980. View Article : Google Scholar
|
|
2
|
Chan MF, Schupak K, Barman C, Chui CS and
Ling CC: Comparison of intensity-modulated radiotherapy with
three-dimensional conformal therapy planning for glioblastoma
muhiforme. Med Dosim. 28:261–265. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Narayana A, Yamade J, Berry S, Shah P,
Hunt M, Gutin PH and Leibel SA: Intensity modulated radiotherapy in
high grade glimas: Clinical and dosimetric result. Int J Radiat
Oncol Biol Phys. 64:892–907. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bragg CM, Conw J and Robinson MH: The role
of intensity-modualated radiotherapy in treatment of parotid
tumors. Int J Radiat Oncol Biol Phys. 52:729–738. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Vanetti E, Clivio A, Nicolini G, Fogliata
A, Ghosh-Laskar S, Agarwal JP, Upreti RR, Budrukkar A, Murthy V,
Deshpande DD, et al: Volumetric modulated arc radiotherapy for
carcinomas of the oro-pharynx, hypo-pharynx and larynx: A treatment
planning comparison with fixed field IMRT. Radiother Oncol.
92:111–117. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Verbakel WF, Cuijpers JP, Hoffmans D,
Bieker M, Slotman BJ and Senan S: Volumetric intensity-modulated
arc therapy vs. Conventional IMRT in head-and-neck cancer: A
comparative planning and dosimetric study. Int J Radiat Oncol Biol
Phys. 74:252–259. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wu Hao, Han Shukui, Sun Yan and Jiang Fan:
Dosimetric comparison of RapidArc with fixed gantry dynamic IMRT
for loco-regionally advanced nasopharyngeal carcinoma. Chinese
Journal of Radiation Oncology. 19:410–413. 2010.
|
|
8
|
Clemente S, Wu B, Sanguineti G, Fusco V,
Ricchetti F, Wong J and McNutt T: SmartArc-based Volumetric
modulated arc therapy for oropharyngeal cancer: A dosimetric
comparison with both intensity-modulated radiation therapy and
helical tomotherapy. Int J Radiat Oncol Biol Phys. 80:1248–1255.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Doornaert P, Verbakel WF, Bieker M,
Slotman BJ and Senan S: Rapid Arc planning and delivery in patients
with locally advanced head-and-neck cancer undergoing
chemoradiotherapy. Int J Radiat Oncol Biol Phys. 79:429–435. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sixel KE and Faddegon BA: Calculation of
x-ray spectra for radiosurgical beams. Med Phys. 22:1657–1661.
1995. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Vassiliev ON, Titt U, Pönisch F, Kry SF,
Mohan R and Gillin MT: Dosimetric properties of photon beams from a
flattening filter free clinical accelerator. Phys Med Biol.
51:1907–1917. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cashmore J: The characterization of
unflattened photon beams from a 6 MV linear accelerator. Phys Med
Biol. 53:1933–1946. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kragl G, Wetterstedt Af S, Knäusl B, Lind
M, McCavana P, Knöös T, McClean B and Georg D: Dosimetric
characteristics of 6 and 10MV unflattened photon beams. Radiother
Oncol. 93:141–146. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Baltas D, Kolotas C, Geramani K, Mould RF,
Ioannidis G, Kekchidi M and Zamboglou N: A conformal index (COIN)
to evaluate implant quality and dose specification in
brachytherapy. Int J Radiat Oncol Biol Phys. 40:515–524. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zwahlen DR, Lang S, Hrbacek J, Glanzmann
C, Kloeck S, Najafi Y, Streller T, Studer G, Zaugg K and Luetolf
UM: The use of photon beams of a flattening filter-free linear
accelerator for hypofractionated volumetric modulated arc therapy
in localized prostate cancer. Int J Radiat Oncol Biol Phys.
83:1655–1660. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Alongi F, Fogliata A, Clerici E, Navarria
P, Tozzi A, Comito T, Ascolese AM, Clivio A, Lobefalo F, Reggiori
G, et al: Volumetric modulated arc therapy with flattening filter
free beams for isolated abdominal/pelvic lymph nodes: Report of
dosimetric and early clinical results in oligometastatic patients.
Radiat Oncol. 7:2042012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cao D, Holmes TW, Afghan MK and Shepard
DM: Comparison of plan quality provided by intensity-modulated arc
therapy and helical tomotherapy. Int J Radial Oncol Biol Phys.
69:240–250. 2007. View Article : Google Scholar
|
|
18
|
Palms D, Vollans E, James K, Nakano S,
Moiseenko V, Shaffer R, McKenzie M, Morris J and Otto K: Volumetrie
modulated arc therapy for delivery of prostate radiotherapy.
Comparison with intensity-modulated radiotherapy and
three-dimensional conformal radiotherapy. Int J Radial Oncol Biol
Phys. 72:996–1001. 2008. View Article : Google Scholar
|