Introduction
Human lung cancer, one of the most common types of
aggressive malignancy, is the primary cause of cancer-associated
mortality worldwide (1,2). In 2015, it was estimated that there
would be 221,200 new cases and 158,040 mortalities due to lung
cancer in the USA (3). Previous
studies have demonstrated that environmental deterioration, tobacco
use, radon exposure and occupational carcinogens are risk factors
for lung cancer (4–7). Lung cancer is divided into two major
clinically relevant groups, small cell lung cancer and non-small
cell lung cancer (NSCLC), according to histological analysis
(8). NSCLC is the predominant type of
lung cancer and accounts for ~83% of all cases (9). NSCLC may be further divided into three
histological subtypes: Squamous cell carcinoma, adenocarcinoma and
large cell carcinoma (10). In spite
of advances in the development of therapeutic strategies for
patients with NSCLC, the prognosis of patients with NSCLC has
remained poor over the past decade and the 5-year survival rate is
≤15% (11,12). It has been demonstrated that the
primary causes of mortality in NSCLC are invasion and metastasis of
cancerous cells (13). In addition,
>80% of patients with NSCLC are diagnosed at the advanced or
distant stages (14). Therefore, it
is essential to understand the underlying molecular mechanisms of
NSCLC and develop therapeutic strategies for patients with
NSCLC.
The dysregulation of microRNAs (miRNAs/miRs) has
been demonstrated in NSCLC (15–17).
miRNAs are a class of short (19–24 nucleotides in length)
endogenous non-protein-coding single-stranded RNAs, which regulate
~1/3 of mRNAs in the human genome (18). miRNAs regulate mRNA expression by
binding the 3′ untranslated regions (3′UTRs) of target mRNAs and
inducing either translational repression or transcript degradation
(19). It has been demonstrated that
miRNAs contribute to various physiological and pathological
processes, including cell proliferation, differentiation,
morphogenesis, cell cycle regulation, apoptosis, migration and
invasion (20). miRNAs are classified
as tumor-suppressive miRNAs and oncogenic miRNAs (21). Tumor suppressive miRNAs are
downregulated in cancer and inhibit carcinogenesis and progression,
whereas oncogenic miRNAs are upregulated in cancer, and enhance the
initiation and development of cancer (22–24).
Therefore, miRNAs have been extensively investigated in cancer
research as therapeutic targets and biomarkers, as they regulate
gene expression and cell biological activities.
In the present study, the expression level of
miR-29a was assessed in NSCLC tissues and cell lines. In addition,
whether the expression level of miR-29a was associated with the
clinicopathological features of patients with NSCLC was
investigated. Furthermore, the effect of miR-29a on cell
proliferation, migration and invasion were determined, and the
underlying molecular mechanism of its functions in NSCLC was
investigated. The results of the present study may inform the
development of a novel therapeutic strategy for NSCLC.
Materials and methods
Clinical specimens
The present study was approved by the Ethics Board
of The Institute of The Second Affiliated Hospital of Xi'an Medical
University (Xi'an, China). Written informed consent was also
obtained from each patient involved in the present study. A total
of 62 pairs of NSCLC tissue specimens and corresponding adjacent
non-tumor lung tissues were obtained from patients who had
undergone surgery at The Second Affiliated Hospital of Xi'an
Medical University between January 2012 and November 2014. Tissues
were immediately snap-frozen in liquid nitrogen and stored at
−80°C. No patient had received radiotherapy or chemotherapy prior
to surgery. The complete clinical data for patients with NSCLC are
presented in Table I.
 | Table I.Comparison of miR-29a expression in
NSCLC and clinicopathological features. |
Table I.
Comparison of miR-29a expression in
NSCLC and clinicopathological features.
|
|
| miR-29a
expression |
|
|---|
|
|
|
|
|
|---|
| Clinical
feature | n | Low | High | P-value |
|---|
| Gender |
|
|
| 0.191 |
|
Male | 30 | 21 | 9 |
|
|
Female | 42 | 23 | 19 |
|
| Age, years |
|
|
| 0.449 |
|
<60 | 32 | 18 | 14 |
|
|
>60 | 40 | 26 | 14 |
|
| Smoking history,
years |
|
|
| 0.117 |
|
<10 | 25 | 15 | 10 |
|
|
>10 | 47 | 29 | 18 |
|
| TNM
classification |
|
|
| 0.003 |
|
I–II | 38 | 17 | 21 |
|
|
III–IV | 34 | 27 | 7 |
|
| Tumor
differentiation |
|
|
| 0.212 |
|
I+II | 45 | 25 | 20 |
|
|
III+IV | 27 | 19 | 8 |
|
| Metastasis |
|
|
| 0.020 |
| No | 29 | 13 | 16 |
|
|
Yes | 43 | 31 | 12 |
|
Cell culture
The human NSCLC cell lines H23, H522, H1299 and
A549, and non-tumorigenic bronchial epithelium cell line BEAS-2B
were purchased from the American Type Culture Collection (Manassas,
VA, USA). The human embryonic kidney cell line HEK293T was
purchased from the Cell Bank of the Type Culture Collection of the
Chinese Academy of Sciences (Shanghai, China). The NSCLC cell lines
and HEK293T cell line were maintained in RPMI-1640 medium (Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with
10% fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific,
Inc.), 100 U/ml penicillin and 100 mg/ml streptomycin (Gibco;
Thermo Fisher Scientific, Inc.) in a cell incubator with 5%
CO2 at 37°C. BEAS-2B cells were maintained in LHC-9
medium (Gibco; Thermo Fisher Scientific, Inc.) containing 10% FBS
at 37°C.
Cell transfection
miR-29a mimics, negative control (NC), miR-29a
inhibitor, NC inhibitor, cell division cycle 42 (CDC42) small
interfering RNA (siRNA) and NC siRNA were obtained from Shanghai
GenePharma Co., Ltd. (Shanghai, China). Cells were seeded into
6-well plates and cultured until the cell density reached 70–90%.
Cell transfection was performed using Lipofectamine™ 2000
(Invitrogen; Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocol. Cell transfection efficiency was
determined using reverse transcription-quantitative polymerase
chain reaction (RT-qPCR).
RNA isolation and RT-qPCR
Total RNA from tissues and cells was isolated using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.), according to the manufacturer's protocol. The miR-29a
expression level was determined using an SYBR PrimeScript miRNA
RT-PCR kit (Takara Bio, Inc., Otsu, Japan) with U6 as the internal
control. The thermocycling conditions were as follows: 42°C for 5
min; 95°C for 10 sec; and 40 cycles of 95°C for 5 sec, 55°C for 30
sec and 70°C for 30 sec. The expression of CDC42 mRNA was
determined using a standard SYBR PrimeScript miRNA RT-PCR kit
(Takara Bio, Inc.) with GAPDH as the control. The thermocycling
conditions were as follows: 42°C for 5 min; 95°C for 10 sec; and 40
cycles of 95°C for 5 sec, 55°C for 30 sec and 70°C for 30 sec.
RT-qPCR was performed using an ABI 7500 Real-time PCR detection
system (Applied Biosystems; Thermo Fisher Scientific, Inc.). The
primer sequences were as follows: miR-29a forward,
5′-ACACTCCAGCTGGGACTGATTTCTTTTGGT-3′ and reverse,
5′-CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGTCAGGTGT-3′; U6 forward,
5′-CTCGCTTCGGCAGCACA-3′ and reverse, 5′-AACGCTTCACGAATTTGCGT-3′;
CDC42 forward, 5′-ACGACCGCTGAGTTATCCAC-3′ and reverse,
5′-TATGGGCCTTGTCTCACACG-3′; and GAPDH forward,
5′-TGCACCACCAACTGCTTAGC-3′ and reverse,
5′-GGCATGCACTGTGGTCATGAG-3′. RT-qPCR results were calculated using
the 2−ΔΔCq method (25).
Each sample was analyzed in triplicate and repeated >3
times.
Cell Counting kit-8 (CCK-8) assay
In vitro cell proliferation was monitored
using a CCK-8 assay (Dojindo Molecular Technologies, Inc.,
Kumamoto, Japan), according to the manufacturer's protocol.
Transfected cells were collected and seeded into 96-well plates at
a density of 3,000 cells/well. Quantification of cell proliferation
was performed daily for 4 days. Α 10 µl volume of CCK-8 assay
solution was added to each well prior to incubation at 37°C for 2 h
in a cell incubator. The absorbance at 450 nm of each well was
determined using a spectrophotometer. Each sample was analyzed in
triplicate.
Cell migration and invasion
assays
In vitro cell migration and invasion assays
were performed using Transwell chambers (EMD Millipore, Billerica,
MA, USA) with an 8-µm pore polycarbonate membrane. For the cell
migration assay, 5×104 transfected cells in 100 µl
FBS-free RPMI-1640 medium were plated in the upper chamber. A 500
µl volume of RPMI-1640 medium containing 20% FBS was added to the
lower chamber as a chemoattractant. For the cell invasion assay,
Transwell chambers were pre-coated with Matrigel (BD Biosciences,
San Jose, CA, USA). Otherwise, the cell invasion assays were
performed according to the procedure of the cell migration assay.
After 24 h of incubation, cells were fixed with 95% ethanol and
stained with 0.1% crystal violet (Beyotime Institute of
Biotechnology, Haimen, China) for 20 min. Non-migrating and
non-invading cells were carefully scraped from upper chambers using
cotton wool. Cells were counted using a light microscope. Each
sample was repeated ≥3 times.
Western blotting
Cells were lysed with radioimmunoprecipitation
buffer (Thermo Fisher Scientific, Inc.). A bicinchoninic acid assay
(Thermo Fisher Scientific, Inc.) was used to determine protein
concentrations. Equal amounts of proteins (20 µg) were separated by
SDS-PAGE (10% gels; Beyotime Institute of Biotechnology) and
transferred onto polyvinylidene fluoride (EMD Millipore) membranes.
The membranes were blocked with 5% non-fat dry milk and incubated
with mouse anti-human CDC42 monoclonal primary antibody (dilution,
1:500; cat. no. sc-8401; Santa Cruz Biotechnology, Dallas, TX, USA)
and rabbit anti-human GAPDH monoclonal primary antibody (dilution,
1:1,000; cat no. 2118; Cell Signaling Technology, Inc., Danvers,
MA, USA). Following incubation at 4°C overnight, the membranes were
incubated with corresponding horseradish peroxidase-conjugated
secondary antibody (both dilution, 1:5,000; CDC42 cat. no. sc-2005;
GAPDH cat. no. sc-2054; both Santa Cruz Biotechnology, Dallas, TX,
USA) for 1 h at room temperature. The membranes were visualized
with enhanced chemiluminescence solution (Pierce; Thermo Fisher
Scientific, Inc.) and analyzed using a FluorChem imaging system
(version 4.1.0; Alpha Innotec, San Leandro, CA, USA). Each sample
was repeated ≥3 times.
miRNA target prediction
The potential target genes of miR-29a were predicted
using TargetScan (www.Targetscan.org) and miRanda (www.microrna.org/microrna/home.do).
Dual-luciferase reporter assay
The luciferase reporter plasmids PGL3-CDC42-3′UTR
wild-type (Wt) and PGL3-CDC42-3′UTR mutant (Mut) were purchased
from Shanghai GenePharma Co., Ltd. HEK293T cells were seeded into
12-well plates and cultured as described above until the cell
density reached 90%. Cells were transfected with miR-29a mimics or
NC, and co-transfection with PGL3-CDC42-3′UTR Wt or
PGL3-CDC42-3′UTR Mut using Lipofectamine 2000 was performed
according to the manufacturer's protocol. After transfection for 48
h, firefly luciferase activity and Renilla luciferase
activity were detected using the Dual-Luciferase Reporter Assay
system (Promega Corporation, Madison, WI, USA), according to the
manufacturer's protocol. The firefly luciferase activity was
normalized to the Renilla luciferase activity. Each assay
was replicated three times.
Statistical analysis
Data are presented as the mean ± standard deviation,
and were analyzed using the Student's t test or one-way analysis of
variance using SPSS software (version 17; SPSS Inc., Chicago, IL,
USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
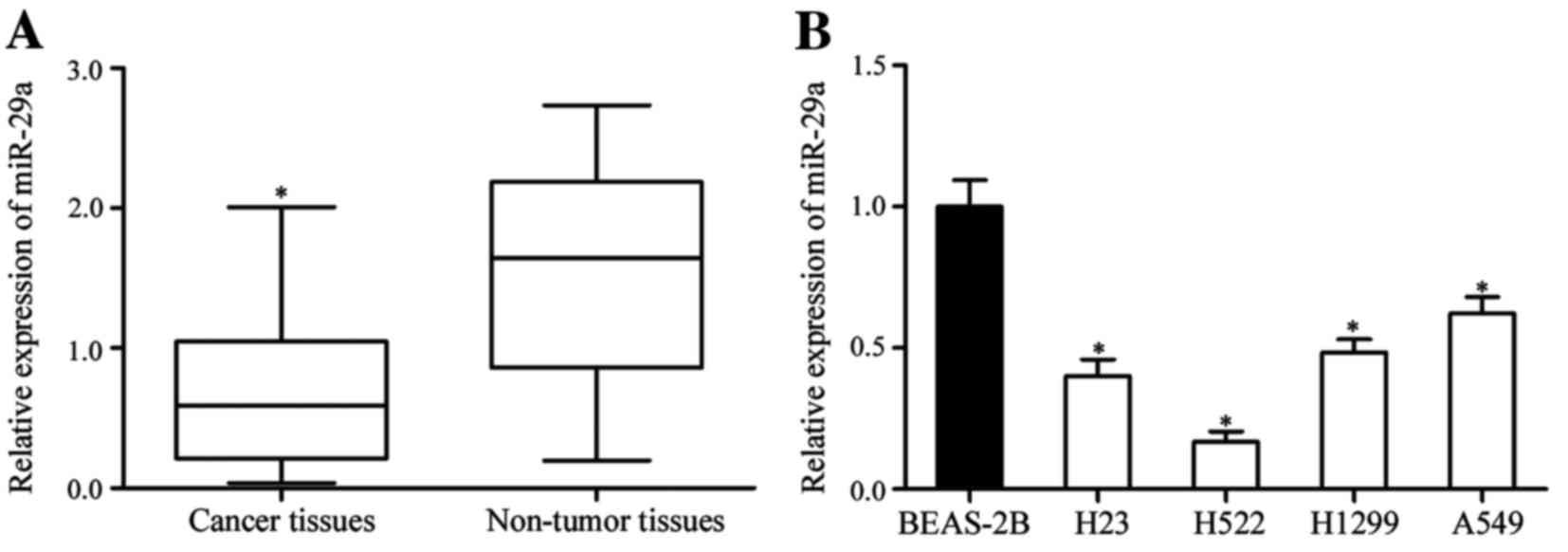
Expression of miR-29a in NSCLC tissues
and cell lines
To investigate the potential function of miR-29a in
NSCLC, the miR-29a expression level in NSCLC tissue specimens and
corresponding adjacent non-tumor lung tissues was determined. The
expression level of miR-29a was significantly decreased in NSCLC
tissue compared with adjacent non-tumor lung tissue (P<0.05;
Fig. 1A). The expression of miR-29a
was also measured in NSCLC cell lines and the non-tumorigenic
bronchial epithelium cell line BEAS-2B. Analysis using RT-qPCR
revealed that the expression of miR-29a was downregulated in all
four NSCLC cell lines compared with BEAS-2B cells (P<0.05;
Fig. 1B). These results suggested
that miR-29a contributes to the initiation and progression of
NSCLC.
miR-29a expression and
clinicopathological features in NSCLC
To investigate the clinical significance of miR-29a
expression in NSCLC, statistical analysis was used to explore the
association between miR-29a and clinicopathological factors. As
presented in Table I, it was
identified that the low expression level of miR-29a was
significantly associated with advanced tumor-node-metastasis (TNM)
classification (P=0.003) and metastasis (P=0.020). However, no
significant association between miR-29a expression level and other
clinicopathological features (gender, age, smoking history and
tumor differentiation) was identified (P>0.05).
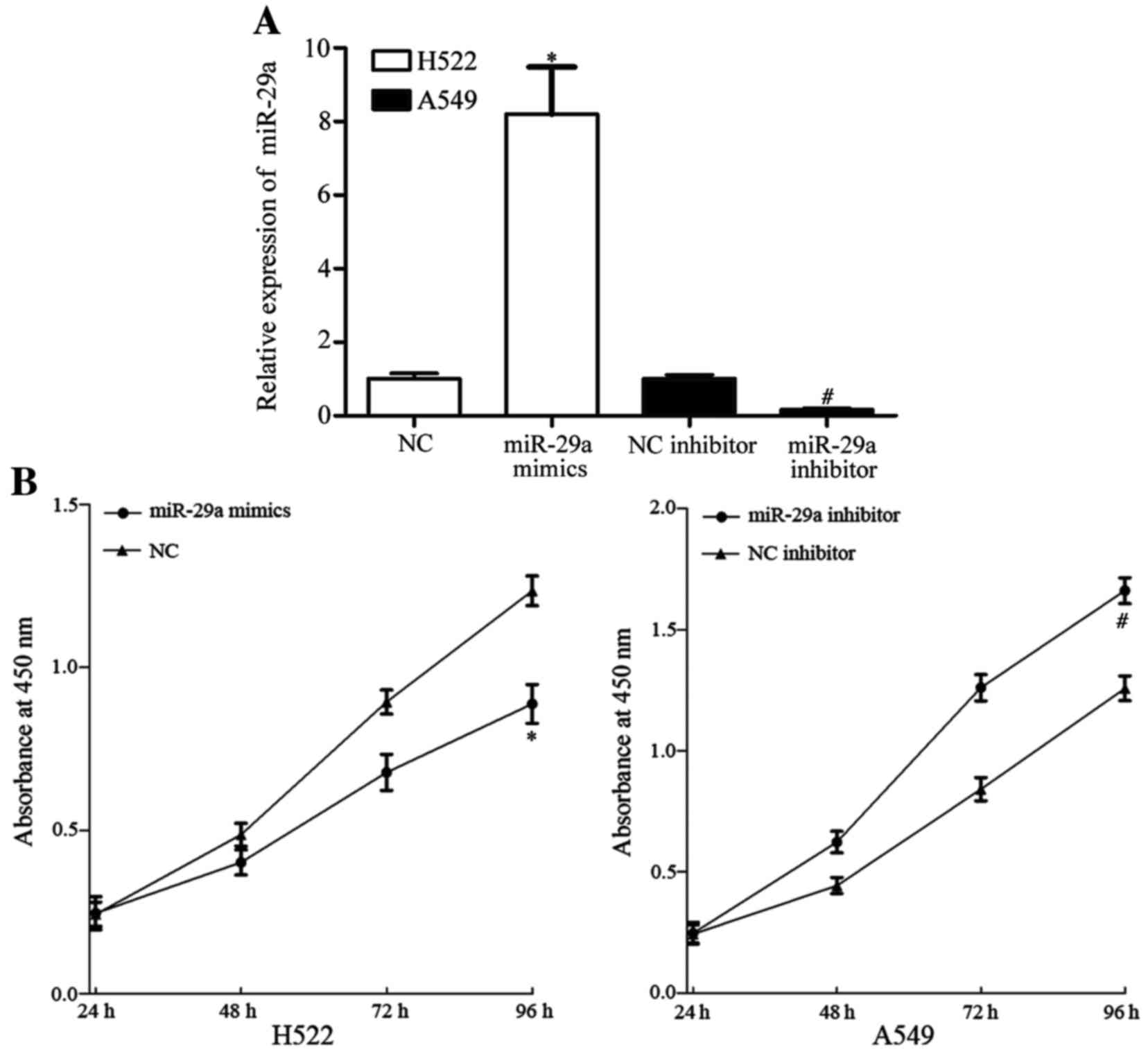
miR-29a inhibits NSCLC cell
proliferation
To investigate the roles of miR-29a expression in
NSCLC, miR-29a mimics, NC siRNA, miR-29a inhibitor and NC inhibitor
were transfected into NSCLC cells. Among the four NSCLC cell lines,
the expression level of miR-29a in H522 cells was the lowest and
that in A549 cells was the highest. Consequently, H522 was
transfected with miR-29a mimics or NC, and A529 was transfected
with miR-29a inhibitor or NC inhibitor. Following transfection for
48 h, the cell transfection efficiency was assessed using RT-qPCR.
As presented in Fig. 2A, miR-29a was
significantly upregulated in H522 cells transfected with miR-29a
mimics, whereas miR-29a was downregulated in A549 cells transfected
with miR-29a inhibitor (P<0.05).
The effect of miR-29a on cell proliferation was
measured using a CCK-8 assay. The cell proliferation assay
demonstrated that miR-29a mimics inhibited H522 cell proliferation
and miR-29a inhibitor enhanced A549 cell proliferation (P<0.05;
Fig. 2B). These results indicate that
miR-29a inhibits cell proliferation in vitro.
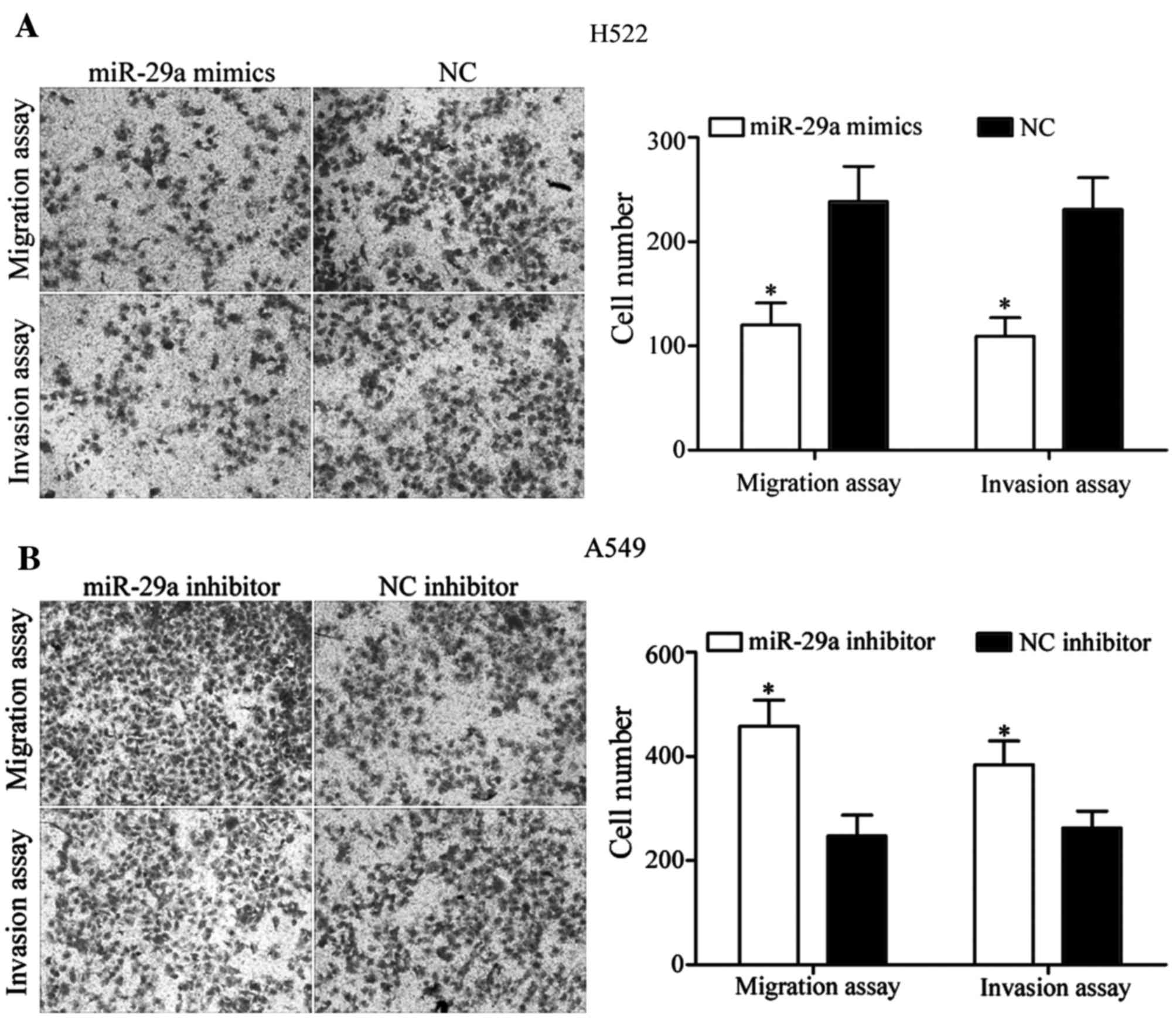
miR-29a inhibits NSCLC cell migration
and invasion
In order to determine whether miR-29a was able to
regulate NSCLC cell migration and invasion, cell migration and
invasion assays were performed using Transwell chambers.
miR-29a mimics suppressed the cell migratory and
invasive ability of H522 cells (P<0.05; Fig. 3A). Furthermore, miR-29a inhibitor
increased the cell migratory and invasive ability of A549 cells
(P<0.05; Fig. 3B). These results
indicate that miR-29a was able to inhibit NSCLC cell migration and
invasion ability in vitro.
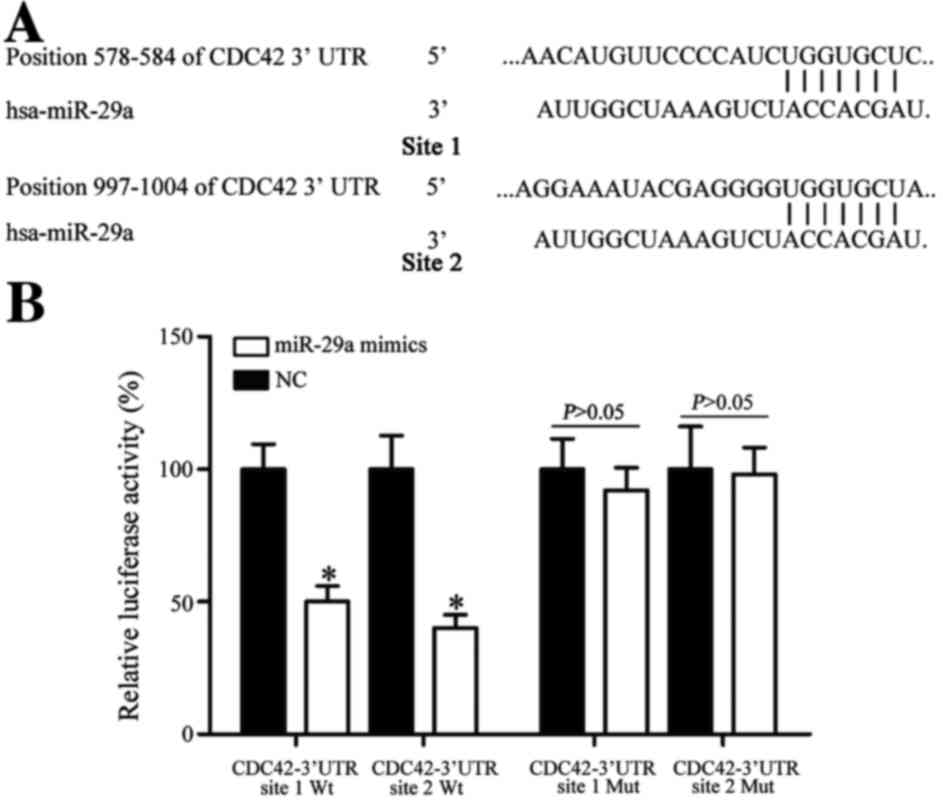
CDC42 is a direct target gene of
miR-29a in vitro
To investigate the molecular mechanism of miR-29a on
NSCLC carcinogenesis, bioinformatics analysis was performed.
TargetScan (www.targetscan.org) and miRanda
(www.microrna.org/microrna) predicted
that CDC42 was a direct target gene of miR-29a (Fig. 4A).
To investigate whether CDC42 was a direct target of
miR-29a, a dual-luciferase reporter assay was used. The results
demonstrated that miR-29a led to a significant decrease in
pGL3-CDC42-3′UTR site 1 Wt and pGL3-CDC42-3′UTR site 2 Wt
luciferase activity in HEK293T cells (P<0.05; Fig. 4B). Mutation of miR-29a-binding sites
(site 1 Mut or site 2 Mut) restored wild-type luciferase activity
of CDC42-3′UTR in HEK293T cells. These results indicate that CDC42
is a direct target gene of miR-29a.
miR-29a regulates CDC42 expression at
the post-transcriptional level
To explore the regulatory effect of miR-29a on
CDC42, RT-qPCR and western blotting were performed to detect the
expression of CDC42 at the mRNA and protein level in response to
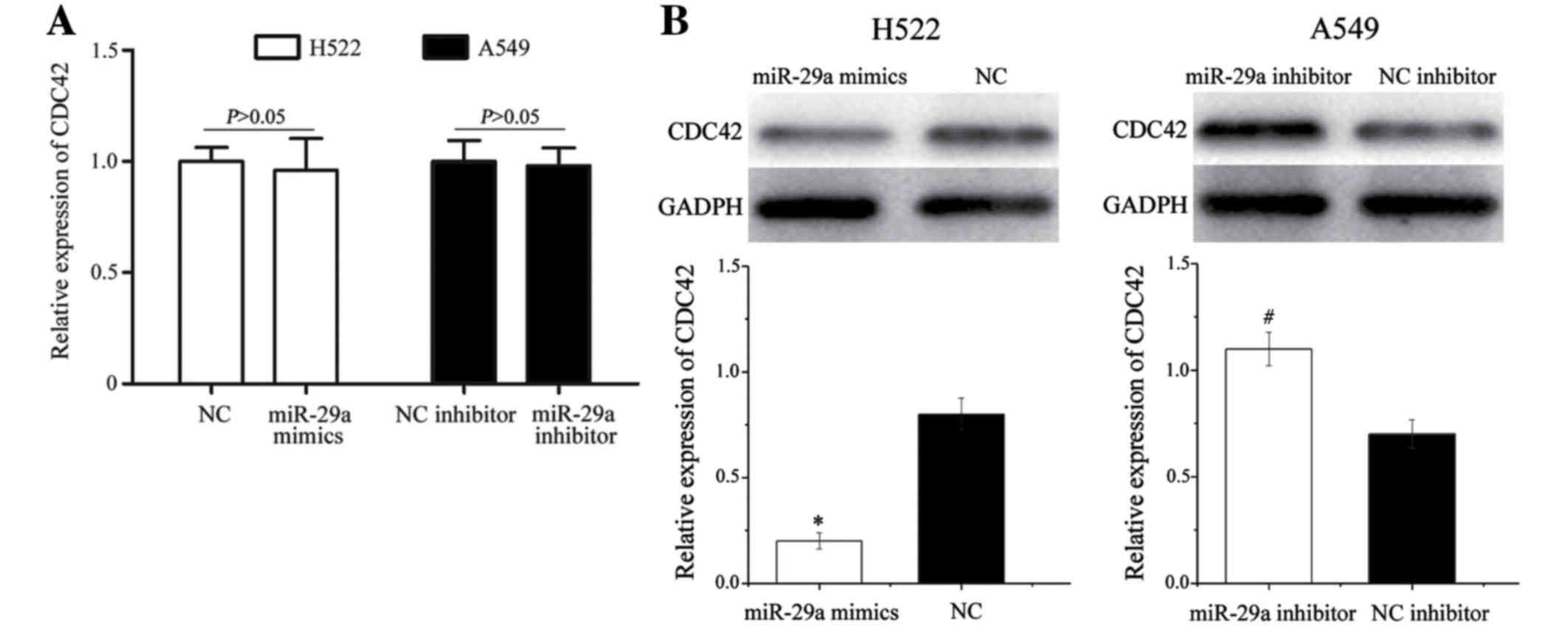
the alterations in miR-29a levels. CDC42 mRNA levels were not
significantly altered during these treatments (P>0.05; Fig. 5A). However, western blot analysis
revealed that CDC42 protein expression was significantly
downregulated in H522 cells transfected with miR-29a mimics,
whereas miR-29a inhibitor significantly increased CDC42 protein
expression in A549 cells (P<0.05; Fig.
5B). These results indicate that miR-29a negatively regulates
CDC42 expression at the post-transcriptional level.
CDC42 is involved in miR29a-induced
effects in NSCLC cells
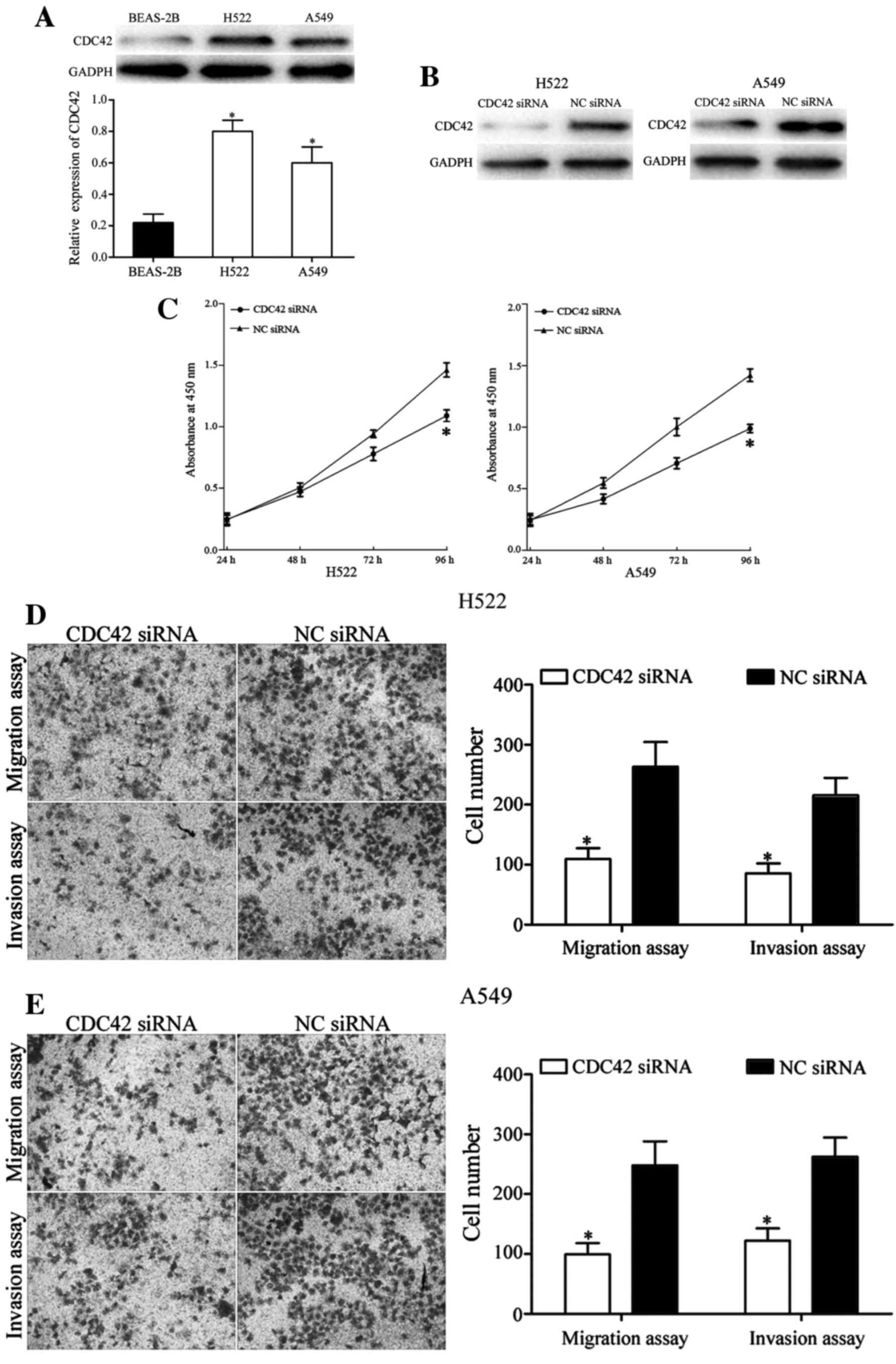
The endogenous expression of CDC42 was detected in
H522, A549 and BEAS-2B cells. Western blot analysis demonstrated
that the expression level of CDC42 was significantly upregulated in
H522 and A549 cells compared with BEAS-2B cells (P<0.05;
Fig. 6A). These results are in
accordance with the downregulation of miR-29a in NSCLC.
To explore further whether the functional effects of
miR-29a on NSCLC cell lines was exerted via CDC42, functional
assays were performed in H522 and A549 cells following transfection
with CDC42 siRNA or NC siRNA. The transfection efficiency was
measured using western blot analysis. The expression level of CDC42
was markedly decreased in H522 and A549 cells transfected with
CDC42 siRNA compared with the NC siRNA groups (Fig. 6B).
The CCK-8 assay demonstrated that silencing of CDC42
decreased cell proliferation in H522 and A549 cells (P<0.05;
Fig. 6C). Furthermore, the cell
migration and invasion assays revealed that knockdown of CDC42 in
H522 and A549 cells significantly suppressed cell migration and
invasion ability, in comparison with the NC siRNA-transfected cells
(P<0.05; Fig. 6D and E). These
results indicate that the roles of CDC42 siRNA are similar to the
functions exerted by miR-29a in NSCLC cells, indicating that CDC42
is a functional target of miR-29a in vitro.
Discussion
miR-29a is a member of the miR-29 family, which
includes miR-29a, miR-29b-1, miR-29b-2 and miR-29c. These miRNAs
are encoded in two clusters: miR-29b-1 and miR-29a in 7q32, and
miR-29b-2 and miR-29c in 1q32 (26,27).
miR-29a has been demonstrated to be downregulated in various types
of cancer, including prostate cancer (28), gastric cancer (29), esophageal carcinoma (30), acute myeloid leukemia (31) and hepatocellular carcinoma (32). However, miR-29a was identified to be
upregulated in colorectal cancer (33). These conflicting studies indicated
that the expression of miR-29a in cancer has tissue-specificity. In
the present study, it was demonstrated that the expression level of
miR-29a was decreased in NSCLC tissues and cell lines. In addition,
the decreased expression level of miR-29a was significantly
associated with advanced TNM classification and metastasis. These
results indicated that miR-29a may have a tumor-suppressive
capacity in the carcinogenesis and progression of NSCLC.
Abnormal expression of miR-29a was demonstrated to
be involved in the malignant phenotype of cancer. For example, in
prostate cancer, upregulated miR-29a significantly inhibited cell
proliferation and enhanced cell apoptosis by directly targeting
histone lysine demethylase 5B (28).
In renal cell carcinoma, miR-29a expression was decreased, and
functioned as a cell migration and invasion suppressor by targeting
lysine oxidase homolog 2 (34).
Tréhoux et al (35)
demonstrated that miR-29a suppressed cell proliferation, migration
and invasion, and sensitized cells to gemcitabine by directly
targeting mucin 1 in pancreatic cancer. In gastric cancer, enforced
expression of miR-29a significantly reduced cell migration and
invasion ability via blockade of roundabout guidance receptor 1
(29). In addition, miR-29 inhibited
gastric cancer cell proliferation and would healing by
downregulation of cyclin-dependent kinase (CDK) 2, CDK4 and CDK6
(36). In oral squamous cell
carcinoma, miR-29a negatively regulated matrix metalloproteinase 2
(MMP2) expression to inhibit cell invasion and enhance apoptosis
(37). However, in colorectal cancer,
miR-29a promoted cell migration and invasion via targeting
Krüppel-like factor 4 to regulate expression of MMP2 and epithelial
cadherin (33). These conflicting
studies suggest that the functions of miR-29a were variable in
tumors and exhibited tissue-specificity. These results also
indicate that miR-29a may serve important roles in these types of
cancer and may function as a potential therapeutic target gene.
In the present study, it was demonstrated that
miR-29a inhibited NSCLC cell proliferation, migration and invasion.
Since miR-29a is involved in NSCLC carcinogenesis and progression,
the potential molecular mechanism contributing to miR-29a-induced
inhibition of NSCLC proliferation, migration and invasion was
investigated. In the present study, an important molecular link
between miR-29a and CDC42 was determined in NSCLC. TargetScan and
miRanda predicted that CDC42 was a direct target gene of miR-29a.
Two putative miR-29a-binding sites were identified covering
nucleotides 578–584 and 997–1004 of the CDC42 3′UTR. A
dual-luciferase reporter assay demonstrated that miR-29a was
directly targeted to the CDC42 3′UTR. RT-qPCR and western blot
analysis demonstrated that miR-29a negatively regulated CDC42
expression at the post-transcriptional level. CDC42 was verified to
be upregulated in NSCLC cell lines, in accordance with the
downregulation of miR-29a. Knockdown of CDC42 also inhibited NSCLC
cell proliferation, migration and invasion. These results suggest
that miR-29a targets CDC42 to suppress NSCLC cell proliferation,
migration and invasion. Identification of the target genes of
miR-29a is important to understand its role in the initiation and
progression of NSCLC, and also for exploring new targeted therapies
for NSCLC.
CDC42, a member of the Rho family of GTPases, is
located at 1p36.1 and encodes a 25-kDa protein (38). It was initially identified in
Saccharomyces cerevisiae as a cell-cycle mutant that
contributed to the regulation of budding and mating projection
(39). Previous studies have
demonstrated that CDC42 is involved in cell proliferation, cell
cycle progression, cytoskeletal remodeling, migration and invasion
(40–43). Consistent with its important functions
in these distinct physiological and pathological processes,
abnormal expression of CDC42 has been identified in numerous
diseases, particularly in human cancer. CDC42 has been identified
to be upregulated in a number of human cancers, including lung
cancer (44). Therefore, it may be
advantageous to investigate novel targeted therapy against CDC42 in
lung cancer. In the present study, it was identified that miR-29a
negatively regulated CDC42 to inhibit NSCLC cell proliferation,
migration and invasion. Therefore, miR-29a may be investigated as a
targeted therapy for NSCLC.
CDC42 has been identified to be regulated by
multiple miRNAs in numerous types of cancer. For example, in
colorectal cancer, miR-137 decreases cell invasion and increases
cell apoptosis by targeting CDC42 (45). In addition, miR-18a targets CDC42 to
function as a tumor suppressor (46).
Furthermore, miR-224 inhibits cell proliferation and invasion via
blockade of CDC42 (47). In
esophageal squamous cell carcinoma, miR-195 suppressed cell
proliferation and invasion via targeting CDC42 (48). In hepatocellular carcinoma, miR-224
promotes cell proliferation, migration and invasion, and inhibited
apoptosis by regulating CDC42 expression (49). In gastric cancer, CDC42 was
demonstrated to be a direct gene of miR-137 (50). In NSCLC, miR-25 and miR-137 were
demonstrated, by targeting CDC42, to be important regulators in
initiation and progression and NSCLC (51,52). To
the best of our knowledge, the present study demonstrates for the
first time that miR-29a serves a suppressive role in NSCLC
proliferation and motility by directly targeting CDC42.
miR-29a/CDC42-based targeted therapy may be a novel treatment for
NSCLC.
In conclusion, the results of the present study
revealed that miR-29a was significantly downregulated in NSCLC and
that decreased expression of miR-29a was associated with advanced
TNM classification and metastasis. In addition, inhibition of
miR-29a and knockdown of CDC42 decreased NSCLC cell proliferation,
migration and invasion. Furthermore, miR-29a negatively regulated
the expression of CDC42 at the post-transcriptional level. These
results indicate that miR-29a targets CDC42 to inhibit NSCLC
carcinogenesis and progression. Therefore, miR-29a may be
investigated as a targeted therapy for NSCLC. Further study is
required to address the potential of miR-29a as an NSCLC
therapy.
References
|
1
|
Ilic N, Petricevic A, Arar D, Kotarac S,
Banovic J, Ilic NF, Tripkovic A and Grandic L: Skip mediastinal
nodal metastases in the IIIa/N2 non-small cell lung cancer. J
Thorac Oncol. 2:1018–1021. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Boffetta P and Nyberg F: Contribution of
environmental factors to cancer risk. Br Med Bull. 68:71–94. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Didkowska J, Manczuk M, McNeill A, Powles
J and Zatonski W: Lung cancer mortality at ages 35–54 in the
European Union: Ecological study of evolving tobacco epidemics.
BMJ. 331:189–191. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ridge CA, McErlean AM and Ginsberg MS:
Epidemiology of lung cancer. Semin Intervent Radiol. 30:93–98.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Paliogiannis P, Attene F, Cossu A, Budroni
M, Cesaraccio R, Tanda F, Trignano M and Palmieri G: Lung cancer
epidemiology in North Sardinia, Italy. Multidiscip Respir Med.
8:452013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ni T, Mao G, Xue Q, Liu Y, Chen B, Cui X,
Lv L, Jia L, Wang Y and Ji L: Upregulated expression of ILF2 in
non-small cell lung cancer is associated with tumor cell
proliferation and poor prognosis. J Mol Histol. 46:325–335. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Peters S, Adjei AA, Gridelli C, Reck M,
Kerr K and Felip E: ESMO Guidelines Working Group: Metastatic
non-small-cell lung cancer (NSCLC): ESMO clinical practice
guidelines for diagnosis, treatment and follow-up. Ann Oncol.
23:(Suppl 7). vii56–vii64. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang T, Zhang DM, Zhao D, Hou XM, Liu XJ,
Ling XL and Ma SC: The prognostic value of osteopontin expression
in non-small cell lung cancer: A meta-analysis. J Mol Histol.
45:533–540. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cai J, Fang L, Huang Y, Li R, Yuan J, Yang
Y, Zhu X, Chen B, Wu J and Li M: miR-205 targets PTEN and PHLPP2 to
augment AKT signaling and drive malignant phenotypes in non-small
cell lung cancer. Cancer Res. 73:5402–5415. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pisters KM, Evans WK, Azzoli CG, Kris MG,
Smith CA, Desch CE, Somerfield MR, Brouwers MC, Darling G, Ellis
PM, et al: Cancer care ontario and American society of clinical
oncology adjuvant chemotherapy and adjuvant radiation therapy for
stages I-IIIA resectable non small-cell lung cancer guideline. J
Clin Oncol. 25:5506–5518. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Verdecchia A, Francisci S, Brenner H,
Gatta G, Micheli A, Mangone L and Kunkler I: EUROCARE-4 Working
Group: Recent cancer survival in Europe: A 2000–02 period analysis
of EUROCARE-4 data. Lancet Oncol. 8:784–796. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sun W, Yuan X, Tian Y, Wu H, Xu H, Hu G
and Wu K: Non-invasive approaches to monitor EGFR-TKI treatment in
non-small-cell lung cancer. J Hematol Oncol. 8:952015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu R, Liu X, Zheng Y, Gu J, Xiong S,
Jiang P, Jiang X, Huang E, Yang Y, Ge D and Chu Y: MicroRNA-7
sensitizes non-small cell lung cancer cells to paclitaxel. Oncol
Lett. 8:2193–2200. 2014.PubMed/NCBI
|
|
16
|
Ma Y, Li X, Cheng S, Wei W and Li Y:
MicroRNA-106a confers cisplatin resistance in non-small cell lung
cancer A549 cells by targeting adenosine triphosphatase-binding
cassette A1. Mol Med Rep. 11:625–632. 2015.PubMed/NCBI
|
|
17
|
Zhong Z, Xia Y, Wang P, Liu B and Chen Y:
Low expression of microRNA-30c promotes invasion by inducing
epithelial mesenchymal transition in non-small cell lung cancer.
Mol Med Rep. 10:2575–2579. 2014.PubMed/NCBI
|
|
18
|
Shi Y, Liu C, Liu X, Tang DG and Wang J:
The microRNA miR-34a inhibits non-small cell lung cancer (NSCLC)
growth and the CD44hi stem-like NSCLC cells. PLoS One.
9:e900222014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li X, Abdel-Mageed AB, Mondal D and Kandil
E: MicroRNA expression profiles in differentiated thyroid cancer, a
review. Int J Clin Exp Med. 6:74–80. 2013.PubMed/NCBI
|
|
20
|
Hwang HW and Mendell JT: MicroRNAs in cell
proliferation, cell death, and tumorigenesis. Br J Cancer.
96:(Suppl). R40–R44. 2007.PubMed/NCBI
|
|
21
|
Tafsiri E, Darbouy M, Shadmehr MB,
Zagryazhskaya A, Alizadeh J and Karimipoor M: Expression of miRNAs
in non-small-cell lung carcinomas and their association with
clinicopathological features. Tumour Biol. 36:1603–1612. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Braun J and Hüttelmaier S: Pathogenic
mechanisms of deregulated microRNA expression in thyroid carcinomas
of follicular origin. Thyroid Res. 4:(Suppl 1). S12011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang B, Pan X, Cobb GP and Anderson TA:
microRNAs as oncogenes and tumor suppressors. Dev Biol. 302:1–12.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Esquela-Kerscher A and Slack FJ:
Oncomirs-microRNAs with a role in cancer. Nat Rev Cancer.
6:259–269. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kriegel AJ, Liu Y, Fang Y, Ding X and
Liang M: The miR-29 family: Genomics, cell biology, and relevance
to renal and cardiovascular injury. Physiol Genomics. 44:237–244.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang Y, Zhang X, Li H, Yu J and Ren X: The
role of miRNA-29 family in cancer. Eur J Cell Biol. 92:123–128.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li J, Wan X, Qiang W, Li T, Huang W, Huang
S, Wu D and Li Y: MiR-29a suppresses prostate cell proliferation
and induces apoptosis via KDM5B protein regulation. Int J Clin Exp
Med. 8:5329–5339. 2015.PubMed/NCBI
|
|
29
|
Liu X, Cai J, Sun Y, Gong R, Sun D, Zhong
X, Jiang S, He X, Bao E, Yang L and Li Y: MicroRNA-29a inhibits
cell migration and invasion via targeting Roundabout homolog 1 in
gastric cancer cells. Mol Med Rep. 12:3944–3950. 2015.PubMed/NCBI
|
|
30
|
Liu C, Duan P, Li B, Huang C, Jing Y and
Yan W: miR-29a activates Hes1 by targeting Nfia in esophageal
carcinoma cell line TE-1. Oncol Lett. 9:96–102. 2015.PubMed/NCBI
|
|
31
|
Wang F, Wang XS, Yang GH, Zhai PF, Xiao Z,
Xia LY, Chen LR, Wang Y, Wang XZ, Bi LX, et al: miR-29a and
miR-142-3p downregulation and diagnostic implication in human acute
myeloid leukemia. Mol Biol Rep. 39:2713–2722. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhu XC, Dong QZ, Zhang XF, Deng B, Jia HL,
Ye QH, Qin LX and Wu XZ: microRNA-29a suppresses cell proliferation
by targeting SPARC in hepatocellular carcinoma. Int J Mol Med.
30:1321–1326. 2012.PubMed/NCBI
|
|
33
|
Tang W, Zhu Y, Gao J, Fu J, Liu C, Liu Y,
Song C, Zhu S, Leng Y, Wang G, et al: MicroRNA-29a promotes
colorectal cancer metastasis by regulating matrix metalloproteinase
2 and E-cadherin via KLF4. Br J Cancer. 110:450–458. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Nishikawa R, Chiyomaru T, Enokida H,
Inoguchi S, Ishihara T, Matsushita R, Goto Y, Fukumoto I, Nakagawa
M and Seki N: Tumour-suppressive microRNA-29s directly regulate
LOXL2 expression and inhibit cancer cell migration and invasion in
renal cell carcinoma. FEBS Lett. 589:2136–2145. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tréhoux S, Lahdaoui F, Delpu Y, Renaud F,
Leteurtre E, Torrisani J, Jonckheere N and Van Seuningen I:
Micro-RNAs miR-29a and miR-330-5p function as tumor suppressors by
targeting the MUC1 mucin in pancreatic cancer cells. Biochim
Biophys Acta. 1853:2392–2403. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhao Z, Wang L, Song W, Cui H, Chen G,
Qiao F, Hu J, Zhou R and Fan H: Reduced miR-29a-3p expression is
linked to the cell proliferation and cell migration in gastric
cancer. World J Surg Oncol. 13:1012015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lu L, Xue X, Lan J, Gao Y, Xiong Z, Zhang
H, Jiang W, Song W and Zhi Q: MicroRNA-29a upregulates MMP2 in oral
squamous cell carcinoma to promote cancer invasion and
anti-apoptosis. Biomed Pharmacother. 68:13–19. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ma J, Xue Y, Liu W, Yue C, Bi F, Xu J,
Zhang J, Li Y, Zhong C and Chen Y: Role of activated Rac1/Cdc42 in
mediating endothelial cell proliferation and tumor angiogenesis in
breast cancer. PLoS One. 8:e662752013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sun N, Ye L, Chang T and Li X and Li X:
microRNA-195-Cdc42 axis acts as a prognostic factor of esophageal
squamous cell carcinoma. Int J Clin Exp Pathol. 7:6871–6879.
2014.PubMed/NCBI
|
|
40
|
Reymond N, Im JH, Garg R, Vega FM, Borda
d'Agua B, Riou P, Cox S, Valderrama F, Muschel RJ and Ridley AJ:
Cdc42 promotes transendothelial migration of cancer cells through
β1 integrin. J Cell Biol. 199:653–668. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Stengel KR and Zheng Y: Essential role of
Cdc42 in Ras-induced transformation revealed by gene targeting.
PLoS One. 7:e373172012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Nobes CD and Hall A: Rho, rac and cdc42
GTPases regulate the assembly of multimolecular focal complexes
associated with actin stress fibers, lamellipodia, and filopodia.
Cell. 81:53–62. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Olson MF, Ashworth A and Hall A: An
essential role for Rho, Rac and Cdc42 GTPases in cell cycle
progression through G1. Science. 269:1270–1272. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Chen QY, Jiao DM, Yao QH, Yan J, Song J,
Chen FY, Lu GH and Zhou JY: Expression analysis of Cdc42 in lung
cancer and modulation of its expression by curcumin in lung cancer
cell lines. Int J Oncol. 40:1561–1568. 2012.PubMed/NCBI
|
|
45
|
Liu M, Lang N, Qiu M, Xu F, Li Q, Tang Q,
Chen J, Chen X, Zhang S, Liu Z, et al: miR-137 targets Cdc42
expression, induces cell cycle G1 arrest and inhibits invasion in
colorectal cancer cells. Int J Cancer. 128:1269–1279. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Humphreys KJ, McKinnon RA and Michael MZ:
miR-18a inhibits CDC42 and plays a tumour suppressor role in
colorectal cancer cells. PLoS One. 9:e1122882014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ke TW, Hsu HL, Wu YH, Chen WT, Cheng YW
and Cheng CW: MicroRNA-224 suppresses colorectal cancer cell
migration by targeting Cdc42. Dis Markers. 2014:6171502014.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Fu MG, Li S, Yu TT, Qian LJ, Cao RS, Zhu
H, Xiao B, Jiao CH, Tang NN, Ma JJ, et al: Differential expression
of miR-195 in esophageal squamous cell carcinoma and miR-195
expression inhibits tumor cell proliferation and invasion by
targeting of Cdc42. FEBS Lett. 587:3471–3479. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Zhang Y, Takahashi S, Tasaka A, Yoshima T,
Ochi H and Chayama K: Involvement of microRNA-224 in cell
proliferation, migration, invasion, and anti-apoptosis in
hepatocellular carcinoma. J Gastroenterol Hepatol. 28:565–575.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Chen Q, Chen X, Zhang M, Fan Q, Luo S and
Cao X: miR-137 is frequently down-regulated in gastric cancer and
is a negative regulator of Cdc42. Dig Dis Sci. 56:2009–2016. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Yang T, Chen T, Li Y, Gao L, Zhang S, Wang
T and Chen M: Downregulation of miR-25 modulates non-small cell
lung cancer cells by targeting CDC42. Tumour Biol. 36:1903–1911.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Zhu X, Li Y, Shen H, Li H, Long L, Hui L
and Xu W: miR-137 inhibits the proliferation of lung cancer cells
by targeting Cdc42 and Cdk6. FEBS Lett. 587:73–81. 2013. View Article : Google Scholar : PubMed/NCBI
|