Introduction
Endometrial cancer is a cancer of the endometrium
(the lining of the uterus), and is a common gynaecologic malignancy
encountered in developed countries and the second most common in
the developing world (1). The leading
treatment method for endometrial cancer is abdominal hysterectomy
(total surgical removal of the uterus), along with removal of the
fallopian tubes and ovaries on both sides, which is known as a
bilateral salpingo-oophorectomy (2).
If the disease is diagnosed at an early stage, the outcome is
favourable, and the overall five-year survival rate in the United
States is >80% (3). However, the
majority of cases of endometrial cancer are diagnosed at a later
stage and the outcome is poor, with a five-year survival rate of
<30% (3). Therefore, it is
critical to develop suitable chemotherapeutic regimens for
late-stage endometrial cancer. It is also important to demonstrate
the molecular mechanism underlying the effect of chemotherapeutic
drugs on endometrial cancer treatment.
Autophagy is a dynamic process involving the
rearrangement of subcellular membranes to sequester the cytoplasm
and organelles for delivery to the lysosome or vacuole, where the
sequestered cargo is degraded and recycled (4). Autophagy has been implicated in the
pathogenesis of cancer, and is commonly referred to as a
‘double-edged sword’ for its role in tumour progression and tumour
suppression (5). Previous studies
have revealed that chemotherapy drugs may regulate autophagy in the
cells of a number of cancer subtypes (6–8). Cisplatin
(CDDP) is the first-line chemotherapeutic drug for endometrial
cancer chemotherapy (9). CDDP-induced
autophagy has been reported in numerous types of cancer cells,
including hepatocellular carcinoma (10), laryngeal cancer (11) and lung adenocarcinoma (12), though the association between CDDP and
autophagy varied between types of cancer cell. Treatment with CDDP
was demonstrated to activate autophagy in hepatocellular carcinoma
and lung adenocarcinoma; however, it suppressed autophagy in
laryngeal cancer (10–12). The present study investigated the
association between CDDP and autophagy in endometrial cancer.
The activation of phosphoinositide 3-kinase (PI3K)
or mammalian target of rapamycin (mTOR) are two of the most common
events in the development of human cancer, including endometrial
cancer (5,13). Alteration of the PI3K/protein kinase B
(AKT)/mTOR signalling pathway is implicated in endometrial cancer
pathogenesis (14). Previous studies
have demonstrated that the PI3K/AKT/mTOR signalling pathway may
repress autophagy in response to insulin-like and other growth
factor signals (15,16). However, it is not clear whether CDDP
regulates cell autophagy in endometrial cancer cells by the
PI3K/AKT/mTOR signalling pathway. In the present study, it was
considered whether CDDP regulated cell autophagy in the endometrial
cancer cell line Ishikawa via the PI3K/AKT/mTOR signalling pathway,
a hypothesis that was confirmed by the results.
Materials and methods
Materials and reagents
The Ishikawa human endometrial cancer cell line was
purchased from the American Type Culture Collection (ATCC;
Manassas, VA, USA). RPMI-1640 medium, fetal bovine serum (FBS),
PBS, sodium pyruvate, trypsin and antibiotics were purchased from
HyClone (GE Healthcare Life Sciences, Logan, UT, USA). CellTiter
96® AQueous One Solution Cell Proliferation Assay kit
was purchased from Promega Corp. (Madison, WI, USA). Polyvinylidene
fluoride (PVDF) membrane was purchased from EMD Millipore
(Billerica, MA, USA). Molecular weight markers were purchased from
Fermentas (Thermo Fisher Scientific, Inc., Pittsburgh, PA, USA).
Bicinchoninic acid (BCA) protein assay kit and
radioimmunoprecipitation assay (RIPA) buffer were obtained from
Beyotime Institute of Biotechnology (Shanghai, China). Freeze-dried
CDDP powder was purchased from Qilu Pharmaceutical Co., Ltd.
(Jinan, China). Mouse monoclonal anti-PI3K p85 antibody (ab40776),
mouse monoclonal anti-AKT1 antibody (ab89402), rabbit polyclonal
anti-AKT1 (phospho-S473) antibody (ab66138), rabbit monoclonal
anti-mTOR antibody (ab32028), rabbit monoclonal anti-mTOR
(phospho-S2448) antibody (ab109268), rabbit polyclonal
anti-microtubule-associated protein 1 light chain 3α (LC3) B
antibody (ab63817) and rabbit polyclonal anti-GAPDH antibody
(ab9485) were all purchased from Abcam (Cambridge, UK). Enhanced
chemiluminescence (ECL) reagent was obtained from EMD Millipore.
Alexa Fluor 488-conjugated donkey anti-rabbit immunoglobulin (Ig)G
secondary antibodies were provided by Santa Cruz Biotechnology,
Inc. (Dallas, TX, USA) (sc-3895). Horseradish peroxidase-conjugated
goat anti-rabbit IgG was purchased from Boster Biological
Technology (BA1055; Wuhan, China). Insulin-like growth factor-1
(IGF-1) was purchased from Sino Biological, Inc. (Beijing,
China).
Cell culture and CDDP treatment
Ishikawa cells were maintained on culture dishes in
90% (v/v) RPMI-1640 medium supplemented with 2 mM L-glutamine and
1.5 g/l sodium bicarbonate with 10% (v/v) FBS. The cells were
cultured in an atmosphere containing 5% CO2 at 37°C in
an incubator. A stock solution of CDDP was prepared in dimethyl
sulfoxide (DMSO) at 1 mg/ml and was further diluted to the final
working concentrations (10, 20, 40 and 80 µg/ml) with
antibiotic-free RPMI-1640 medium.
Cell proliferation assays
Cell proliferation was evaluated using the CellTiter
96® AQueous One Solution Cell Proliferation Assay kit,
according to the manufacturer's protocol. Ishikawa cells
(1×104 cells) were seeded onto 96-well plates. Following
4 h of incubation, 10 µl CDDP in 1% DMSO was added to the wells at
10, 20, 40 and 80 µg/ml, whereas 10 µl 1% DMSO was used as the
negative control. Briefly, following CDDP treatment for 0, 6, 12,
24 and 48 h, 20 µl CellTiter 96® AQueous One Solution
reagents were added to each well and incubated for 4 h at 37°C. The
absorbance was determined at 490 nm using a Multiskan MK3
microplate reader (Thermo Fisher Scientific, Inc., Waltham, MA,
USA). The experiments were repeated three times, and the rate of
cell proliferation inhibition was evaluated using the following
formula: Inhibition rate (%) = 1 - (mean absorbance of treated
cells/mean absorbance of the negative control) × 100.
Transmission electron microscopy
The autophagy of Ishikawa cells was evaluated by
autophagosome screening under a JEM-1010 transmission electron
microscope (Matsunaga Manufacturing, Co., Ltd., Gifu, Japan). At
the end of the intervention, the cells were digested with 0.25%
trypsin and collected in centrifuge tubes, followed by
centrifugation at 100 × g for 10 min, then 80 × g for 10 min, at
4°C. The supernatant was discarded, and 2.5% glutaraldehyde was
added to the tube to fix the cells. Following a 2-h period of
fixation, dehydration, and embedding, ultra-thin sections (70 nm)
were prepared using a microtome and each section was mounted on a
copper grid. Samples were contrasted with 4% aqueous uranyl acetate
(10 min) and then Reynolds lead citrate (2 min). The autophagosomes
were observed under a transmission electron microscope and
imaged.
Western blot analysis
Each group of Ishikawa cells was washed twice with
ice-cold PBS, and resuspended in ice-cold RIPA buffer supplemented
with 1 mmol/l phenylmethanesulfonyl fluoride and a cocktail of
protease inhibitors (dilution, 1:100; Beyotime Institute of
Biotechnology, Nantong, China). Samples were centrifuged at 4°C for
20 min at 800 × g. Supernatants were recovered and total protein
was quantified using the aforementioned BCA protein assay kit.
Equal amounts of protein were loaded and separated on 10%
SDS-polyacrylamide gels and transferred to PVDF membranes. The
membranes were blocked for 1 h at room temperature with 5% milk in
TBS supplemented with 0.05% Tween-20 (TBST), incubated for 1 h with
the corresponding primary antibody [anti-PI3K p85, dilution,
1:5,000; anti-phosphorylated (p)-AKT1, dilution, 1:2,000;
anti-total (t)-AKT1, dilution, 1:1,000; anti-p-mTOR, dilution,
1:2,000; anti-t-mTOR, dilution, 1:5,000; and anti-GAPDH, dilution
1:5,000], washed three times with TBST, incubated with horseradish
peroxidase-conjugated goat anti-rabbit IgG (dilution, 1:20,000; 40
min at 37°C), washed three times with TBST and visualized using the
aforementioned ECL reagent. GAPDH served as an internal loading
control.
Immunofluorescence microscopy
Ishikawa cells treated with 0, 10, 20, 40 or 80
µg/ml were grown on coverslips. After 0, 12 or 24 h, the cells were
washed with PBS and fixed for 20 min with 4% paraformaldehyde at
room temperature. The cells were then permeabilized for 5 min in
0.2% Triton X-100 and washed with PBS. In order to block
non-specific background staining, the samples were incubated at
37°C in a solution containing 10% normal goat serum (Boster
Biological Technology) for 30 min. Upon washing with PBS, the cells
were incubated for 1 h at room temperature with primary rabbit
polyclonal anti-LC3B antibody diluted in PBS supplemented with 1%
bovine serum albumin (Boster Biological Technology). The cells were
then rinsed with PBS and incubated with Alexa Fluor 488-conjugated
donkey anti-rabbit IgG secondary antibodies for 60 min at room
temperature. The nuclei were counterstained with DAPI for 5 min at
room temperature. Upon washing with PBS, the stained cells were
mounted with a fluorescent-mounting medium (Dako; Agilent
Technologies, Inc., Santa Clara, CA, USA) and examined using a TCS
SP2 AOBS confocal laser scanning microscope (Leica Microsystems
GmbH, Wetzlar, Germany).
Statistical analysis
All results are presented as the mean ± standard
deviation, and were analysed using SPSS version 19.0 software (IBM
SPSS, Armonk, NY, USA). A one-way analysis of variance was used to
determine statistical significance. P<0.05 was considered to
indicate a statistically significant difference.
Results
Effect of CDDP treatment on Ishikawa
cell proliferation
Prior to investigating the effect of CDDP on
Ishikawa cell autophagy, the effect of CDDP on Ishikawa cell
proliferation was evaluated, and a suitable treatment concentration
and time were determined by the results of the cell proliferation
assays. Ishikawa cells were treated with 10, 20, 40 and 80 µg/ml
CDDP for 12, 24, 48 and 72 h. The cells were then harvested and
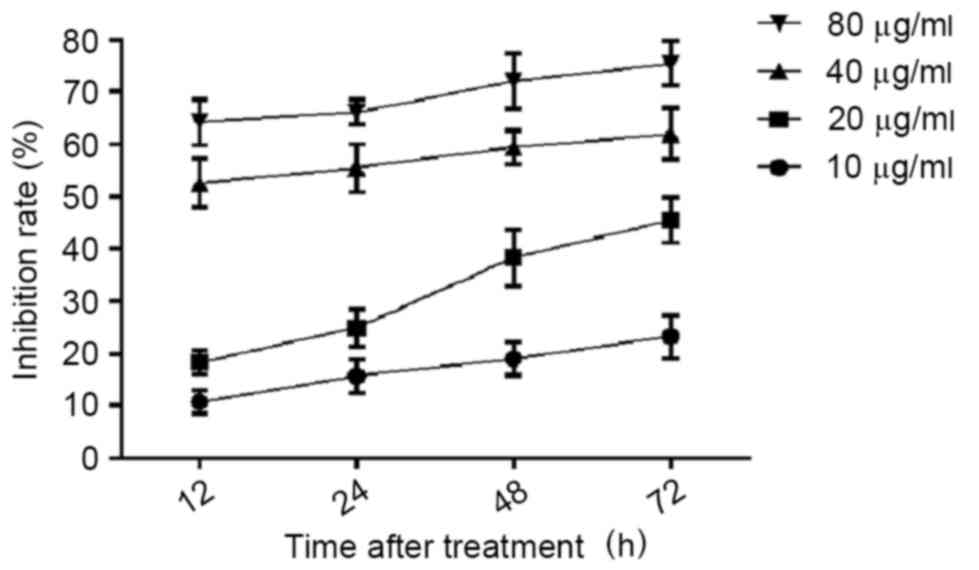
subjected to cell proliferation assays. As presented in Fig. 1, the proliferation inhibition of
Ishikawa cells treated with CDDP demonstrated dose- and
time-dependent results. For the 10 µg/ml CDDP treatment, the
proliferation inhibition rates were 18.27±2.25, 24.88±3.58,
38.27±5.34 and 45.43±4.25 following 12, 24, 48 and 72 h of
treatment, respectively. For the 20 µg/ml CDDP treatment, the
proliferation inhibition rate was ≤20% at all time-points. For the
40 and 80 µg/ml CDDP treatment, the proliferation inhibition rate
was <50% at all time points. Based on these results, 20 µg/ml
CDDP treatment for 12 or 24 h was selected as the concentration and
time periods for the subsequent assays.
Promotion of cell autophagy induced by
CDDP treatment in Ishikawa cells
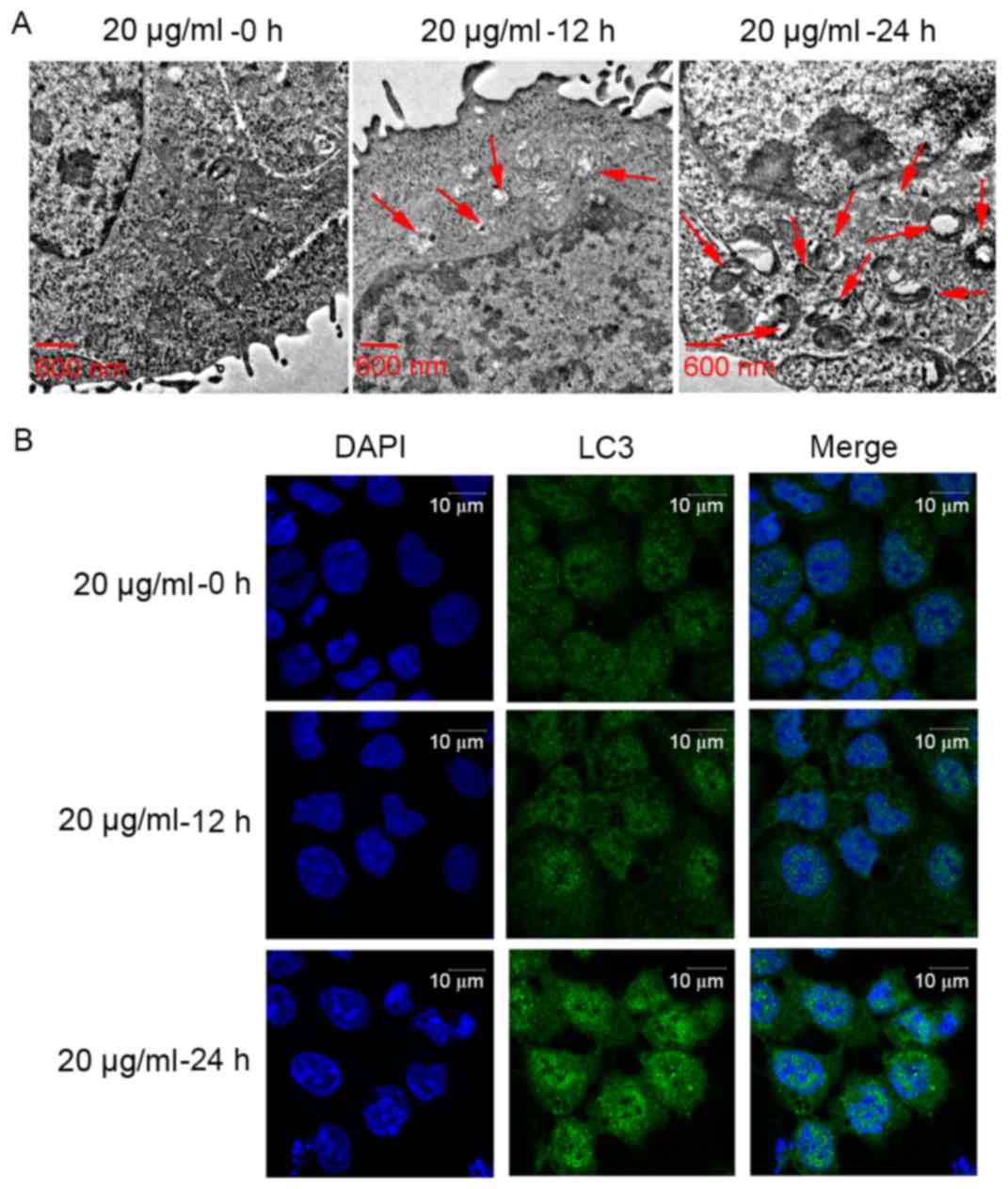
To detect the effect of CDDP on Ishikawa cell
autophagy, the formation of autophagosomes was observed using a
transmission electron microscope. As presented in Fig. 2A, the number of intracellular
autophagosomes following 20 µg/ml CDDP treatment for 12 h increased
compared with that of the control group (no treatment). In
addition, the number of intracellular autophagosomes following 20
µg/ml CDDP treatment for 24 h was higher than that following 20
µg/ml CDDP treatment for 12 h. Furthermore, in order to determine
the effect of CDDP treatment on cell autophagy, the expression
level of the autophagy-related gene (ATG) LC3 (17), was examined using immunofluorescence
microscopy. The LC3 protein expression levels following 20 µg/ml
CDDP treatment for 12 h were increased compared with those of the
group that did not receive treatment. The LC3 protein expression
level following 20 µg/ml CDDP treatment for 24 h was higher than
that following 20 µg/ml CDDP treatment for 12 h. In brief, CDDP
treatment may promote cell autophagy in a time-dependent manner in
Ishikawa cells. Based on these results, 20 µg/ml CDDP treatment for
24 h was selected as the concentration and time to be used for the
following assays.
Inactivation of the PI3K/AKT/mTOR
signalling pathway by CDDP treatment
To investigate the mechanism underlying the effect
of CDDP treatment on autophagy in Ishikawa cells, the protein
expression level of PI3K p85, p-AKT1, t-AKT1, p-mTOR and t-mTOR
were evaluated by the western blot analysis. As presented in
Fig. 3, the t-AKT1 and t-mTOR
expression levels remained constant; however, the expression levels
of p-Akt and p-mTOR were reduced in cells treated with 20 µg/ml
CDDP for 24 h. Reduction in the expression level of PI3K p85 was
also observed.
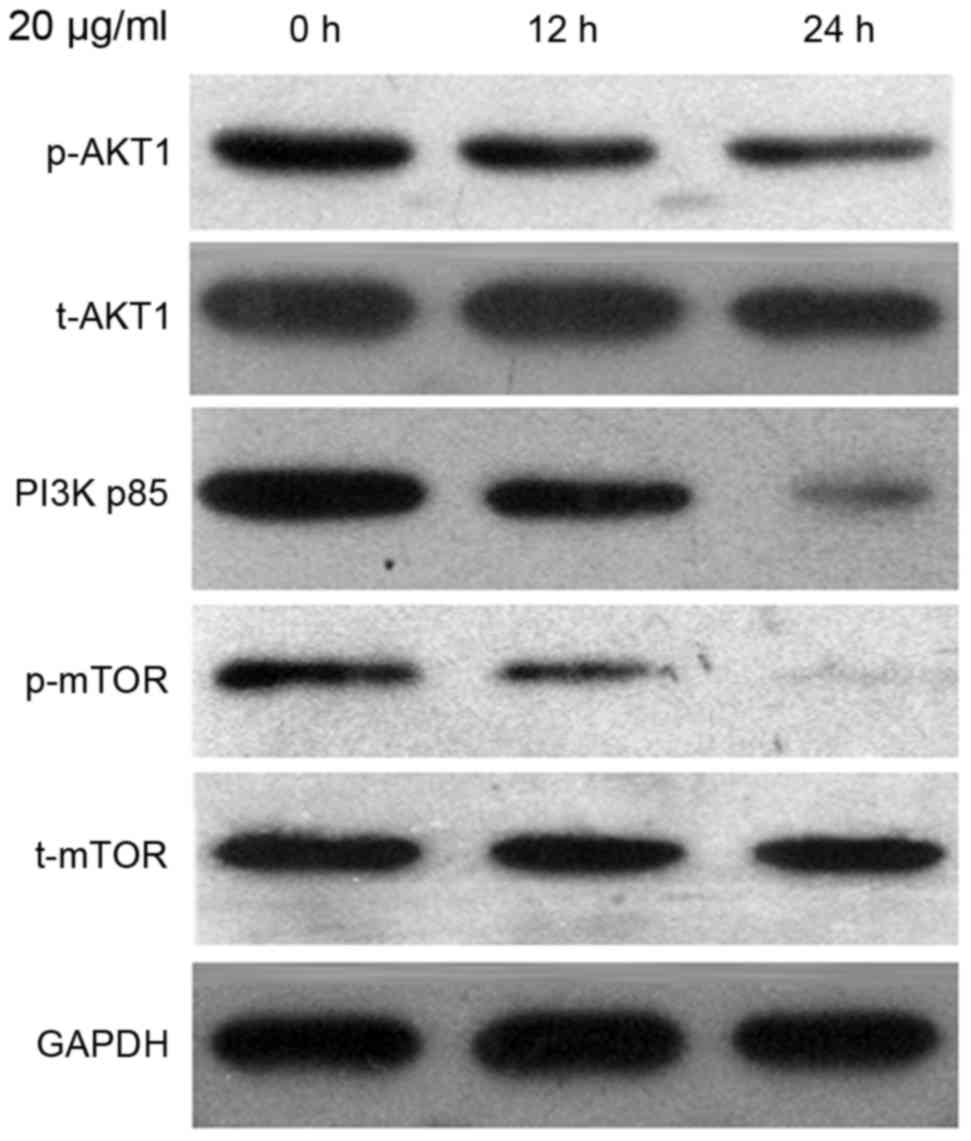 | Figure 3.The protein expression level of PI3K
p85, p-AKT1, t-AKT1, p-mTOR and t-mTOR were detected using western
blot analysis. Ishikawa cells were treated with 20 µg/ml CDDP for
12 and 24 h, followed by harvesting for western blot analysis.
PI3K, phosphoinositide 3-kinase; p-, phosphorylated; t-, total;
AKT, protein kinase B; mTOR, mammalian target of rapamycin; CDDP,
cisplatin. |
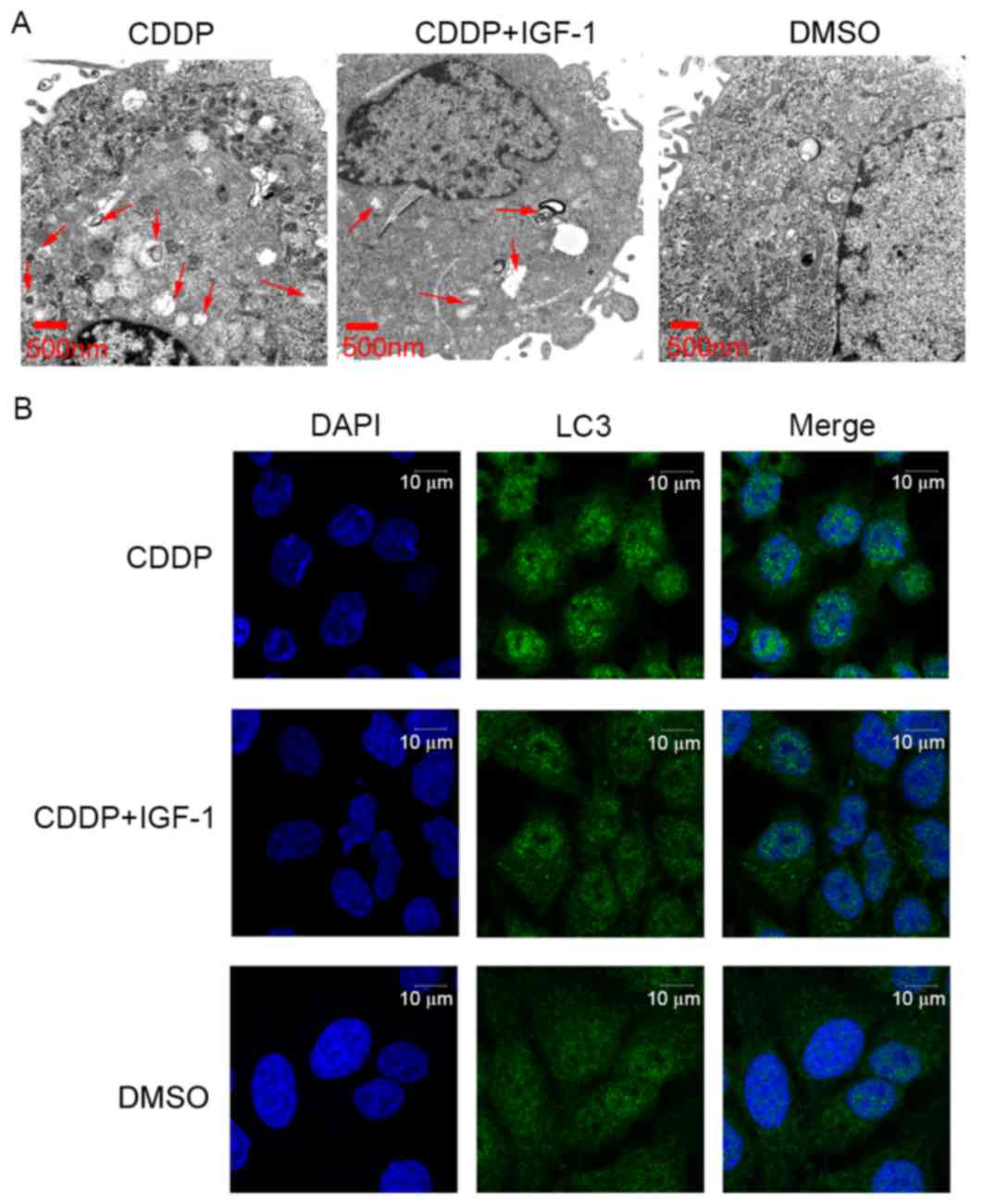
IGF-1 co-treatment reverses the effect
of CDDP on cell autophagy in Ishikawa cells
To further evaluate the effect of CDDP on cell
autophagy in Ishikawa cells via the PI3K/AKT/mTOR signalling
pathway, the cells were co-treated with a PI3K activator, IGF-1, at
100 ng/ml. As presented in Fig. 4,
the number of intracellular autophagosomes following treatment with
20 µg/ml CDDP and IGF-1 co-treatment for 24 h was decreased
compared with that in the 20 µg/ml CDDP treatment group. However,
the number of intracellular autophagosomes following 20 µg/ml CDDP
and IGF-1 co-treatment was higher than that in the cells treated
with DMSO alone. Furthermore, the expression level of the ATG LC3
was examined using immunofluorescence microscopy. The LC3 protein
expression levels following 20 µg/ml CDDP and IGF-1 co-treatment
were decreased compared with those in the 20 µg/ml CDDP treatment
group. However, the LC3 protein expression level following 20 µg/ml
CDDP and IGF-1 co-treatment was higher than that in the DMSO-only
group. In brief, IGF-1 co-treatment may partially reverse the
effect of CDDP on cell autophagy in Ishikawa cells.
Discussion
CDDP is a highly effective chemotherapeutic agent
used for the treatment of human solid tumours, including
endometrial cancer (18). However,
the cytotoxicity induced by CDDP is an important obstacle in its
utility and therapeutic profile. CDDP and other platinum-based
compounds are considered as cytotoxic drugs that kill cancer cells
by damaging the DNA, inhibiting DNA synthesis and mitosis, and
inducing apoptotic cell death (19).
CDDP chemotherapy is also associated with substantial side effects,
including hepatotoxic, nephrotoxic, cardiotoxic, neurotoxic and/or
hematotoxic damage (19). The
molecular mechanism underlying the cytotoxic effects induced by
CDDP was widely discussed in previous studies, and numerous
molecular mechanisms are explained, including the induction of
oxidative stress, which is characterized by reactive oxygen species
production and lipid peroxidation; induction of p53 signalling and
cell cycle arrest; downregulation of proto-oncogenes and
anti-apoptotic proteins; and activation of intrinsic and extrinsic
pathways of apoptosis (19–22). In previous studies, autophagy, a
highly regulated self-degradation process of eukaryotic cells, was
identified as one of the molecular mechanisms underlying CDDP
cytotoxicity in certain types of cancer cells and in normal cells
(10,23). Autophagy is a context-dependent
tumour-suppressing mechanism that can also promote tumour cell
survival under stress conditions and treatment resistance (24). The present study aimed to discuss the
association between autophagy and CDDP in endometrial cancer.
Therefore, the current results will aid in the explanation of
molecular mechanisms underlying CDDP cytotoxicity in endometrial
cancer.
The dynamic process of autophagy involves membrane
formation and fusion, including autophagosome formation,
autophagosome-lysosome fusion and the degradation of
intra-autophagosomal contents by lysosomal hydrolases (25). The present study used transmission
electron microscopy to detect the formation of intracellular
autophagosomes in Ishikawa cells following CDDP treatment. The
results revealed that CDDP treatment may increase the number of
intracellular autophagosomes in a time-dependent manner in Ishikawa
cells. To further determine the effect of CDDP treatment on cell
autophagy, the expression level of the ATG LC3 (15) was examined using immunofluorescence
microscopy. The results demonstrated that CDDP treatment may
upregulate LC3 protein expression level. Together, these results
revealed that CDDP treatment promoted cell autophagy in the
Ishikawa endometrial cancer cells. The results of the present study
are in accord with previous studies (10,12). In
Huh7 and HepG2 hepatocellular carcinoma cells, CDDP treatment
activated autophagy (10), and in
human lung adenocarcinoma cells, LC3-II was increased in the
A549/DDP CDDP-resistant cells compared with that in A549 cells
(12).
PI3K/AKT/mTOR signalling pathway activation is
heavily implicated in endometrial cancer pathogenesis (14). The results of the present study
revealed that CDDP treatment may inactivate the PI3K/AKT/mTOR
signalling pathway. In addition co-treatment with a PI3K activator,
IGF-1, may reverse the effect of CDDP on cell autophagy in Ishikawa
cells. The association between CDDP and the PI3K/AKT/mTOR
signalling pathway was also discussed in other cells. In non-small
cell lung cancer cells, platycodin-D induced autophagy in NCI-H460
and A549 cells via inhibition of the PI3K/AKT/mTOR signalling
pathway (26), while in human
cervical cancer cells, licochalcone A induced autophagy via
inactivation of the PI3K/AKT/mTOR signalling pathway (27). Based on these results, the present
study hypothesized that CDDP may regulate cell autophagy in the
Ishikawa endometrial cancer cells by the PI3K/AKT/mTOR signalling
pathway.
Acknowledgements
The present study was supported by the General
Guidance Project of Guangzhou City Health Bureau (grant no.
20151A010106).
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Magrina JF, Mutone NF, Weaver AL, Magtibay
PM, Fowler RS and Cornella JL: Laparoscopic lymphadenectomy and
vaginal or laparoscopic hysterectomy with bilateral
salpingo-oophorectomy for endometrial cancer: Morbidity and
survival. Am J Obstet Gynecol. 181:376–381. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
National Cancer Institute: Cancer Stat
Facts: Endometrial Cancer. 2015.
|
|
4
|
Klionsky DJ and Emr SD: Autophagy as a
regulated pathway of cellular degradation. Science. 290:1717–1721.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
White E and DiPaola RS: The double-edged
sword of autophagy modulation in cancer. Clin Cancer Res.
15:5308–5316. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Du F, Feng Y, Fang J and Yang M:
MicroRNA-143 enhances chemosensitivity of Quercetin through
autophagy inhibition via target GABARAPL1 in gastric cancer cells.
Biomed Pharmacother. 74:169–177. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu YP, Dong FX, Chai X, Zhu S, Zhang BL
and Gao DS: Role of autophagy in capsaicin-induced apoptosis in
U251 Glioma Cells. Cell Mol Neurobiol. 36:737–743. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nazim UM and Park SY: Genistein enhances
TRAIL-induced cancer cell death via inactivation of autophagic
flux. Oncol Rep. 34:2692–2698. 2015.PubMed/NCBI
|
|
9
|
Zhang Y, Jiang F, Bao W, Zhang H, He X,
Wang H and Wan X: SOX17 increases the cisplatin sensitivity of an
endometrial cancer cell line. Cancer Cell Int. 16:292016.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xu N, Zhang J, Shen C, Luo Y, Xia L, Xue F
and Xia Q: Cisplatin-induced downregulation of miR-199a-5p
increases drug resistance by activating autophagy in HCC cell.
Biochem Biophys Res Commun. 423:826–831. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kang R, Wang ZH, Wang BQ, Zhang CM, Gao W,
Feng Y, Bai T, Zhang HL, Huang-Pu H and Wen SX: Inhibition of
autophagy-potentiated chemosensitivity to cisplatin in laryngeal
cancer Hep-2 cells. Am J Otolaryngol. 33:678–684. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ren JH, He WS, Nong L, Zhu QY, Hu K, Zhang
RG, Huang LL, Zhu F and Wu G: Acquired cisplatin resistance in
human lung adenocarcinoma cells is associated with enhanced
autophagy. Cancer Biother Radiopharm. 25:75–80. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen J, Zhao KN, Li R, Shao R and Chen C:
Activation of PI3K/Akt/mTOR pathway and dual inhibitors of PI3K and
mTOR in endometrial cancer. Curr Med Chem. 21:3070–3080. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Slomovitz BM and Coleman RL: The
PI3K/AKT/mTOR pathway as a therapeutic target in endometrial
cancer. Clin Cancer Res. 18:5856–5864. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lum JJ, Bauer DE, Kong M, Harris MH, Li C,
Lindsten T and Thompson CB: Growth factor regulation of autophagy
and cell survival in the absence of apoptosis. Cell. 120:237–248.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Levine B and Kroemer G: Autophagy in the
pathogenesis of disease. Cell. 132:27–42. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen Y, Li X, Wu X, He C, Guo L, Zhang S,
Xiao Y, Guo W and Tan B: Autophagy-related proteins LC3 and
Beclin-1 impact the efficacy of chemoradiation on esophageal
squamous cell carcinoma. Pathol Res Pract. 209:562–567. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Youn CK, Kim J, Park JH, Do NY and Cho SI:
Role of autophagy in cisplatin-induced ototoxicity. Int J Pediatr
Otorhinolaryngol. 79:1814–1819. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dasari S and Tchounwou PB: Cisplatin in
cancer therapy: Molecular mechanisms of action. Eur J Pharmacol.
740:364–378. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jiang Y, Guo C, Vasko MR and Kelley MR:
Implications of apurinic/apyrimidinic endonuclease in reactive
oxygen signaling response after cisplatin treatment of dorsal root
ganglion neurons. Cancer Res. 68:6425–6434. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
DeHaan RD, Yazlovitskaya EM and Persons
DL: Regulation of p53 target gene expression by cisplatin-induced
extracellular signal-regulated kinase. Cancer Chemother Pharmacol.
48:383–388. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhu H, Luo H, Zhang W, Shen Z, Hu X and
Zhu X: Molecular mechanisms of cisplatin resistance in cervical
cancer. Drug Des Devel Ther. 10:1885–1895. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kaushal GP, Kaushal V, Herzog C and Yang
C: Autophagy delays apoptosis inrenal tubular epithelial cells in
cisplatin cytotoxicity. Autophagy. 4:710–712. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kubisch J, Turei D, Földvári-Nagy L, Dunai
ZA, Zsákai L, Varga M, Vellai T, Csermely P and Korcsmáros T:
Complex regulation of autophagy in cancer-integrated approaches to
discover the networks that hold a double-edged sword. Semin Cancer
Biol. 23:252–261. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tanida I: Autophagosome formation and
molecular mechanism of autophagy. Antioxid Redox Signal.
14:2201–2214. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhao R, Chen M, Jiang Z, Zhao F, Xi B,
Zhang X, Fu H and Zhou K: Platycodin-D induced autophagy in
non-small cell lung cancer cells via PI3K/Akt/mTOR and MAPK
Signaling Pathways. J Cancer. 6:623–631. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tsai JP, Lee CH, Ying TH, Lin CL, Hsueh JT
and Hsieh YH: Licochalcone A induces autophagy through
PI3K/Akt/mTOR inactivation and autophagy suppression enhances
Licochalcone A-induced apoptosis of human cervical cancer cells.
Oncotarget. 6:28851–28866. 2015.PubMed/NCBI
|