Introduction
Colorectal cancer (CRC) is one of the most common
types of malignancy, it is the third most common cancer in males
and the second most common in females, worldwide (1). In 2015 it was estimated that there would
be 93,090 novel cases of CRC and 49,700 CRC-associated mortalities
in the USA (2). The molecular
mechanism underlying CRC carcinogenesis and progression involves
numerous factors, including genetic instability, hereditary
components, increased age, male gender, increased intake of fat,
alcohol or red meat, obesity, smoking and a lack of physical
exercise (3–5). Currently, the standard therapeutic
treatments for CRC are surgical resection, radiotherapy and
chemotherapy (6). Despite progress in
the development of treatments for CRC, the 5-year overall survival
rate has not improved significantly (7–9). The poor
prognosis of CRC is associated with an advanced stage of cancer and
tumor recurrence and metastasis following surgical resection
(10). Patients with advanced stage
CRC and metastasis have been treated with 5-fluorouracil
(5-FU)-associated chemotherapy, following surgery (11). However, not every case of CRC responds
to 5-FU based chemotherapy and the response rate to this treatment
is only between 10 and 20% (12).
Therefore, overcoming chemoresistance, improving the
chemosensitivity of CRC and enhancing the curative effect of
chemotherapy, is required for the treatment of CRC.
A number of previous studies have demonstrated that
microRNAs (miRNAs) are downregulated in numerous types of human
cancer, including CRC (13–15). miRNAs are endogenous,
non-protein-coding small RNAs, 18–25 nucleotides in length
(16). miRNAs serve important
regulatory roles in human, animal, plant and DNA viruses by binding
to the 3′untranslated regions (3′UTRs) of target mRNAs, resulting
in mRNA degradation or translational repression at the
translational or post-transcriptional levels (17,18).
miRNAs regulate the expression of ≥20% of human genes, and
contribute to a variety of important physiological and pathological
processes including, cell cycle, proliferation, migration,
invasion, apoptosis, differentiation and development (18–20). A
previous study demonstrated that miRNAs are able to function as
oncogenes by repressing the expression of tumor target suppressor
genes, or tumor suppressors by repressing the expression of target
oncogenes (21). Furthermore, miRNAs
have previously been identified to serve important functions in the
regulation of chemoresistance (22,23). This
suggested that miRNAs may be novel potential therapeutic targets
for chemoresistance of CRC.
The present study revealed that miRNA (miR)-330 was
significantly downregulated in CRC tissues and cell lines. In
addition, miR-330 inhibited CRC cell proliferation and enhanced the
chemosensitivity of CRC cells to 5-FU by increasing the rate of CRC
cell apoptosis induced by 5-FU. Furthermore, thymidylate synthase
(TYMS) was identified as a direct target gene of miR-330 in
CRC. These results have therapeutic implications and may be
exploited for further treatment of CRC.
Materials and methods
Clinical samples
The present study was approved by the Ethics
Committee of The Military General Hospital of Beijing PLA (Beijing,
China). Written informed consent was obtained from all patients,
prior to enrollment in the present study. The patients with CRC had
not received chemotherapy or radiotherapy prior to surgery. A total
of 59 pairs of CRC tissues and their normal adjacent tissues (NATs)
were obtained from patients with CRC, who underwent surgical
resection at The Military General Hospital of Beijing PLA. CRC
tissues and the NATs were rapidly frozen in liquid nitrogen and
stored in at −80°C.
Cell culture and transfection
A total of four human CRC cell lines (HCT116, HT29,
SW480 and SW620), the normal human colon epithelium cell line FHC
and the HEK293T cell line were obtained from the American Type
Culture Collection (Manassas, VA, USA). All cell lines were
maintained in Dulbecco's modified Eagle's medium or RPMI-1640
supplemented with 10% fetal bovine serum (all Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA), 100 mg/ml penicillin and 100
mg/ml in a humidified atmosphere of 5% CO2 at 37°C.
In order to investigate the function of miR-330 in
CRC, CRC cells were transfected with miR-330 mimics or the negative
scrambled control (NC), purchased from GenePharma, Inc. (Sunnyvale,
CA, USA). Furthermore, TYMS small interfering (si)RNA and NC
siRNA were obtained from Guangzhou RiboBio Co., Ltd. (Guangzhou,
China) and transfected into CRC cells. The sequence of the miR-330
mimic was 5′-GCAAAGCACACGGCCUGCAGAGA-3′ and the sequence of the
miR-NC mimic was 5′-UUCUCCGAACGUGUCACGUTT-3′. The sequence of the
TYMS siRNA was 5′-TACGTCCAAGGTCGGGCAGGAAGA-3′ and the NC siRNA
sequence was 5′-AACAGGCACACGTCCCAGCGT-3′. Cell transfection was
performed using Lipofectamine™ 2000 (Invitrogen; Thermo Fisher
Scientific, Inc), according to the manufacturer's protocol.
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was isolated using TRIzol®
reagent (Invitrogen; Thermo Fisher Scientific, Inc.), according to
the manufacturer's protocol. In order to detect miR-330 expression
level, reverse transcription was performed using the
TaqMan® MicroRNA Reverse Transcription kit (Applied
Biosystems; Thermo Fisher Scientific, Inc.), followed by RT-qPCR
using the TaqMan miRNA assay (Applied Biosystems; Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocol. The
temperature protocol for reverse transcription was as follows: 16°C
for 30 min; 42°C for 30 min; and 85°C for 5 min. The thermocycling
conditions for qPCR were as follows: 95°C for 10 min; 40 cycles of
denaturation at 95°C for 15 sec and annealing at 60°C for 1 min;
followed by a final elongation step at 72°C for 10 min.
For analysis of TYMS mRNA expression level,
reverse transcription was performed using the M-MLV Reverse
Transcription system (Promega Corp., Madison, WI, USA), according
to the manufacturer's protocol. The temperature protocol for
reverse transcription was as follows: 95°C for 2 min; 20 cycles of
94°C for 1 min, 55°C for 1 min and 72°C for 2 min; and 72°C for 5
min. Subsequently, SYBR®-Green Master mix was used to
determine the mRNA expression level. The thermocycling conditions
of qPCR were as follows: 95°C for 10 min; and 40 cycles of 95°C for
15 sec and 60°C for 1 min. U6 small nuclear RNA was used for
normalization of miRNA expression and GADPH was used as an internal
control for mRNA expression. RT-qPCR was performed using a 7500
Real-Time PCR system (Applied Biosystems; Thermo Fisher Scientific,
Inc.). The relative expression of miR-330a and TYMS was analyzed by
use of the 2−ΔΔCq method (24). This assay was performed in triplicate
and repeated three times.
CCK-8 assay
Cell proliferation rates were evaluated using the
Cell Counting Kit-8 (CCK-8; Dojindo Molecular Technologies, Inc.,
Kumamoto, Japan) assay. Cells were seeded into 96-well plates at a
density of 3,000 cells/well. Subsequently, cells were transfected
and incubated in a humidified atmosphere of 5% CO2 at
37°C for 24–96 h. Every 24 h post-transfection, CCK-8 assays was
performed. A total of 10 µl CCK8 assay solution was added to each
well. Cells were incubated for 2 h at 37°C and the absorbance of
each well was determined at 450 nm by an automatic multi-well
spectrophotometer (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
All experiments were performed in triplicate.
Chemosensitivity assay
Cells were seeded into 96-well plates at a density
of 3,000 cells/well. Subsequently, cells were transfected and
incubated for 48 h in a humidified atmosphere of 5% CO2
at 37°C. Cells were then treated with 5-FU (Sigma-Aldrich; Merck
Millipore, Darmstadt, Germany) at various concentrations (0–32 µM)
at room temperature. Following incubation for a further 48 h, a
chemosensitivity assay was performed using a CCK8 assay, as
aforementioned. The dose-response curve was charted at various
concentrations. All experiments were performed in triplicate.
Cell apoptosis assay
Cells were seeded into 6-well plates at a confluence
of between 60 and 70%. Cells were transfected and incubated for 24
h in a humidified atmosphere of 5% CO2 at 37°C. Cells
were subsequently treated with 5-FU at a concentration of 8 µM.
Following a 48-h incubation, the rate of cell apoptosis was
determined by Annexin V-fluorescein isothiocyanate (FITC; BD
Biosciences, Franklin Lakes, NJ, USA; cat. no. 556419) and
propidium iodide (PI; BD Biosciences; cat. no. 556463) staining,
according to the manufacturer's protocol. In brief, cells were
harvested and washed with ice-cold PBS (Gibco; Thermo Fisher
Scientific, Inc.) three times. Cells were resuspended in 100 µl
binding buffer, followed by treatment with 2 µl Annexin V-FITC and
5 µl of PI at room temperature. Following a 15 min incubation at
room temperature in the dark, 400 µl of binding buffer was added
and the mixture was analyzed by flow cytometry (BD Biosciences,
Franklin Lakes, NJ, USA) using FCS Express software (version 3.0;
De Novo Software, Glendale, CA, USA).
Western blotting
Transfected cells were washed with ice-cold PBS
three times and lysed with radioimmunoprecipitaion assay lysis
buffer (Beyotime Institute of Biotechnology, Haimen, China)
supplemented with protease inhibitors (Promega Corp.). Protein
concentration was quantified using a bicinchoninic acid assay kit
(Pierce; Thermo Fisher Scientific, Inc.). Equal amounts of protein
(20 µg) were separated by 10% SDS-PAGE, followed by transference to
polyvinylidene difluoride membranes. The membranes were blocked in
TBS-Tween-20 containing 5% non-fat dry milk for 2 h at room
temperature, followed by incubation with primary antibodies
overnight at 4°C. Subsequently, the membranes were incubated with
the goat anti-mouse horseradish peroxidase-conjugated secondary
antibody (1:3,000 dilution; cat. no. sc-ab6789; Abcam, Cambridge,
MA, USA) for 2 h at room temperature. Protein bands were visualized
using enhanced chemiluminescence solution (Pierce; Thermo Fisher
Scientific, Inc.). The primary antibodies used in the present study
were mouse anti-human monoclonal TYMS (cat. no. sc-33679)
and mouse anti-human GADPH (cat. no. sc-59540) (both 1:1,000
dilution; both Santa Cruz Biotechnology, Inc., Dallas, TX, USA).
GADPH was used as an internal control. Band intensities were imaged
using the FluorChem imaging system (alpha innotec GmbH, Kasendorf,
Germany) and analyzed using Image Lab version 5.2.1 (Bio-Rad
Laboratories, Inc.).
Dual-luciferase® reporter
assay
The Dual-Luciferase Reporter assay (Promega
Corporation) was performed in HEK293T cells. PGL3-TYMS-3′UTR
wild-type (Wt) and PGL3-TYMS-3′UTR mutant (Mut) were purchased from
GenePharma, Inc. Cells were seeded into 24-well plates at a
confluence of between 60 and 70%. Following overnight incubation,
cells were co-transfected with miR-330 mimics or NC, and
PGL3-TYMS-3′UTR Wt or PGL3-TYMS-3′UTR Mut, using Lipofectamine
2000. The firefly and Renilla luciferase activities were
determined by Dual-Luciferase reporter assay 48 h following
transfection. Luciferase activity was measured at a wavelength of
560 nm using an xMark™ Microplate Absorbance Spectrophotometer
(Bio-Rad Laboratories, Inc.). Renilla luciferase activities
were used as an internal control. All experiments were performed in
triplicate.
Statistical analysis
Data are presented as the mean ± standard deviation,
and compared with the Student's t-test or a one-way analysis of the
variance using SPSS 16.0 statistical software (SPSS, Inc., Chicago,
IL, USA). Two-tailed P<0.05 was considered to indicate a
statistically significant difference.
Results
miR-330 expression level in CRC
tissues and cell lines
In order to investigate whether miR-330 was
downregulated in CRC tissues, the miR-330 expression level was
determined by RT-qPCR in CRC tissues and paired NATs. As presented
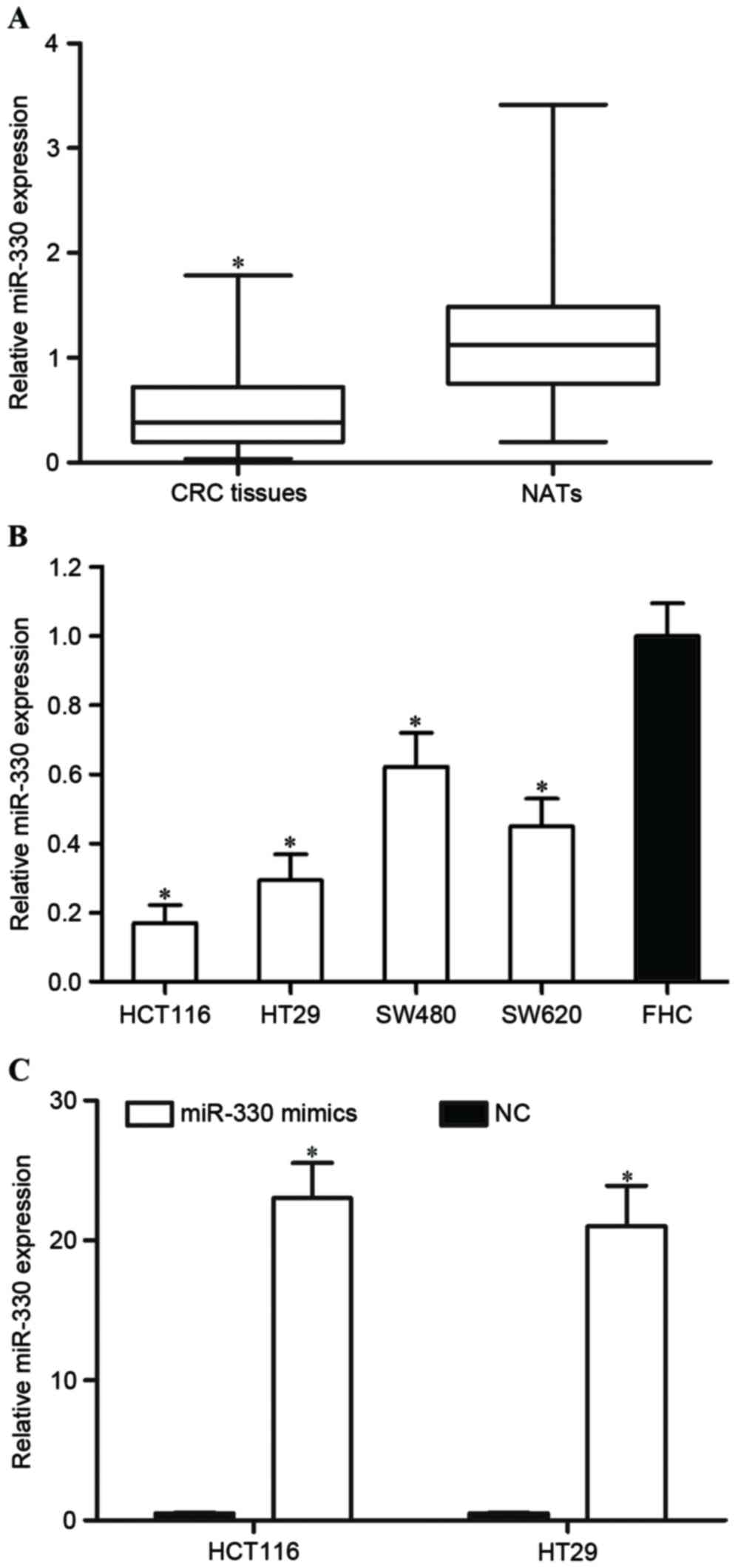
in Fig. 1A, miR-330 was significantly
downregulated in CRC tissues compared with paired NATs (P<0.05).
Furthermore, the expression level of miR-330 was evaluated in CRC
and FHC cell lines. Consistent with the result that the miR-330
expression level was decreased in CRC tissues, miR-330 was
downregulated in the HCT116, HT29, SW480 and SW620 cell lines,
compared with the FHC cell line (all P<0.05; Fig. 1B). These findings suggested that
miR-330 may act as a tumor suppressor in CRC.
HCT116 and HT29 expression levels were lower
compared with SW480 and SW620 expression levels. Therefore, the
present study transfected miR-330 mimics or NC into HCT116 and HT29
cells in order to explore the functions of miR-330 in CRC cells.
RT-qPCR was performed to determine the miR-330 expression level
following transfection. As presented in Fig. 1C, miR-330 was significantly
upregulated in miR-330 mimic transfected HCT116 and HT29 cells,
compared with in NC cells (both P<0.05).
miR-330 inhibited cell proliferation
in HCT116 and HT29 cells
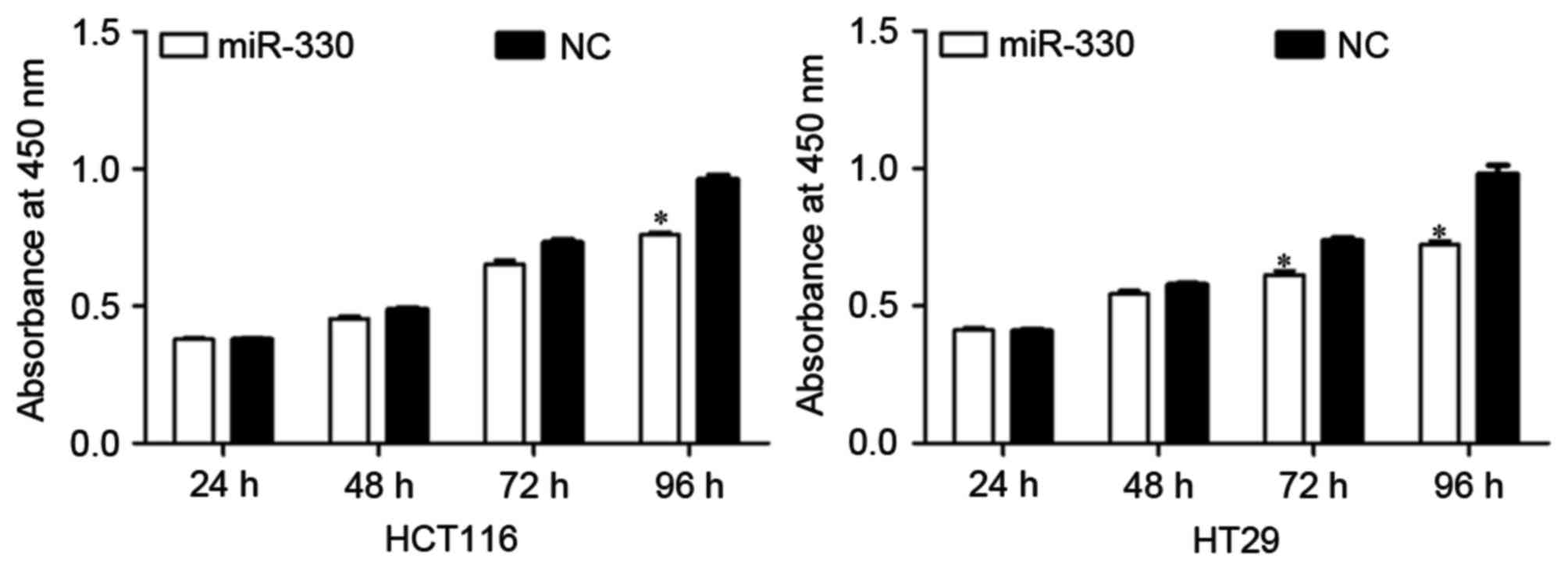
The effect of miR-330 expression on CRC cell
proliferation was evaluated by performing a CCK-8 assay. As
presented in Fig. 2, the upregulation
of miR-330 resulted in significant growth inhibition of HCT116 and
HT29 cells (both P<0.05) at 96 h compared with the NC. The
results suggested that miR-330 may be a negative regulator of cell
proliferation in CRC cells.
miR-330 enhanced cell chemosensitivity
to 5-FU in HCT116 and HT29 cells
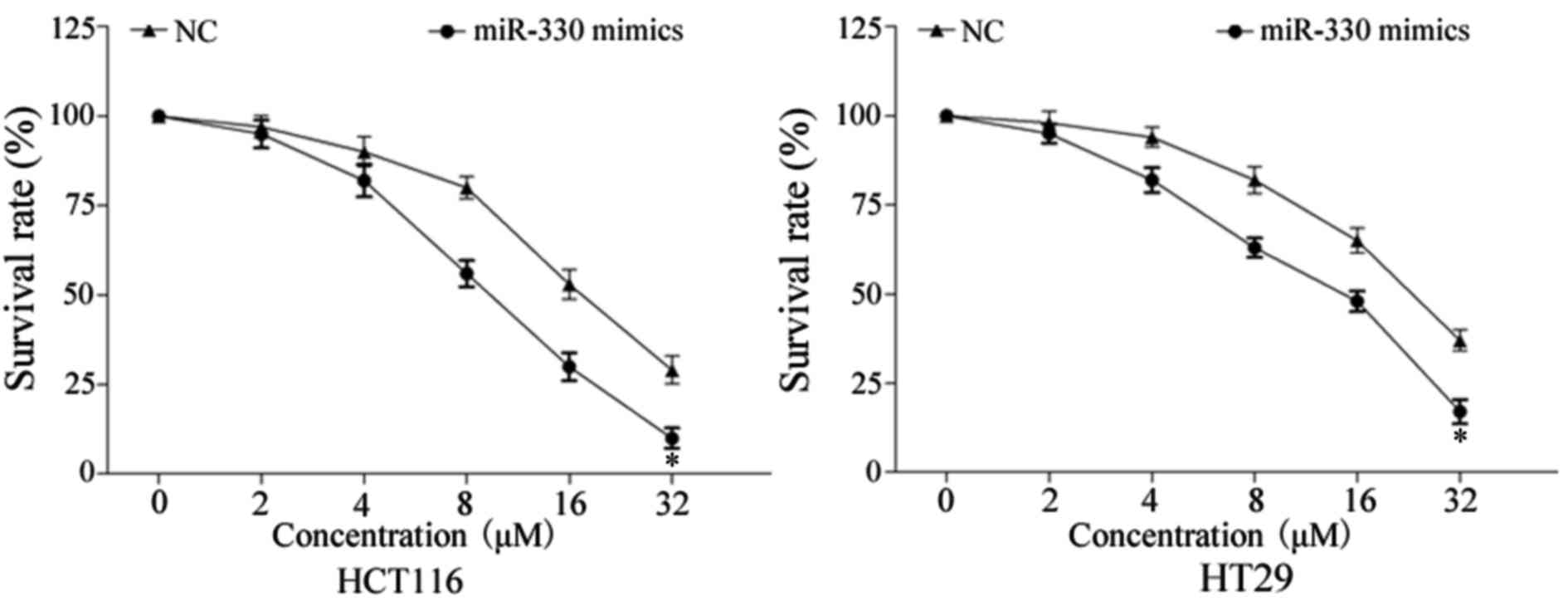
In order to evaluate the effect of miR-330 on CRC
cell chemosensitivity to 5-FU, a chemosensitivity assay was
performed. As presented in Fig. 3,
enforced miR-330 expression decreased the survival rate of HCT116
and HT29 cells, compared with NC-transfected HCT116 and HT29 cells
(P<0.05). These results indicated that miR-330 increased cell
chemosensitivity of CRC cells to 5-FU.
miR-330 increased the rate of cell
apoptosis induced by 5-FU in HCT116 and HT29 cells
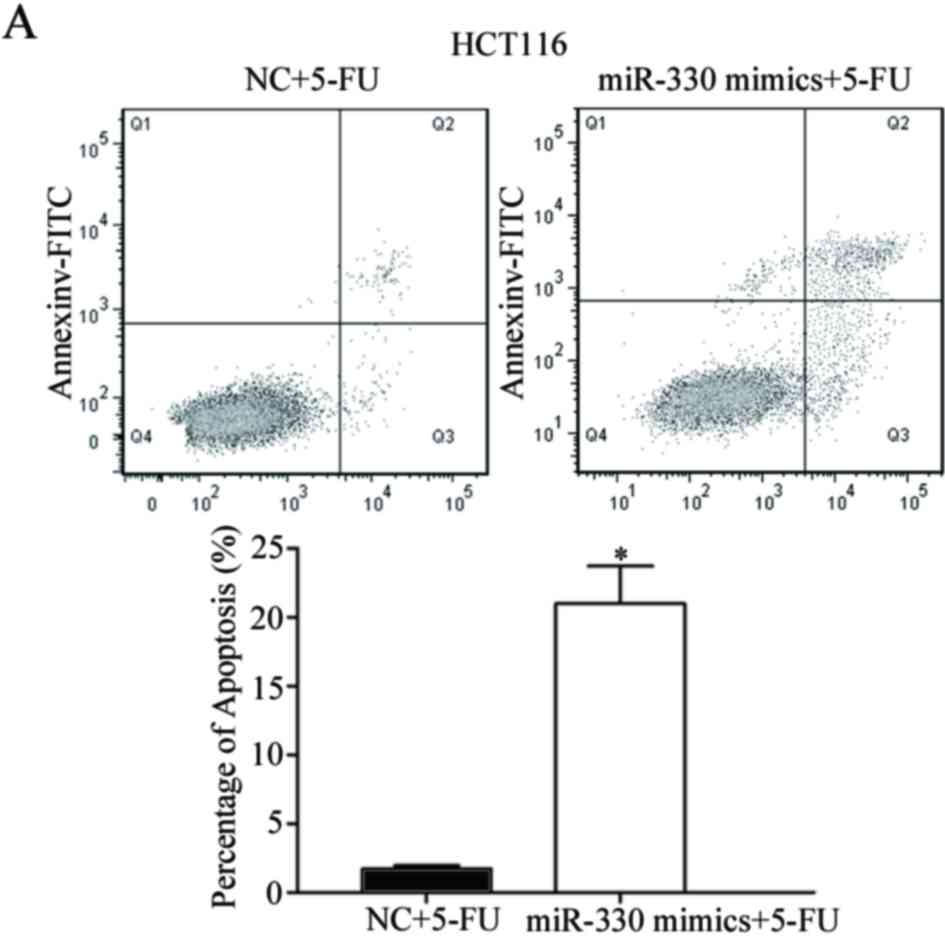
Cell-cycle arrest at the G1 phase and/or
apoptosis of cancer cells is enhanced by 5-FU (25,26).
Therefore, the rate of cell apoptosis induced by 5-FU was
evaluated. As presented in Fig. 4,
the overexpression of miR-330 significantly increased the rate of
apoptosis of HCT116 and HT29 cells induced by 5-FU (P<0.05)
compared with the NC. This supports the observation that miR-330
increases HCT116 and HT29 cell chemosensitivity to 5-FU. These
results suggested that miR-330 increased cell chemosensitivity of
HCT116 and HT29 cells to 5-FU via the cell apoptosis pathway.
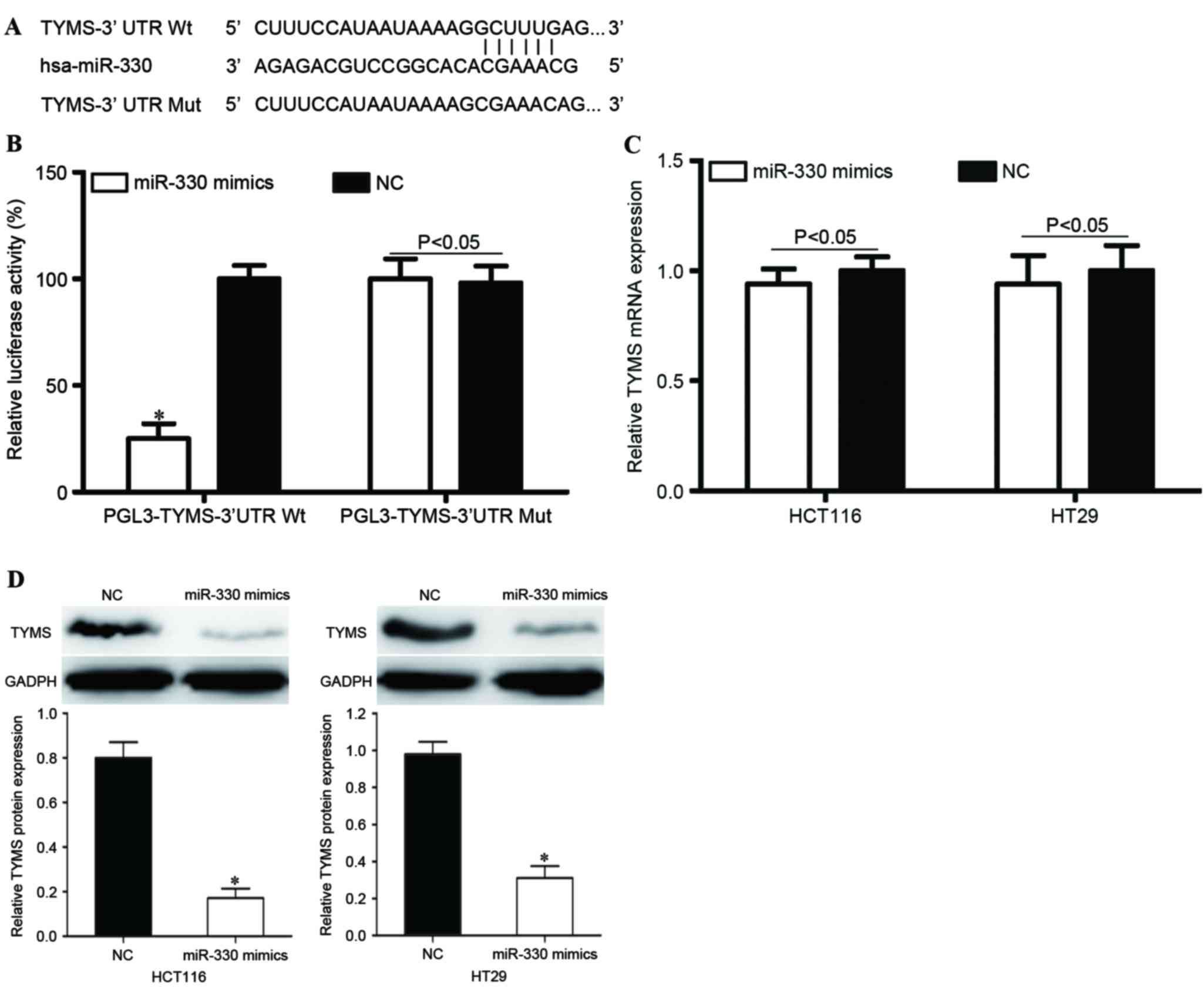
TYMS was a direct target gene of
miR-330 in vitro
In order to investigate the molecular mechanisms
underlying the functions of miR-330 in CRC cells, bioinformatic
predication algorithms (TargetScan; www.targetscan.org) were used to determine the direct
target gene of miR-330. Amongst the hypothetical target mRNAs
identified for miR-330, TYMS was selected for further
investigation (Fig. 5A). In addition,
a Dual-Luciferase reporter assay was performed to determine whether
TYMS was a direct target mRNA of miR-330 in vitro. As
presented in Fig. 5B, miR-330
significantly inhibited PGL3-TYMS-3′UTR Wt luciferase activity in
HEK293T cells (P<0.05) compared with the NC, whereas
PGL3-TYMS-3′UTR Mut demonstrated no significant response to miR-330
(P>0.05).
In order to further investigate the potential
effects of miR-330 in the regulation of TYMS, RT-qPCR and
western blot analysis were performed to evaluate TYMS mRNA
and protein levels following transfection with miR-330 mimics or
NC. As presented in Fig. 5C,
TYMS mRNA expression levels were not significantly altered
following transfection with miR-330 mimics (P>0.05). However,
TYMS protein expression was significantly downregulated in miR-330
mimic-transfected HCT116 and HT29 cells, compared with NC groups
(both P<0.05; Fig. 5D). This
indicated that miR-330 regulated TYMS expression at the
post-transcriptional level. Therefore, TYMS was a direct
target gene of miR-330 in CRC cells.
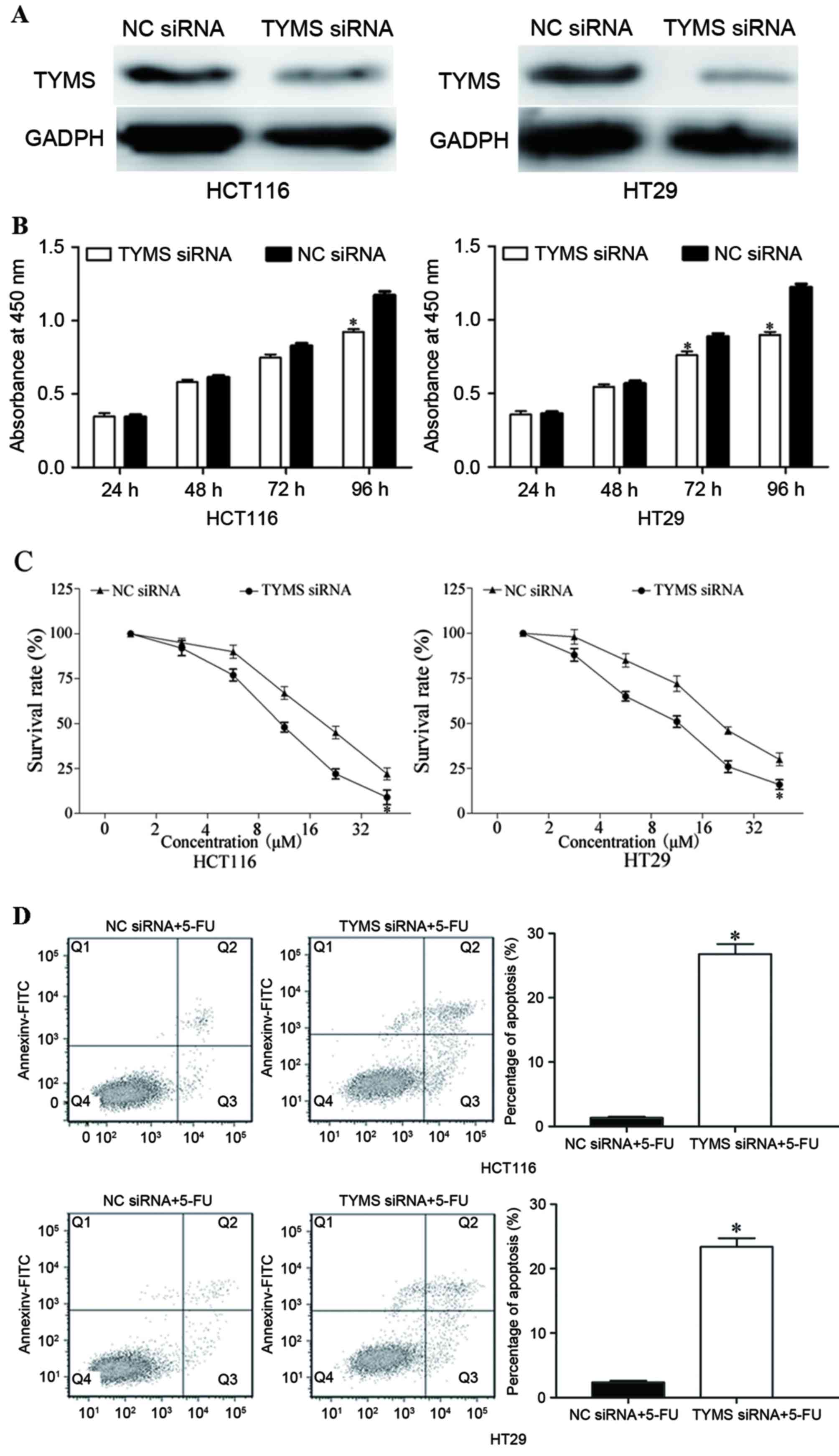
TYMS served a role in miR-330-mediated
functions in HCT116 and HT29 cells
In order to investigate the function of TYMS
in CRC, HCT116 and HT29 cells were transfected with TYMS
siRNA or NC siRNA. As presented in Fig.
6A, TYMS siRNA decreased the expression level of TYMS in
HCT116 and HT29 cells (P<0.05). Following transfection with
TYMS siRNA, CCK-8, chemosensitivity and cell apoptosis
assays were performed. As presented in Fig. 6B, relative cell growth was
significantly suppressed in TYMS siRNA transfected HCT116
and HT29 cells, compared with NC siRNA cells (both P<0.05). In
addition, chemosensitivity assays demonstrated that TYMS
siRNA significantly improved chemosensitivity of HCT116 and HT29
cells to 5-FU (both P<0.05; Fig.
6C). Furthermore, TYMS siRNA significantly increased the
rate of HCT116 and HT29 cell apoptosis induced by 5-FU (both
P<0.05; Fig. 6D). These results
revealed that the effects of TYMS siRNA were similar to
those induced by miR-330 in CRC cells, suggesting that TYMS
was a functional target of miR-330 in CRC cells.
Discussion
CRC is the third leading cause of cancer-associated
mortality worldwide, and the 5-year survival rate is mostly
dependent on the stage of cancer, resulting in a survival rate of
between 10 and 95% (27,28). In China, the incidence rate of CRC is
relatively lower; however, an increased prevalence in recent years
has been observed (29). For patients
with CRC following surgical resection, 5-FU based chemotherapy is
the adjuvant treatment (30). In
addition, chemotherapy is the first-line of treatment for a number
of patients with metastatic CRC (31). However, numerous CRC cases develop
5-FU resistance, which is a major challenge to the effective
treatment of cancer (32). Therefore,
it is essential to explore the molecular mechanism underlying
chemoresistance in CRC and investigate novel therapeutic strategies
for patients with CRC.
miR-330 has been identified to be downregulated in
prostate cancer, and inhibited cell migration and invasion through
the downregulation of SP1 (33).
However, miR-330 was also revealed to be upregulated in human
esophageal cancer cells (34),
non-small cell lung cancer cells (35) and glioblastoma cells (36). In previous functional studies, miR-330
was identified to be an oncogene. In esophageal cancer, miR-330
targeted programmed cell death protein 4 in order to enhance cell
proliferation, migration and invasion, and to decrease the rate of
cisplatin-induced apoptosis (34).
Liu et al (35) demonstrated
that miR-330 increased non-small cell lung cancer cell
proliferation by directly targeting early growth response protein 2
(35). In glioblastoma tissues, the
upregulation of miR-330 promoted cell growth, migration and
invasion, and suppressed cell apoptosis by regulating SH3 domain
containing GRB2 like 2 (36). These
conflicting results revealed that the expression and functions of
miR-330 were tissue-type dependent. The present study demonstrated
that miR-330 was downregulated in CRC tissues and cell lines. In
addition, enforced miR-330 expression inhibited CRC cell
proliferation and enhanced the chemosensitivity of CRC cells to
5-FU by the cell apoptosis pathway. These findings may facilitate
the development of a novel strategy for 5-FU-based
chemotherapy.
In order to explore the molecular mechanisms of
miR-330 underlying the cellular response to 5-FU, the present study
identified that TYMS was a direct target gene of miR-330 in
CRC. TargetScan predicated that TYMS contained a miR-330
seed match at position 192–197 of the TYMS-3′UTR. miR-330 decreased
the luciferase activity of TYMS-3′UTR. However, co-transfection of
miR-330 mimics and TYMS-3′UTR Mut did not induce a decrease in
luciferase activity. Subsequently, miR-330 inhibited TYMS
expression at the post-transcriptional level. Finally, functional
studies revealed that TYMS siRNA enhanced cell
chemosensitivity of CRC cells to 5-FU and mediated the suppressive
role of miR-330 in CRC cell growth, and enhanced cell apoptosis due
to exposure to 5-FU. Identification of miR-330 target genes is
essential for understanding its function in chemoresistance.
In CRC chemotherapy, 5-FU is the most commonly
administered chemotherapeutic agent alone or combined with other
chemotherapeutic agents (37). The
structure of 5-FU is similar to pyrimidine, a nucleoside required
for DNA replication (38). Therefore,
5-FU primarily affects nucleoside metabolism and may be
incorporated into RNA and DNA molecules. Consequently, 5-FU
enhances cell-cycle arrest at the G1 phase and/or
apoptosis in cancer cells (25,26).
TYMS, a cytosolic enzyme that alters the methylation of
deoxyuridine monophosphate to deoxythymidine monophosphate, is an
important therapeutic target of 5-FU (39). Previous studies verified that patients
with CRC and low TYMS expression have an improved response
to 5-FU and 5-year overall survival rate (40,41). In
comparison, the upregulation of TYMS in patients with CRC
induces 5-FU chemoresistance (42,43). The
present study demonstrated that the downregulation of TYMS
significantly inhibited CRC cell proliferation and enhanced cell
chemosensitivity of CRC cells to 5-FU by inducing apoptosis under
5-FU exposure. Therefore, TYMS may serve as a predictive
biomarker of the cellular response to 5-FU and a therapeutic target
for 5-FU-based chemotherapy. The present study provides evidence
for the use of 5-FU in combination with miR-330 in the
chemotherapeutic treatment of CRC.
In conclusion, the present study revealed that
miR-330 was downregulated in CRC tissues and cell lines. Increased
expression of miR-330 suppressed CRC cell proliferation. In
addition, the present study demonstrated that miR-330 enhanced the
chemosensitivity of CRC cells to 5-FU by the cell apoptosis
pathway. Furthermore, TYMS was identified as a direct target
gene of miR-330 in CRC. These results have therapeutic implications
and may be exploited for further treatment of CRC.
References
|
1
|
Siegel R, Desantis C and Jemal A:
Colorectal cancer statistics, 2014. CA Cancer J Clin. 64:104–117.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Andrews L: Dietary flavonoids for the
prevention of colorectal cancer. Clin J Oncol Nurs. 17:671–672.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Altobelli E, Lattanzi A, Paduano R,
Varassi G and di Orio F: Colorectal cancer prevention in Europe:
Burden of disease and status of screening programs. Prev Med.
62:132–141. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sugarbaker PH: Colorectal cancer:
Prevention and management of metastatic disease. Biomed Res Int.
2014:7828902014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yu H, Gao G, Jiang L, Guo L, Lin M, Jiao
X, Jia W and Huang J: Decreased expression of miR-218 is associated
with poor prognosis in patients with colorectal cancer. Int J Clin
Exp Pathol. 6:2904–2911. 2013.PubMed/NCBI
|
|
7
|
Sun Z, Zhou N, Han Q, Zhao L, Bai C, Chen
Y, Zhou J and Zhao RC: MicroRNA-197 influences 5-fluorouracil
resistance via thymidylate synthase in colorectal cancer. Clin
Transl Oncol. 17:876–883. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Brouquet A and Nordlinger B: Metastatic
colorectal cancer outcome and fatty liver disease. Nat Rev
Gastroenterol Hepatol. 10:266–267. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kuipers EJ, Rösch T and Bretthauer M:
Colorectal cancer screening-optimizing current strategies and new
directions. Nat Rev Clin Oncol. 10:130–142. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chaffer CL and Weinberg RA: A perspective
on cancer cell metastasis. Science. 331:1559–1564. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cunningham D, Atkin W, Lenz HJ, Lynch HT,
Minsky B, Nordlinger B and Starling N: Colorectal cancer. Lancet.
375:1030–1047. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
De Angelis PM, Svendsrud DH, Kravik KL and
Stokke T: Cellular response to 5-fluorouracil (5-FU) in
5-FU-resistant colon cancer cell lines during treatment and
recovery. Mol Cancer. 5:202006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zheng K, Liu W, Liu Y, Jiang C and Qian Q:
MicroRNA-133a suppresses colorectal cancer cell invasion by
targeting Fascin1. Oncol Lett. 9:869–874. 2015.PubMed/NCBI
|
|
14
|
Yuan W, Sui C, Liu Q, Tang W, An H and Ma
J: Up-regulation of microRNA-145 associates with lymph node
metastasis in colorectal cancer. PLoS One. 9:e1020172014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang Y, Wang Z, Chen M, Peng L, Wang X,
Ma Q, Ma F and Jiang B: MicroRNA-143 targets MACC1 to inhibit cell
invasion and migration in colorectal cancer. Mol Cancer. 11:232012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
He L and Hannon GJ: MicroRNAs: Small RNAs
with a big role in gene regulation. Nat Rev Genet. 5:522–531. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
O'Hara SP, Mott JL, Splinter PL, Gores GJ
and LaRusso NF: MicroRNAs: Key modulators of posttranscriptional
gene expression. Gastroenterology. 136:17–25. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ambros V: The functions of animal
microRNAs. Nature. 431:350–355. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Broderick JA and Zamore PD: MicroRNA
therapeutics. Gene Ther. 18:1104–1110. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Calin GA and Croce CM: MicroRNA signatures
in human cancers. Nat Rev Cancer. 6:857–866. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lu F, Zhang J, Ji M, Li P, Du Y, Wang H,
Zang S, Ma D, Sun X and Ji C: miR-181b increases drug sensitivity
in acute myeloid leukemia via targeting HMGB1 and Mcl-1. Int J
Oncol. 45:383–392. 2014.PubMed/NCBI
|
|
23
|
Nagano H, Tomimaru Y, Eguchi H, Hama N,
Wada H, Kawamoto K, Kobayashi S, Mori M and Doki Y: MicroRNA-29a
induces resistance to gemcitabine through the Wnt/β-catenin
signaling pathway in pancreatic cancer cells. Int J Oncol.
43:1066–1072. 2013.PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Thomas DM and Zalcberg JR: 5-fluorouracil:
A pharmacological paradigm in the use of cytotoxics. Clin Exp
Pharmacol Physiol. 25:887–895. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Noordhuis P, Holwerda U, Van der Wilt CL,
Van Groeningen CJ, Smid K, Meijer S, Pinedo HM and Peters GJ:
5-Fluorouracil incorporation into RNA and DNA in relation to
thymidylate synthase inhibition of human colorectal cancers. Ann
Oncol. 15:1025–1032. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Coget J, Borrini F, Susman S and Sabourin
JC: Colorectal carcinomas in 2013: The search for powerful
prognostic markers is still on the go! Cancer Biomark. 14:145–150.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li T, Gao F and Zhang XP: miR-203 enhances
chemosensitivity to 5-fluorouracil by targeting thymidylate
synthase in colorectal cancer. Oncol Rep. 33:607–614.
2015.PubMed/NCBI
|
|
29
|
Xu AG, Yu ZJ, Jiang B, Wang XY, Zhong XH,
Liu JH, Lou QY and Gan AH: Colorectal cancer in Guangdong province
of China: A demographic and anatomic survey. World J Gastroenterol.
16:960–965. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ades S: Adjuvant chemotherapy for colon
cancer in the elderly: Moving from evidence to practice. Oncology
(Williston Park). 23:162–167. 2009.PubMed/NCBI
|
|
31
|
McEwan DG, Brunton VG, Baillie GS, Leslie
NR, Houslay MD and Frame MC: Chemoresistant KM12C colon cancer
cells are addicted to low cyclic AMP levels in a phosphodiesterase
4-regulated compartment via effects on phosphoinositide 3-kinase.
Cancer Res. 67:5248–5257. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
He Y, Wang J, Wang J, Yung VY, Hsu E, Li
A, Kang Q, Ma J, Han Q, Jin P, et al: MicroRNA-135b regulates
apoptosis and chemoresistance in colorectal cancer by targeting
large tumor suppressor kinase 2. Am J Cancer Res. 5:1382–1395.
2015.PubMed/NCBI
|
|
33
|
Mao Y, Chen H, Lin Y, Xu X, Hu Z, Zhu Y,
Wu J, Xu X, Zheng X and Xie L: microRNA-330 inhibits cell motility
by downregulating Sp1 in prostate cancer cells. Oncol Rep.
30:327–333. 2013.PubMed/NCBI
|
|
34
|
Meng H, Wang K, Chen X, Guan X, Hu L,
Xiong G, Li J and Bai Y: MicroRNA-330-3p functions as an oncogene
in human esophageal cancer by targeting programmed cell death 4. Am
J Cancer Res. 5:1062–1075. 2015.PubMed/NCBI
|
|
35
|
Liu X, Shi H, Liu B, Li J, Liu Y and Yu B:
miR-330-3p controls cell proliferation by targeting early growth
response 2 in non-small-cell lung cancer. Acta Biochim Biophys Sin
(Shanghai). 47:431–440. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Qu S, Yao Y, Shang C, Xue Y, Ma J, Li Z
and Liu Y: MicroRNA-330 is an oncogenic factor in glioblastoma
cells by regulating SH3GL2 gene. PLoS One. 7:e460102012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Heidelberger C, Chaudhuri NK, Danneberg P,
Mooren D, Griesbach L, Duschinsky R, Schnitzer RJ, Pleven E and
Scheiner J: Fluorinated pyrimidines, a new class of
tumour-inhibitory compounds. Nature. 179:663–666. 1957. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Liu Y, Du F, Zhao Q, Jin J, Ma X and Li H:
Acquisition of 5-fluorouracil resistance induces
epithelial-mesenchymal transitions through the Hedgehog signaling
pathway in HCT-8 colon cancer cells. Oncol Lett. 9:2675–2679.
2015.PubMed/NCBI
|
|
39
|
Lehman NL: Future potential of thymidylate
synthase inhibitors in cancer therapy. Expert Opin Investig Drugs.
11:1775–1787. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Popat S, Matakidou A and Houlston RS:
Thymidylate synthase expression and prognosis in colorectal cancer:
A systematic review and meta-analysis. J Clin Oncol. 22:529–536.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lenz HJ, Hayashi K, Salonga D, Danenberg
KD, Danenberg PV, Metzger R, Banerjee D, Bertino JR, Groshen S,
Leichman LP and Leichman CG: p53 point mutations and thymidylate
synthase messenger RNA levels in disseminated colorectal cancer: An
analysis of response and survival. Clin Cancer Res. 4:1243–1250.
1998.PubMed/NCBI
|
|
42
|
Copur S, Aiba K, Drake JC, Allegra CJ and
Chu E: Thymidylate synthase gene amplification in human colon
cancer cell lines resistant to 5-fluorouracil. Biochem Pharmacol.
49:1419–1426. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Cho YB, Chung HJ, Lee WY, Choi SH, Kim HC,
Yun SH and Chun HK: Relationship between TYMS and ERCC1 mRNA
expression and in vitro chemosensitivity in colorectal cancer.
Anticancer Res. 31:3843–3849. 2011.PubMed/NCBI
|