Introduction
Lung cancer is the leading cause of
cancer-associated mortality, with high incidence rates worldwide
(1). The majority of lung cancers are
malignant carcinomas, and non-small cell lung cancer (NSCLC)
accounts for >80% of these (2).
Although advances in treatment have occurred in the past few years,
the survival rate for patients with NSCLC remains at ~15% due to
cancer metastasis and high recurrence rates (3). Therefore, it is necessary to further
investigate the molecular mechanisms underlying the initiation of
NSCLC development.
Changes to the expression of microRNAs (miRNAs/miRs)
in tumors are a focus being studied at present and it has been
demonstrated that the dysregulation of miRNAs is involved in the
malignancy of lung cancer (4,5). miRNAs are endogenous non-coding 19–23
nucleotide long RNA molecules that are located on chromosome 1p22.
It has been revealed that miRNAs can downregulate gene expression
by binding to the 3′ untranslated regions (3′UTRs) of mRNA
(6), thus affecting the
proliferation, and metastasis of numerous types of cancer, such as
gastric (7), breast (8) and lung cancer (9,10). The
expression of miR-137 has been identified to be significantly
downregulated in lung cancer compared with healthy lung tissue
(11). Similar results have been
identified in glioblastoma (12) and
colorectal cancer (13). Notably, the
downregulation of miR-137 in lung cancer cells may be rescued
following treatment with a DNA methylation inhibitor. Furthermore,
miR-137 has a tumor suppressor function by directly targeting cell
division cycle 42 (CDC42) mRNA to inhibit the proliferation and
invasion of colorectal cancer cells (10). miR-137 has been revealed to be
upregulated following preoperative capecitabine chemoradiotherapy
in rectal cancer. In addition, a previous study reported that
higher levels of miR-137 were associated with a poor response to
therapy (14).
There has been a focus on identifying various tumor
suppressor genes and oncogenes regulated by miRNAs in lung cancer,
which can effect cancer cell survival and proliferation, such as
solute carrier family 22 member 18 (SLC22A18) (15), KIT proto-oncogene receptor tyrosine
kinase (16), kruppel like factor 8
(17), epidermal growth factor
receptor and MET proto-oncogene receptor tyrosine kinase (18). Novel oncogenes and tumor suppressor
genes are continuously being identified. Steroid receptor
coactivator-3 (SRC3, also called NCOA3) (19) is a member of the SRC p1600 family that
includes SRC1 (also called NCOA1 or F-SRC-1) and SRC2 (also called
TIF2, GRIP1 or NCOA2) (20), which
have been reported to be important oncogenes that are overexpressed
in lung cancer, in which they promote tumor development and
progression. In addition, SRC3 levels have been demonstrated to be
elevated in breast cancer cells (21), a glucocorticoid-sensitive SCLC model
(22), pancreatic adenocarcinoma
(23), NSCLC cell lines (24) and NSCLC cells in vivo (25). Substantial data has demonstrated that
overexpression of SRC3 may promote carcinoma cell proliferation,
which is regulated by various miRNAs, such as miR-17-5, −17-92 and
−195 (19,26).
Although a number of studies investigating the
effects of miR-137 in lung cancer have been performed, the role of
miR-137 in cell proliferation remains unclear. Whether the
regulation of miR-137 is associated with the overexpression of SRC3
in lung cancer remains to be further investigated, which may aid in
the development of novel strategies for the early diagnosis and
treatment of lung cancer.
Materials and methods
Human tissue samples
NSCLC tissue and healthy tissue samples were
collected between September 2014 and March 2015 during routine
therapeutic surgery at the Department of Respiratory Medicine of
Shaanxi Provincial People's Hospital (Xi'an, China). Written
informed consent was obtained from all patients and the present
study was approved by the Shaanxi Provincial People's Hospital
Institutional Review Board.
Cell culture and transfection
Human NSCLC cell lines A549 and NCI-H838 (American
Type Culture Collection, Manassas, VA, USA) were stored in the lab
of Shaanxi Provincial People's Hospital. The A549 and NCI-H838
cells were cultured in Dulbecco's modified Eagle medium (DMEM)
supplemented with 10% fetal bovine serum (FBS) (both Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA), streptomycin (100
µg/ml) and penicillin (100 U/ml). A549 and NCI-H838 cells were
incubated in a humidified atmosphere containing 5% CO2
at 37°C. miR-137 mimic (5′-UAUUGCUUGAGAAUACACGUAG-3′) and scramble
control mimic (5′-UUCUCCGAACGUGUCACGUTT-3′) were purchased from
Guangzhou RiboBio Co., Ltd. (Guangzhou, China). Oligonucleotides
were transfected into A549 and NCI-H838 cells when 80% confluency
was achieved at a final concentration of 50 nM using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol.
Reverse transcription
semi-quantitative polymerase chain reaction (PCR)
Total RNA was extracted from healthy lung and NSCLC
tissue samples using TRIzol® reagent (Invitrogen; Thermo
Fisher Scientific, Inc.). Total RNA (1 µg) was reverse-transcribed
into cDNA using ReverTra Ace transcriptase with random primers (100
ng) (both from Toyobo Co., Ltd., Osaka, Japan). Reverse
transcription thermal cycling conditions were 10 min at 25°C,
followed by 40 min at 37°C and 10 min at 70°C. Semi-quantitative
PCR were performed using the StepOne™ Real-time PCR system (Thermo
Fisher Scientific, Inc.) with the SYBR Green master mix (Applied
Biosystems; Thermo Fisher Scientific, Inc.). Each PCR contained 100
ng cDNA and thermal cycling conditions were 2 min at 50°C, followed
by 10 min at 95°C and forty cycles of 10 sec at 95°C and 1 min at
60°C. The relative expression of miR-137, SRC3, proliferating cell
nuclear antigen (PCNA), cyclin E, cyclin A1, cyclin A2 and p21
genes were validated using semi-quantitative PCR analysis. The
primers used were as follows: miR-137 forward,
5′-GCGCTTATTGCTTAAGAATAC-3′ and reverse, 5′-CAGTGCAGGGTCCGAGGT-3′;
SRC3 forward, 5′-CGTCCTCCATATAACCGAGC-3′ and reverse,
5′-TCATAGGTTCCATTCTGCCG-3′; PCNA forward,
5′-CCGGGACCTTAGCCATATTG-3′ and reverse,
5′-GCTGAACTGGCTCATTCATCTC-3′; cyclin E1 forward,
5-'GCATCACAACAGAATATCATAA-3′ and reverse,
5′-AAGCACCATCAGTAACATAA-3′; cyclin A1 forward, GACCTGTCACTGTCTTGTAC
and reverse, CGTTTGGAGTGGTAGAAATC; cyclin A2 forward,
5′-CACGTACCTTAGGGAAATGG-3′ and reverse, 5′-CCAAATGCAGGGTCTCATTC-3′;
p21 forward, 5′-AAGACCATGTGGACCTGTCA-3′ and reverse,
5′-CGTTTGGAGTGGTAGAAATCTG-3′. The expression level of β-actin was
used as an internal control with the following primers: Forward,
5′-GTGGACATCCGCAAAGAC-3′ and reverse,
5′-AAAGGGTGTAACGCAACTA-3′.
Soft agar colony formation assay
Colony formation assays in soft agar were performed
to assess the proliferation ability of NSCLC cells following
transfection with miRNA-137 mimic and scramble mimic. Logarithmic
phase cells were treated with 0.25% trypsin. Six-well plates were
coated with 0.5% agarose containing 2X DMEM, 10% FBS and
antibiotics. A 0.35% agarose containing the same supplements was
placed on top of the previously prepared layer, followed by a
suspension solution of 4×103 cells/well was subsequently placed on
top of that, then the plate was incubated for 21 days at 37°C in a
humidified atmosphere with 5% CO2. Cells were stained
with crystal violet and the number of foci was counted by eye using
a Tanon 5200 chemiluminescent imaging system (Tanon Science and
Technology Co., Ltd., Shanghai, China).
MTT assay
NSCLC A549 and NCI-H838 cell proliferation following
miR-137 mimic and scramble mimic transfection was assessed using an
MTT assay. NSCLC cells at a density of 4×103 cells/well were seeded
into 96-well plates following counting. Then, 5 mg/ml MTT was added
into each well every 24 h for 7 days and incubated at 37°C for 4 h.
Then, crystals were dissolved with dimethyl sulfoxide, and the
absorbance of each well was measured with a microplate reader set
at 560 nm and replaced back into incubator.
Cell cycle analysis
Flow cytometry was used to determine the cell cycle
distribution and analyzed using ModFit LT™ software (Verity
Software House, Inc., Topsham, ME, USA) with propidium iodide (PI)
staining. Following transfection with miR-137 mimic or scramble
mimic, A549 and NCI-H838 cells were seeded at a density of 1×106
cells/well into 6-well plates. Cells were subsequently fixed with
70% ice-cold ethanol for 12 h at 4°C and then harvested through
centrifugation at (149 × g), 4°C for 5 min. Cells were then
washed with PBS three times and stained with 50 µg/ml PI containing
50 µg/ml RNase A, prior to being incubated in the dark for 30 min
at room temperature.
Luciferase reporter assays
To observe the binding of miR-137 to SRC3 mRNA, the
wild-type 3′-UTR and mutant 3′-UTR segments of SRC3 mRNA were
amplified using the KOD-Plus PCR kit according to the
manufacturer's protocol (Toyobo Co., Ltd.) and inserted into the
pGL3-basic luciferase vector (Promega Corporation, Madison, WI,
USA). The mutated putative miR-137 binding site in the SRC3 3′-UTR
was generated using a Site-Directed Gene Mutagenesis kit (Beyotime
Institute of Biotechnology, Haimen, China) according to the
manufacturer's protocol. NSCLC cells were seeded at a density of
~1×105 cells/well into 24-well plates and cultured for 24 h prior
to transfection. Co-transfections with 500 ng pGL3-Basic firefly
luciferase reporter and 100 nM miR-137 mimic or scramble mimic into
cells using Lipofectamine 2000 for 4 h at 37°C was performed.
Luciferase activity was measured at 48 h following transfection
using a Luciferase Reporter Gene Assay kit (Promega
Corporation).
Western blot analysis
A total of 48 h following transfection with miR-137
mimic or scramble mimic, total protein was extracted from the cell
lysates following lysis using a RIPA buffer (150 mM NaCl, 1%
Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS, 50 mM Tris, pH
8.0) and quantification using a BCA Protein Assay kit (Bio-Rad
Laboratories, Inc., Hercules, CA, USA) of human NSCLC A549 and
NCI-H838 cells. Total protein was separated on a 12% SDS-PAGE gel
(20 µg/lane) and transferred to nitrocellulose membranes. The
membranes were blocked with 5% non-fat milk for 2 h at room
temperature, then incubated at 4°C for 12 h with primary antibodies
directed against SRC3 (cat. no. 2126; Cell Signaling Technology,
Inc., Danvers, MA, USA) and β-actin (cat. no. A1978; Sigma; Thermo
Fisher Scientific, Inc.) (both dilution 1:1,000). Following washing
with TBS-Tween 20, membranes were incubated at 37°C for 1 h with
horseradish peroxidase-conjugated secondary antibody (cat. no.
7074; dilution, 1:2,000; Cell Signaling Technology, Inc.) and then
visualized using a Pierce™ ECL Western Blotting kit (Thermo Fisher
Scientific, Inc.).
Statistical analysis
Each experiment was repeated ≥3 times. Statistical
analysis was performed using SPSS software (version 12.0; SPSS,
Inc., Chicago, IL, USA). All results are presented as the mean ±
standard deviation and were compared using a two-tailed Student's
t-test. Linear correlation analysis was used to analyze the
correlation between the expression of protein and miRNA in the
clinical samples. P<0.05 was considered to indicate a
statistically significant difference.
TargetScan Prediction
miRNA and UTR targeting predictions were queried
using the target prediction database, TargetScan (http://www.targetscan.org).
Results
Negative correlation between miR-137
and SRC3 expression in NSCLC tissue samples
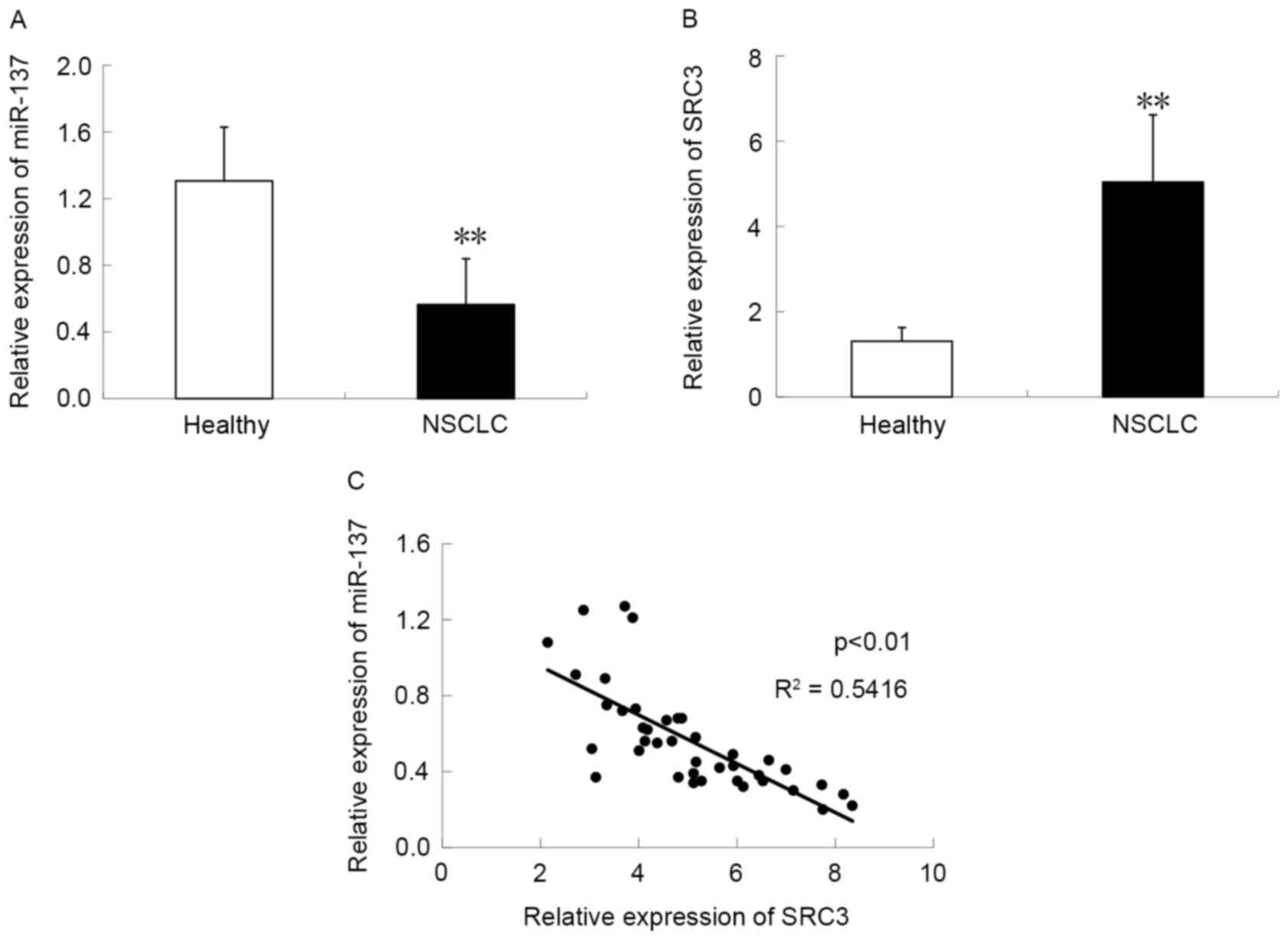
miR-137 expression was measured in 40 healthy lung
fibroblast and NSCLC tissue samples using semi-quantitative PCR.
The data demonstrated that compared with healthy lung tissue
samples, the NSCLC tissue group had significantly reduced
endogenous miR-137 expression (P<0.05; Fig. 1A), suggesting a role for miR-137 in
NSCLC. The expression of SRC3 mRNA was investigated and was
significantly increased in NSCLC tissue compared with adjacent
healthy tissue samples (P<0.05; Fig.
1B). Furthermore, to evaluate miR-137 modulation of SRC3 at the
gene expression level, the association between the expression of
miR-137 and SRC3 was investigated in NSCLC tissue samples. It was
demonstrated that miR-137 expression was negatively correlated with
SRC3 expression in NSCLC (R2=0.5416; P<0.01; Fig. 1C), indicating that miR-137 regulates
the expression of SRC3.
miR-137 inhibits NSCLC cell
proliferation
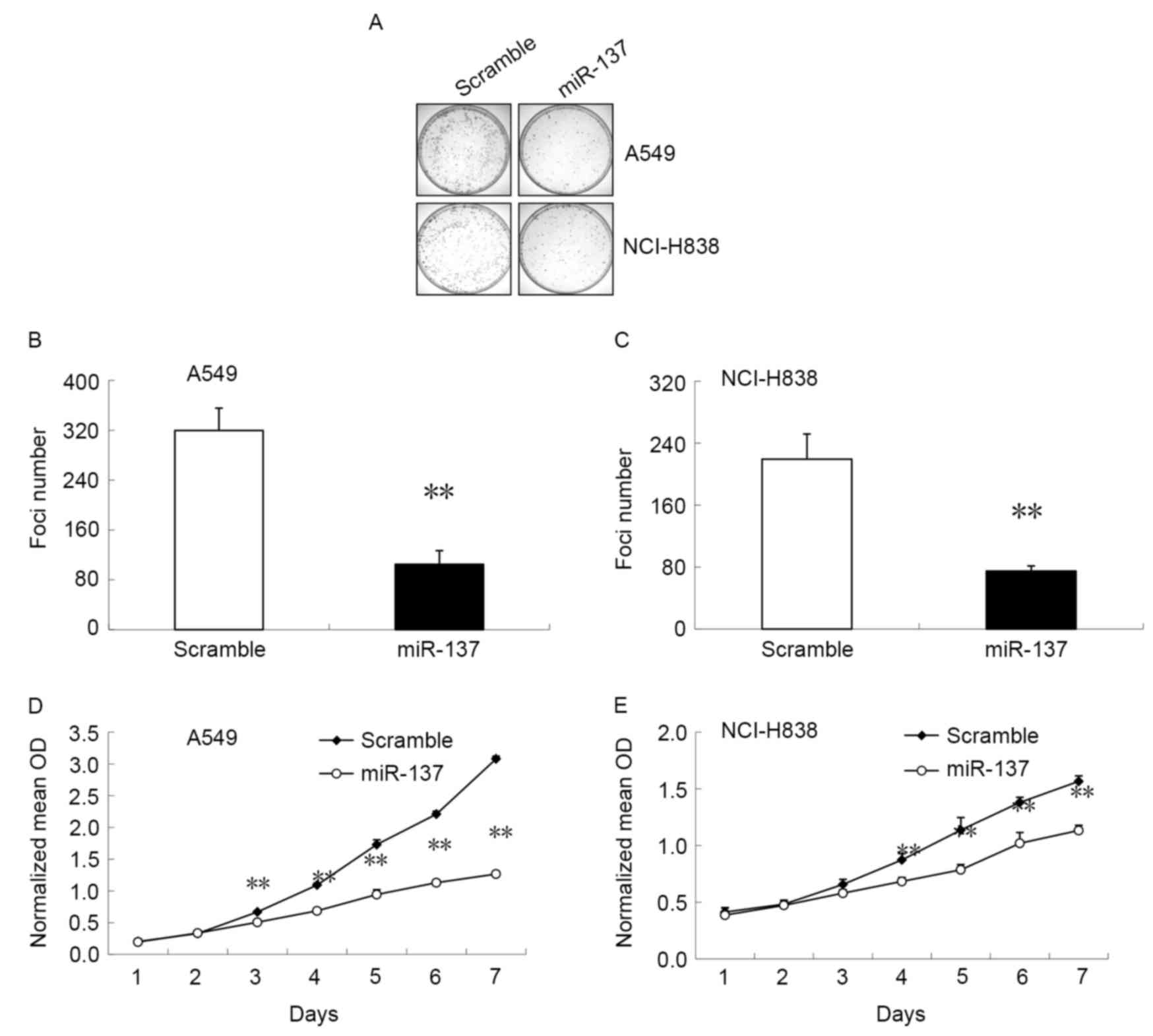
In order to evaluate the association between miR-137
expression and NSCLC cell growth, miR-137 mimic or scramble mimic
were transfected into A549 and NCI-H838 cells. Cell proliferation
was measured using a soft agar colony formation assay (Fig. 2A-C) and an MTT assay (Fig. 2D and E). The results demonstrated that
cells transfected with miR-137 mimic had a significant decrease in
foci number (P<0.05; Fig. 2B and
C) and significantly reduced metabolic activity (P<0.05;
Fig. 2D and E) by day 4 compared with
cells transfected with the scramble mimic. These results indicate
that miR-137 can induce cell colony formation and efficiently
inhibit the proliferation of NCI-H838 and A549 NSCLC cells.
miR-137 induces cell cycle arrest and
reduces the mRNA expression of cell cycle-associated proteins in
NSCLC cells
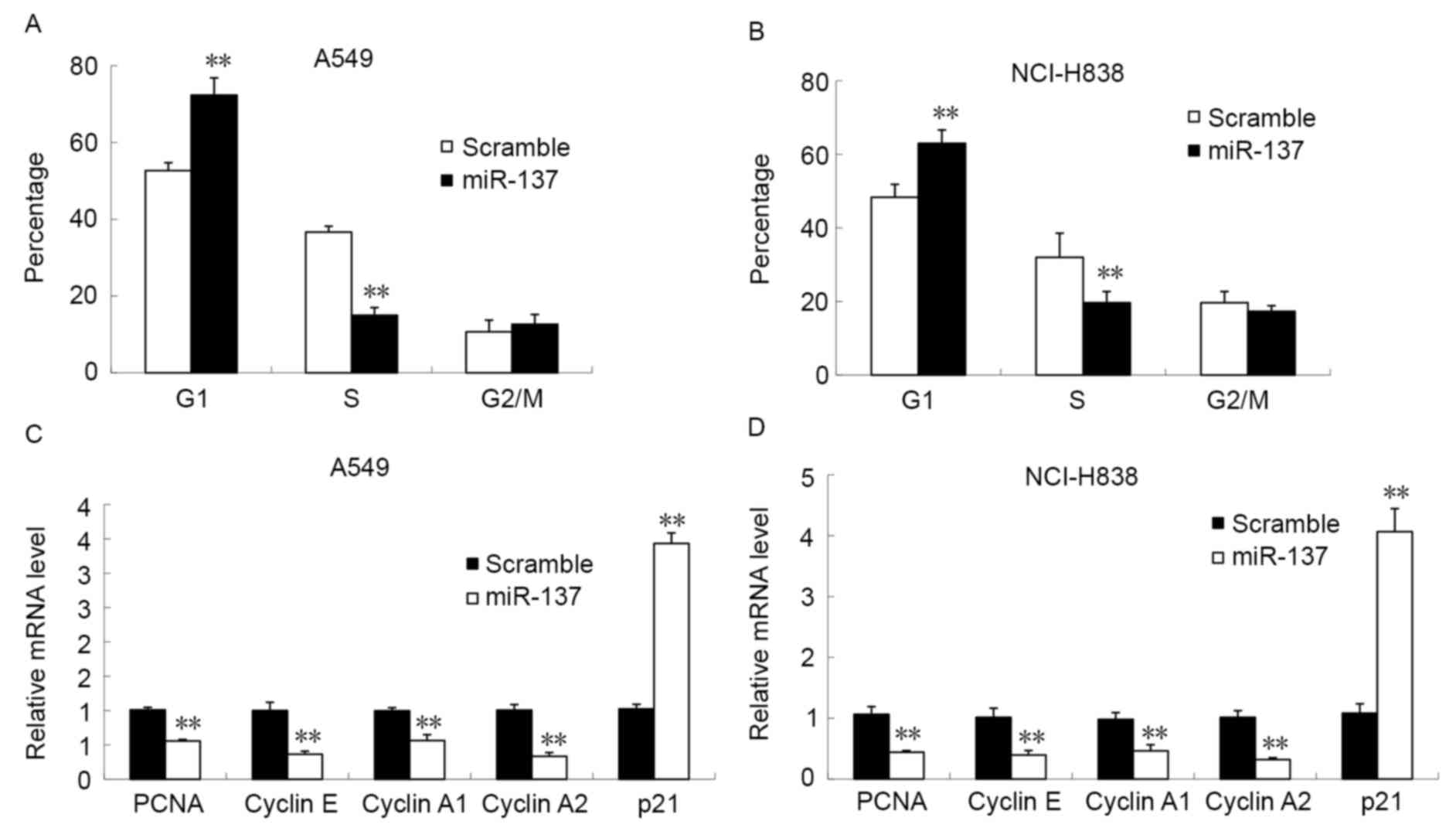
The cell cycle distribution of A549 and NCI-H838
cells was analyzed using flow cytometry following transfection with
miR-137 mimic or scramble mimic (Fig.
3). Following transfection with miR-137 there was a significant
reduction in the percentage of cells in the S-phase of the cell
cycle and a significant increase in the number of G1
cells in A549 (Fig. 3A) and NCI-H838
(Fig. 3B) cells compared with in the
scramble mimic control groups (all P<0.05). However, no
significant differences were observed in the percentage of cells in
the G2/M phase of the cell cycle between the miR-137
mimic- and scramble mimic-transfected groups. To further ascertain
the molecular mechanisms underlying miR-137-induced G1
cell cycle arrest, the expression level of PCNA, cyclin E, cyclin
A1, cyclin A2 and p21 in the NSCLC cells lines was measured. This
demonstrated that miR-137 mimic transfection significantly
decreased the mRNA expression of PCNA, cyclin E, cyclin A1 and
cyclin A2, and significantly increased the expression of p21, in
A549 (Fig. 3C) and NCI-H838 (Fig. 3D) cells compared with the scramble
mimic group (all P<0.05). This data indicates that
overexpression of miR-137 results in G1 phase arrest and
affects the expression of cell cycle-associated proteins.
miR-137 targets SRC3 mRNA in NCI-H838
and A549 cells
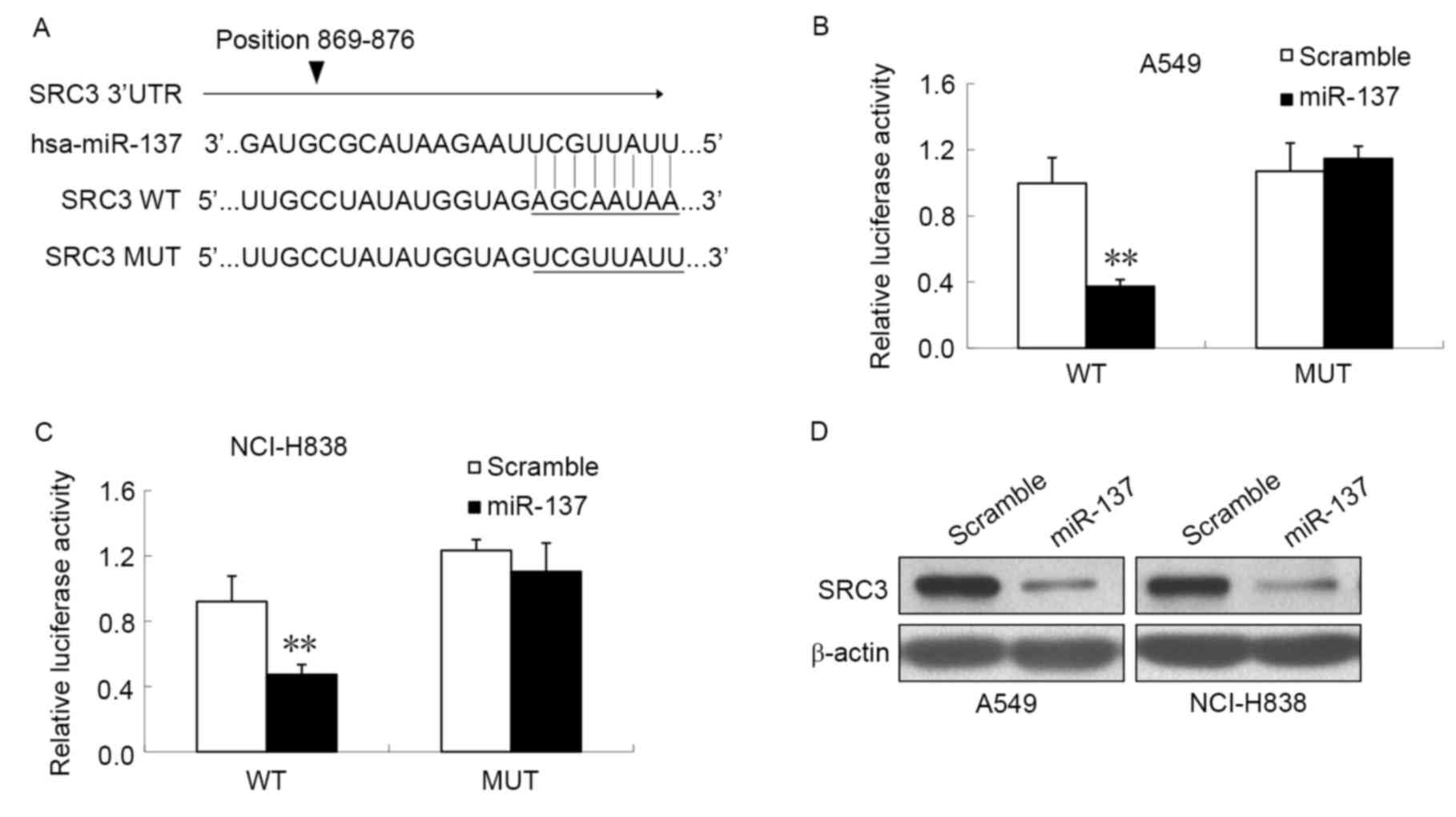
To investigate whether miR-137 directly targeted
SRC3 mRNA in NCI-H838 and A549 cells, the specific locations of the
binding sites were searched for using TargetScan, which predicted a
putative binding site for miR-137 within the 3′UTR of SRC3 TR at
nucleotides 869–876 (Fig. 4A). To
further understand the association between miR-137 and the 3′UTR of
SRC3, luciferase reporter genes were constructed with the 3′UTR of
SRC3, with or without mutations at the predicted miR-137 binding
regions, and the luciferase activity following miR-137 mimic
transfection was measured. As illustrated in Fig. 4B, miR-137 mimic transfection resulted
in a significant reduction in luciferase activity when
co-transfected with the wild-type SRC3 3′-UTR compared with the
scramble control in A549 cells (P<0.05). However, no significant
difference in luciferase activity was observed in cells
co-transfected with mutant SRC3 3′-UTR and miR-137 compared with
the scramble control. Similar results were also observed in the
NCI-H838 cell line (Fig. 4C).
Finally, western blot analysis was performed to analyze SRC3
protein expression following transfection with miR-137 mimic or
scramble mimic (Fig. 4D). The results
revealed a notable decrease in SRC3 protein expression in NCI-H838
cells and A549 cells transfected with miR-137 mimic compared with
scramble mimic, which suggests that SRC3 may be a target of miR-137
in NSCLC. These results suggest that miR-137 binds to the 3′-UTR of
SRC3 and thus reduces the protein expression of SRC3.
Discussion
An increasing number of studies have identified that
miR-137 is frequently downregulated in numerous types of cancer
cell, such as in brain stem tumor (27), colorectal cancer (28) and NSCLC (9) cells. However, several studies have
reported that miR-137 is upregulated in lung cancer tissue
(29), suggesting that miR-137 may
serve different roles in different tumor microenvironments.
Therefore, investigating the mechanisms underlying the involvement
of miR-137 in different types of cancer may have important clinical
implications.
In the present study, miR-137 was identified to be
significantly downregulated in NSCLC samples compared with adjacent
healthy tissue, which is consistent with previous studies performed
by Zhu et al (10) and Bi
et al (9). Previous studies
have demonstrated that a low expression of miR-137 inhibits cell
growth in vivo and in vitro through G1
phase cell cycle arrest, and impairs cell migration and invasion
(7,27). To investigate the effect of miR-137 on
NSCLC cell growth, the colony forming and proliferation ability of
cells was determined using soft agar colony formation assays and
MTT assays, respectively. Transfection of miR-137 mimic resulted in
a significant decrease in foci number and metabolic activity
compared with the scramble mimic control groups. The results of the
present study confirm that miR-137 induces G1/S cell
cycle arrest; however, what causes cells to remain in the quiescent
state remains unclear.
PCNA is a DNA polymerase sliding clamp that serves a
role in DNA replication and repair (30). A previous study demonstrated that a
high PCNA index resulted in an increase in cyclin E2 and
proliferative activity in pancreatic cancer cells (31). Low levels of cyclins, particularly
cyclin A and cyclin E have been suggested as a characteristic
marker of cellular quiescence (32).
For instance, the downregulation of cyclin E2, through regulation
by miR-26a, was demonstrated to induce cell cycle arrest, inhibit
cell proliferation and decrease tumor growth in pancreatic cancer
(31). As a cell cycle arrest
protein, p21 is able to protect cells from apoptosis and induces
G1 phase cell cycle arrest by inactivating cyclin
A/cyclin dependent kinase (CDK)2 complexes (33). Furthermore, ectopic expression of
miR-218 was demonstrated to induce G1/S checkpoint
arrest, which resulted in the inhibition of glioma cell
proliferation through the upregulation of p21 (34). Therefore, the expression of PCNA,
cyclin E, cyclin A1, cyclin A2 and p21 were investigated in the
present study. The results of the current study demonstrated that
miR-137 mimic transfection significantly decreased the mRNA
expression of PCNA, cyclin E, cyclin A1, cyclin A2, and
significantly increased expression of p21 compared with the
control. This further indicates that miR-137 induces NSCLC cell
G1 phase arrest and suppresses cell proliferation by
dysregulating the expression of different cell cycle proteins.
The inhibition of cell proliferation induced by low
level miR-137 expression in NSCLC cell lines typically depends on
the regulation of various target genes. For instance, miR-137 was
revealed to suppress cell growth, migration and invasion in NSCLC
cells through targeting paxillin (9),
which encodes a focal adhesion-associated protein. Ectopic
expression of miR-137 in lung cancer cells was demonstrated to
significantly induce G1 phase cell cycle arrest,
resulting in a significant decrease in cell growth in vivo
and in vitro through downregulation of CDC42 and CDK6. A
previous study revealed that miR-137 negatively regulates
estrogen-related receptor α expression, an orphan nuclear receptor
that was identified as a novel biomarker of breast cancer, and is
involved in the repression of breast cancer cell proliferation and
migration (8). Previous studies have
identified SLC22A18 (15) and
carboxyl-terminal binding protein 1 (35) as other target genes regulated by
miR-137 in tumor cells.
Although SRC3 has been reported to be a target of
miR-17-5p in breast cancer cells (19) and miR-195 in hepatocellular carcinoma
cells (26), the association between
miR-137 and SRC3 has not yet been experimentally validated in
NSCLC, to the best of our knowledge. Similar to previous identified
target genes of miR-137 (8), in the
present study SRC3 was demonstrated to be overexpressed in NSCLC
samples, and a significantly negative correlation was identified
between the expression of miR-137 and SRC3 in NSCLC tissue. In
order to investigate the mechanisms of whether miR-137 inhibited
cell proliferation through modulating SRC3 expression, a series of
experiments were performed. TargetScan was used to predict a
putative binding site for miR-137 within the SRC3 3′-UTR at
nucleotides 869–876. In addition, wild-type SRC3 3′-UTR luciferase
activity was significantly decreased following transfection with
miR-137 mimic, but not in the mutant construct. Further experiments
demonstrated that the expression of SRC3 was significantly reduced
upon transfection with miR-137 mimic in A549 and NCI-H838
cells.
In conclusion, the results of the current study
demonstrate that miR-137 inhibits cell proliferation in NSCLC by
targeting the 3′UTR of SRC. Therefore, miR-137 may aid in providing
novel diagnostic and therapeutic options for the treatment of
patients with NSCLC in the future.
References
|
1
|
Jemal A, Center MM, DeSantis C and Ward
EM: Global patterns of cancer incidence and mortality rates and
trends. Cancer Epidemiol Biomarkers Prev. 19:1893–1907. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yu JL, Simmons C, Victor JC, Han D,
Hogeveen S, Leighl N and Verma S: Impact of new chemotherapeutic
and targeted agents on survival in stage IV non-small cell lung
cancer. Oncologist. 16:1307–1315. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
He L and Hannon GJ: MicroRNAs: Small RNAs
with a big role in gene regulation. Nat Rev Genet. 5:522–531. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Du L, Borkowski R, Zhao Z, Ma X, Yu X, Xie
XJ and Pertsemlidis A: A high-throughput screen identifies miRNA
inhibitors regulating lung cancer cell survival and response to
paclitaxel. RNA Biol. 10:1700–1713. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Guo HL, Ingolia NT, Weissman JS and Bartel
DP: Mammalian microRNAs predominantly act to decrease target mRNA
levels. Nature. 466:835–840. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen QJ, Chen XB, Zhang MZ, Fan QX, Luo SX
and Cao XG: miR-137 is frequently down-regulated in gastric cancer
and is a negative regulator of Cdc42. Dig Dis Sci. 56:2009–2016.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhao YY, Li YP, Lou GY, Zhao L, Xu Z,
Zhang Y and He F: MiR-137 targets estrogen-related receptor alpha
and impairs the proliferative and migratory capacity of breast
cancer cells. PLoS One. 7:e391022012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bi Y, Han Y, Bi H, Gao F and Wang X:
miR-137 impairs the proliferative and migratory capacity of human
non-small cell lung cancer cells by targeting paxillin. Hum Cell.
27:95–102. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhu X, Li Y, Shen H, Li H, Long L, Hui L
and Xu W: miR-137 inhibits the proliferation of lung cancer cells
by targeting Cdc42 and Cdk6. FEBS Lett. 587:73–81. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dacic S, Kelly L, Shuai Y and Nikiforova
MN: miRNA expression profiling of lung adenocarcinomas: Correlation
with mutational status. Mod pathol. 23:1577–1582. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ciafrè SA, Galardi S, Mangiola A, Ferracin
M, Liu CG, Sabatino G, Negrini M, Maira G, Croce CM and Farace MG:
Extensive modulation of a set of microRNAs in primary glioblastoma.
Biochem Biophys Res Commun. 334:1351–1358. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bandrés E, Cubedo E, Agirre X, Malumbres
R, Zárate R, Ramirez N, Abajo A, Navarro A, Moreno I, Monzó M and
García-Foncillas J: Identification by Real-time PCR of 13 mature
microRNAs differentially expressed in colorectal cancer and
non-tumoral tissues. Mol Cancer. 5:292006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Svoboda M, Izakovicova Holla L, Sefr R,
Vrtkova I, Kocakova I, Tichy B and Dvorak J: Micro-RNAs miR125b and
miR137 are frequently upregulated in response to capecitabine
chemoradiotherapy of rectal cancer. Int J Oncol. 33:541–547.
2008.PubMed/NCBI
|
|
15
|
Zhang B, Liu T, Wu T, Wang Z, Rao Z and
Gao J: microRNA-137 functions as a tumor suppressor in human
non-small cell lung cancer by targeting SLC22A18. Int J Biol
Macromol. 74:111–118. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li P, Ma L, Zhang Y, Ji F and Jin F:
MicroRNA-137 down-regulates KIT and inhibits small cell lung cancer
cell proliferation. Biomed Pharmacother. 68:7–12. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shi H, Ji Y, Zhang D, Liu Y and Fang P:
MiR-135a inhibits migration and invasion and regulates EMT-related
marker genes by targeting KLF8 in lung cancer cells. Biochem
Biophys Res Commun. 465:125–130. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhen Q, Liu J, Gao L, Liu J, Wang R, Chu
W, Zhang Y, Tan G, Zhao X and Lv B: MicroRNA-200a targets EGFR and
c-Met to inhibit migration, invasion and gefitinib resistance in
non-small cell lung cancer. Cytogenet Genome Res. 146:1–8. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hossain A, Kuo MT and Saunders GF:
Mir-17-5p regulates breast cancer cell proliferation by inhibiting
translation of AIB1 mRNA. Mol Cell Biol. 26:8191–8201. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xu J and OMalley BW: Molecular mechanisms
and cellular biology of the steroid receptor coactivator (SRC)
family in steroid receptor function. Rev Endocr Metab Disord.
3:185–192. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Anzick SL, Kononen J, Walker RL, Azorsa
DO, Tanner MM, Guan XY, Sauter G, Kallioniemi OP, Trent JM and
Meltzer PS: AIB1, a steroid receptor coactivator amplified in
breast and ovarian cancer. Science. 277:965–968. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Waters CE, Stevens A, White A and Ray DW:
Analysis of co-factor function in a glucocorticoid-resistant small
cell carcinoma cell line. J Endocrinol. 183:375–383. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Henke RT, Haddad BR, Kim SE, Rone JD, Mani
A, Jessup JM, Wellstein A, Maitra A and Riegel AT: Overexpression
of the nuclear receptor coactivator AIB1, (SRC-3) during
progression of pancreatic adenocarcinoma. Clin Cancer Res.
10:6134–6142. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Suen CS, Berrodin TJ, Mastroeni R, Cheskis
BJ, Lyttle CR and Frail DE: A transcriptional coactivator, steroid
receptor coactivator-3, selectively augments steroid receptor
transcriptional activity. J Biol Chem. 273:27645–27653. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang H, Zhang D, Wu W, Zhang J, Guo D,
Wang Q, Jing T, Xu C, Bian X and Yang K: Overexpression and
gender-specific differences of SRC-3 (SRC-3/AIB1) immunoreactivity
in human non-small cell lung cancer: an in vivo study. J Histochem
Cytochem. 58:1121–1127. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jiang HL, Yu H, Ma X, Xu D, Lin GF, Ma DY
and Jin JZ: MicroRNA-195 regulates steroid receptor coactivator-3
protein expression in hepatocellular carcinoma cells. Tumour Biol.
35:6955–6960. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Silber J, Lim DA, Petritsch C, Persson AI,
Maunakea AK, Yu M, Vandenberg SR, Ginzinger DG, James CD, Costello
JF, et al: miR-124 and miR-137 inhibit proliferation of
glioblastoma multiforme cells and induce differentiation of brain
tumor stem cells. Bmc Med. 6:142008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Balaguer F, Link A, Lozano JJ, Cuatrecasas
M, Nagasaka T, Boland CR and Goel A: Epigenetic Silencing of
miR-137 is an early event in colorectal carcinogenesis. Cancer Res.
70:6609–6618. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Huang T, Jiang M, Kong XY and Cai YD:
Dysfunctions associated with methylation, microRNA expression and
gene expression in lung cancer. PLoS One. 7:e434412012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shiomi N, Mori M, Tsuji H, Imai T, Inoue
H, Tateishi S, Yamaizumi M and Shiomi T: Human RAD18 is involved in
S phase-specific single-strand break repair without PCNA
monoubiquitination. Nucleic Acids Res. 35:e92007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Deng J, He M, Chen L, Chen C, Zheng J and
Cai Z: The loss of miR-26a-mediated post-transcriptional regulation
of cyclin E2 in pancreatic cancer cell proliferation and decreased
patient survival. PLoS One. 8:e764502013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cheung TH and Rando TA: Molecular
regulation of stem cell quiescence. Nat Rev Mol Cell Bio.
14:329–340. 2013. View
Article : Google Scholar
|
|
33
|
Levkau B, Koyama H, Raines EW, Clurman BE,
Herren B, Orth K, Roberts JM and Ross R: Cleavage of p21
(Cip1/Waf1) and p27 (Kip1) mediates apoptosis in endothelial cells
through activation of Cdk2: Role of a caspase cascade. Mol Cell.
1:553–563. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jun GJ, Zhong GG and Ming ZS: miR-218
inhibits the proliferation of glioma U87 cells through the
inactivation of the CDK6/cyclin D1/p21(Cip1/Waf1) pathway. Oncol
Lett. 9:2743–2749. 2015.PubMed/NCBI
|
|
35
|
Deng Y, Deng H, Bi F, Liu J, Bemis LT,
Norris D, Wang XJ and Zhang Q: MicroRNA-137 targets
carboxyl-terminal binding protein 1 in melanoma cell lines. Int J
Biol Sci. 7:133–137. 2011. View Article : Google Scholar : PubMed/NCBI
|