Introduction
Photodynamic therapy (PDT) is a photochemical
modality, which has been suggested to be a potential novel
treatment for various types of cancer, including esophageal cancer,
glioblastoma and breast cancer (1–3). In the
PDT process, the photosensitizer (PS) serves a critical function.
It selectively concentrates at the tumor tissue and is activated by
light irradiation of an appropriate wavelength. This is followed by
the generation of reactive oxygen species (ROS), which result in in
cellular destruction (4,5), in particular by inducing cell apoptosis.
The mitochondrial pathway has been demonstrated to be involved in
cell apoptosis induced by PDT (6,7).
Aloe-emodin (AE) has been revealed to possess
antitumor activity and induce apoptosis in various types of cancer
(8–10). However, long term use of AE may lead
to adverse reactions such as acute renal failure (11). Additionally, AE was found to exhibit
fluorescence with a maximum excitation wavelength of 430 nm
(12,13), which indicates that AE has the
potential to be a novel PS. Given the antitumor activity of AE and
the potential to be a novel PS, AE-induced PDT (with a low
concentration of AE) may be applied as a new therapeutic modality
for the treatment of tumor. AE-induced PDT with a lower
concentration of AE compared with AE used alone may decrease the
aforementioned side effect (acute renal failure) when AE is used
alone (14). However, as a potential
PS, the effect of AE-induced photodynamic activity on tumor cells
has been rarely studied (14).
PDT-induced apoptosis may be activated via the
mitochondrial pathway (6,7) and is associated with the inhibitive
effect of PDT on cancer cells (4,5). The
authors of the present study hypothesized that the AE-induced
photodynamic effect on cancer cell may be associated with apoptosis
and the mitochondrial pathway. In order to investigate the
AE-induced photodynamic effect on cancer cells, human gastric
cancer cells were selected. Apoptosis and expression levels of
caspases involved in the mitochondrial pathway were evaluated in
the present study.
Materials and methods
Reagents and cell line
AE was purchased from Jiangxi Tiangong Technology
Co., Ltd. (Nanchang, China). Dimethyl sulfoxide (DMSO) and the MTT
kit were obtained from Sigma-Aldrich; Merck KGaA (Darmstadt,
Germany). The light emitting diode (LED) was supplied by Chongqing
Jingyu Laser Biology Research Institute Co., Ltd. (Chongqing,
China). RPMI-1640 medium was purchased from Hyclone (GE Healthcare
Life Sciences Logan, UT, USA). Fetal bovine serum (FBS) was
obtained from Biological Industries Co., Ltd. (Kibbutz Beit-Haemek,
Israel). The SGC-7901 gastric cancer cell line was supplied by the
China Center for Type Culture Collection at Wuhan University
(Wuhan, China). The One Step TUNEL apoptosis assay kit (Beyotime
Institute of Biotechnology, Shanghai, China) and annexin V
fluorescein isothiocyanate apoptosis detection kit (Beijing Biosea
Biotechnology Co., Ltd., Beijing, China) were used for detection of
cell apoptosis. DAPI was purchased from Nanjing KeyGen Biotech,
Co., Ltd. (Nanjing, China). Antibodies anti-caspase-3 (catalog no.
9662) and anti-caspase-9 (catalog no. 9502) for western blot
analysis were obtained from Cell Signaling Technology, Inc.
(Danvers, MA, USA). Anti-β-actin (catalog no. 4E8H3) antibody for
western blot analysis was purchased from Santa Cruz Biotechnology
Inc., (Dallas, TX, USA). The secondary antibodies used included
horseradish peroxidase (HRP)-labeled goat anti-rabbit
immunoglobulin G (IgG) antibody (catalog no. 20320021), which was
purchased from Bioworld Technology, Inc., (St. Louis Park, MN, USA)
and HRP-labeled goat anti-mouse IgG antibody (catalog no. BA1050),
which was purchased from Boster Biological Technology, Ltd (Wuhan,
China).
Cell culture
SGC-7901 gastric cancer cells were cultured in
RPMI-1640 medium supplemented with 10% FBS. The cells were
incubated at 37°C in a humidified atmosphere containing 5%
CO2 for 1–2 days to attach and colonize. The cells at
70–80% confluence were used for subsequent experiments.
MTT assay to detect the effect of AE
on human gastric cancer cell viability
The SGC-7901 cells (5×103 cells/well)
were grown in a 96-well plate and were cultured for 24 h and
randomly assigned into control and AE groups. In the AE group,
various final concentrations (0.1, 1.0, 10, 20, 30, 40 and 50 µM)
of AE with RPMI-1640 medium were added into the wells, and an
equivalent amount of the medium without AE was added to the control
group. Subsequently, the cells in the two groups were cultured in
the dark for 24 h. Following this, the cells were incubated in
medium with MTT (5 µg/ml, 20 µl for each well) for 4 h, and 150 µl
DMSO was added, followed by incubation in a shaker at room
temperature for 10 min. The iEMS Analyzer (Thermo Fisher
Scientific, Waltham, MA, USA) was used to analyze the optical
density (OD) of each well at a wavelength of 570 nm, in triplicate.
The survival ratio of the cells (OD of the AE group/OD of the
control group ×100%) was calculated.
MTT assay to determine the effect of
AE-induced PDT on human gastric cancer cell viability
The SGC-7901 cells (5×103 cells/well)
grown in a 96-well plate were cultured for 24 h and randomly
assigned into the illumination group and the AE-induced PDT group.
In the AE-induced PDT group, RPMI-1640 medium containing 10 µM AE
was added into the wells, and the equivalent amount of the medium
without AE was added in the light control group. The cells in the
two groups were cultured in the dark for 6 h at 37°C. Subsequently,
the medium was replaced with 10% FBS. The cells were illuminated by
an LED, which produced UV light with a wavelength of 430 nm and
energy density of 40 mW/cm2. The final energy densities
of 0, 0.8, 1.6, 3.2, 6.4, 12.8 and 25.6 J/cm2 were
achieved with various illumination times (0, 20, 40, 80, 160, 320
and 640 sec, respectively). Following this, the cells were cultured
for 24 h at 37°C in the dark. Subsequently, the cells were
incubated in medium with MTT (5 µg/ml, 20 µl for each well) for 4
h, and 150 µl DMSO was added, followed by incubation in a shaker at
room temperature for 10 min. The iEMS Analyzer was used to analyze
the OD of each well at a wavelength of 570 nm in triplicate. The
cell viability (OD of the illumination group or the AE-induced PDT
group/OD of the control group ×100%) was calculated.
Grouping
According to the results of the above experiments,
the concentration of 10 µM AE coupled with an energy density of
12.8 J/cm2 was selected. The cells were randomly
assigned into four groups as follows: Group I, control group
without AE and light; group II, test group with 10 µM AE and 0
J/cm2 light; group III, test group with 12.8
J/cm2 light but without 10 µM AE; and group IV,
AE-induced PDT group with 10 µM AE and 12.8 J/cm2 light.
The SGC-7901 cells (1×105 cells/well) grown in a 6-well
plate were cultured in darkness for 24 h. The medium was
subsequently replaced with 10% FBS. The cells in groups III and IV
were illuminated with UV light with a wavelength of 430 nm. The
energy density received by the cells was 12.8 J/cm2 at
40 mW/cm2 with 320 sec illumination. The cells were
cultured for 12 h in the dark and were then harvested for the
following detections.
TUNEL assay to determine the rate of
apoptosis of the human gastric cancer cells
The harvested cells were washed in PBS and were
fixed in 4% paraformaldehyde for 30 min. PBS supplemented with 0.1%
Triton X-100 was added and the incubation was performed for 2 min
on ice. Following two washes in PBS, 50 µl TUNEL reaction mixture
was added and incubation was performed at 37°C for 60 min. Then the
cells were washed in PBS three times and stained with 50 µl DAPI
for 10 min. Following washing in PBS three times, the cells were
sealed with 50% glycerol and images were captured using a
fluorescence microscope.
Determination of cell death by flow
cytometry analysis
At 12 h post treatment, cells were trypsinized,
washed with PBS, resuspended in 500 µl annexin V binding buffer
(Beijing Biosea Biotechnology Co., Ltd., Beijing, China) at a
density of 106 cells/ml. Subsequently, a total of 5 µl
annexin V and 5 µl propidium iodide (PI) were added, and the
mixture was gently agitated. The cells were incubated at room
temperature for 15 min in lightproof conditions, and the BD
FACSCalibur flow cytometry system (BD Biosciences, Franklin Lakes,
NJ, USA) was subsequently used. The acquired flow cytometry data
were analyzed by using the FlowJo software (version 10.0, FlowJo,
LLC, Ashland, OR, USA). Each assay condition was performed in
triplicate.
Western blot analysis for caspase-3
and caspase-9
The cells were harvested and washed twice in PBS.
Subsequently, total protein lysate was extracted by using
radio-immunoprecipitation assay buffer (Beyotime Institute of
Biotechnology) supplemented with 1% phenylmethanesulfonyl fluoride
(Beyotime Institute of Biotechnology) for 5 min at 4°C. The
solution was centrifuged at 12,000 × g for 15 min at 4°C,
and the supernatant was collected. The enhanced bicinchoninic acid
assay protein assay kit (Beyotime Institute of Biotechnology) was
used to quantify the protein concentration. Equal amounts of
protein (50 µg) for each group was separated by 10% SDS-PAGE. The
protein bands were transferred to a nitrocellulose membrane, and
the membrane was then blocked with 5% non-fat dry milk in TBS
supplemented with 0.1% Tween-20 (TBST) for 1 h at room temperature.
Following three washes with TBST, the membrane was incubated
overnight with primary antibodies for caspase-3 (1:1,000),
caspase-9 (1:1,000) and β-actin (1:1,000), respectively at 4°C. The
membrane was subsequently washed with TBST for three times and
incubated with secondary antibodies for caspase-3 (HRP-labeled goat
anti-rabbit IgG antibody; 1:8,000), caspase-9 (HRP-labeled goat
anti-rabbit IgG antibody; 1:8,000) and β-actin (HRP-labeled goat
anti-mouse IgG antibody; 1:2,000), respectively, at room
temperature for 1 h. The immunoreactive bands of protein were
developed using an enhanced chemiluminescence substrate (Nanjing
KeyGen Biotech. Co., Ltd.), and images were captured using the
G:BOX iChemi XR gel documentation system (Syngene, Frederick, MD,
USA).
Statistical analysis
The data are expressed as the mean ± standard
deviation. The statistical software program, SPSS version 17.0 for
windows (SPSS, Inc., Chicago, IL, USA), was used to perform one-way
analysis of variance (ANOVA) and Student-Newman-Keuls
t-test. If the Levene test used for the homogeneity of
variance revealed heterogeneity prior to ANOVA, the Kruskal-Wallis
test and Wilcoxon test were used. P<0.05 was considered to
indicate a statistically significant difference.
Results
Effect of AE on human gastric cancer
cell viability
The effect of AE on human gastric cancer cell
viability was observed in the AE group and was revealed to be
dose-dependent. An AE concentration of 20 µM and above induced a
significant inhibitory effect compared with the control group
(P<0.05; Fig. 1). According to
this result, a 10 µM concentration of AE was selected for the
following experiments.
Treatment with 10 µM AE induced PDT in
human gastric cancer cells
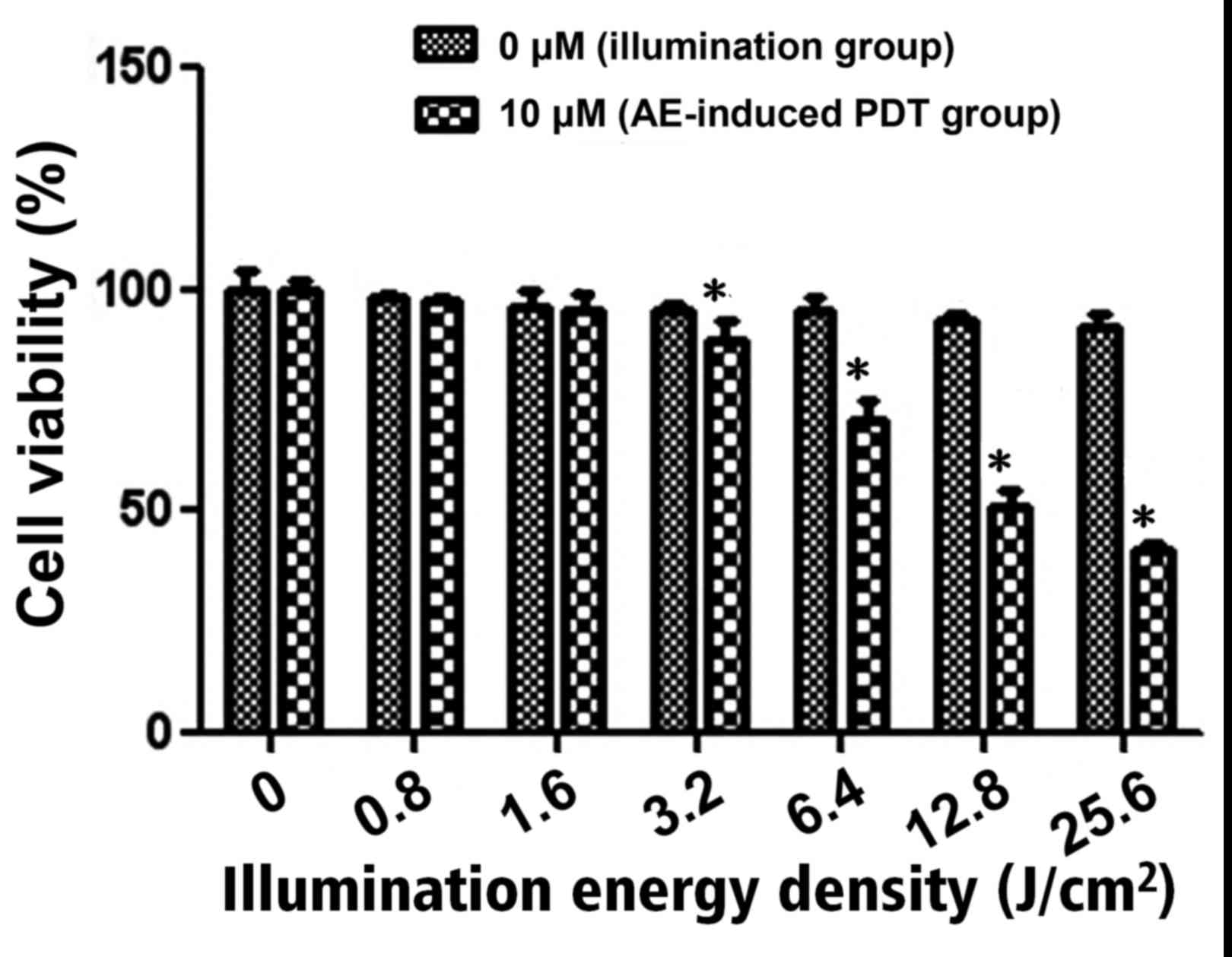
It was demonstrated that there was no significant
difference in cell viability in the illumination group following
exposure to different energy densities (P>0.05; Fig. 2). However, in the AE-induced PDT
group, when the energy density was 3.2 J/cm2 or above,
cell viability was significantly decreased compared with the
illumination group at the same energy density, and the inhibition
effect was energy-dependent (P<0.05; Fig. 2). The half-maximal inhibitory
concentration (IC50) in the 10 µM AE-induced PDT group
was observed when the energy density reached 12.8 J/cm2.
According to the results, the 12.8 J/cm2 energy density
was selected for the following experiments.
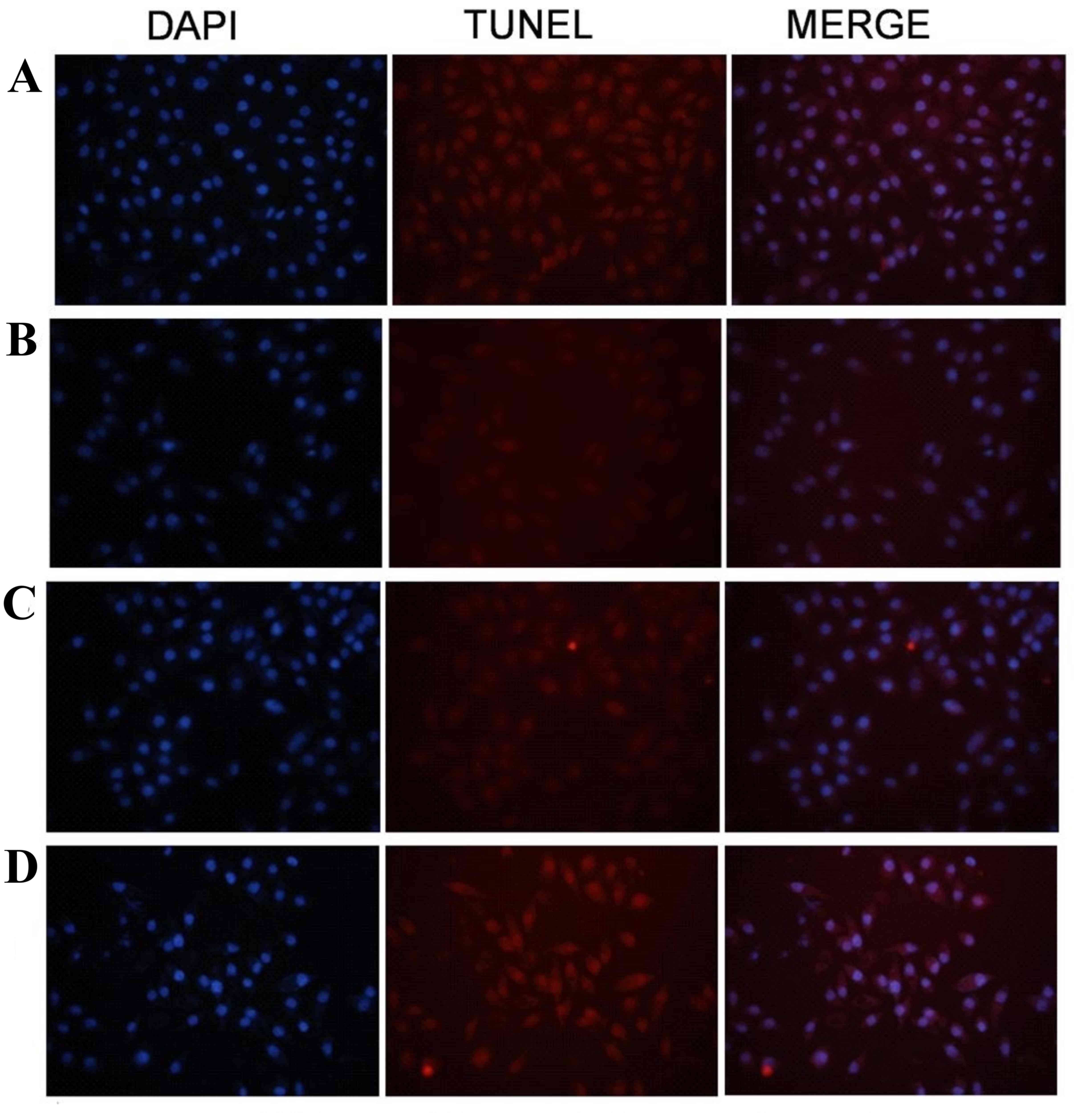
TUNEL assay results revealed that
apoptosis was induced in the human gastric cancer cells in the
AE-induced PDT group
Cells in group IV, which were exposed to 10 µM
AE-induced PDT (12.8 J/cm2 illumination), were observed
using a TUNEL assay and a fluorescence microscope. The adherent
cells changed from flagstone shapes to oval shapes. Typical
morphological changes of cell apoptosis were observed, including
cell shrinkage, nucleus fragmentation and typical apoptotic bodies
(Fig. 3).
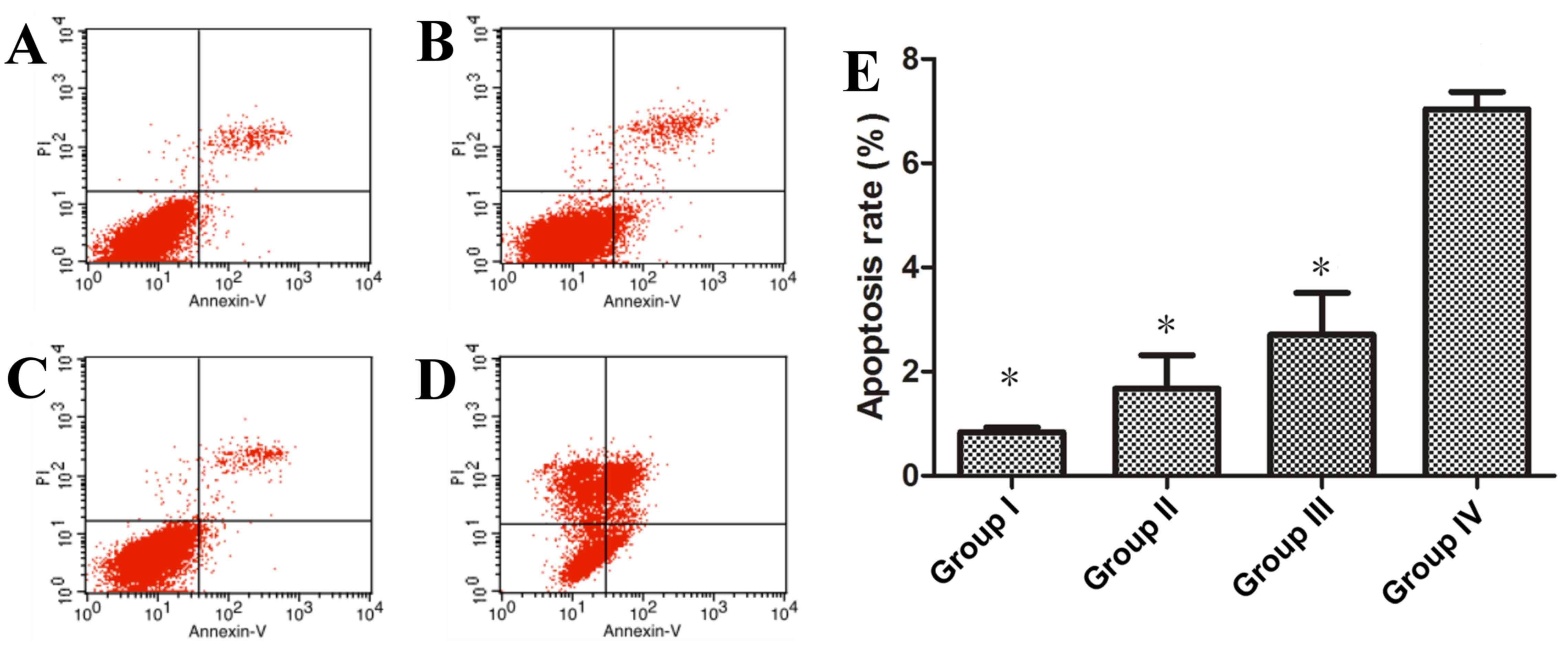
The rate of apoptosis increased in
SGC-7901 human gastric cancer cells in the AE-induced PDT
group
The rate of apoptosis of SGC-7901 human gastric
cancer cells in all groups was determined by flow cytometry
analysis (Fig. 4). The rate of cell
apoptosis in group IV, which underwent 10 µM AE-induced PDT (12.8
J/cm2 illumination), was significantly higher when
compared with groups I, II and III (P<0.05; Fig. 4). No significant differences were
identified between the apoptosis rates of groups I, II and III,
(P>0.05; Fig. 4).
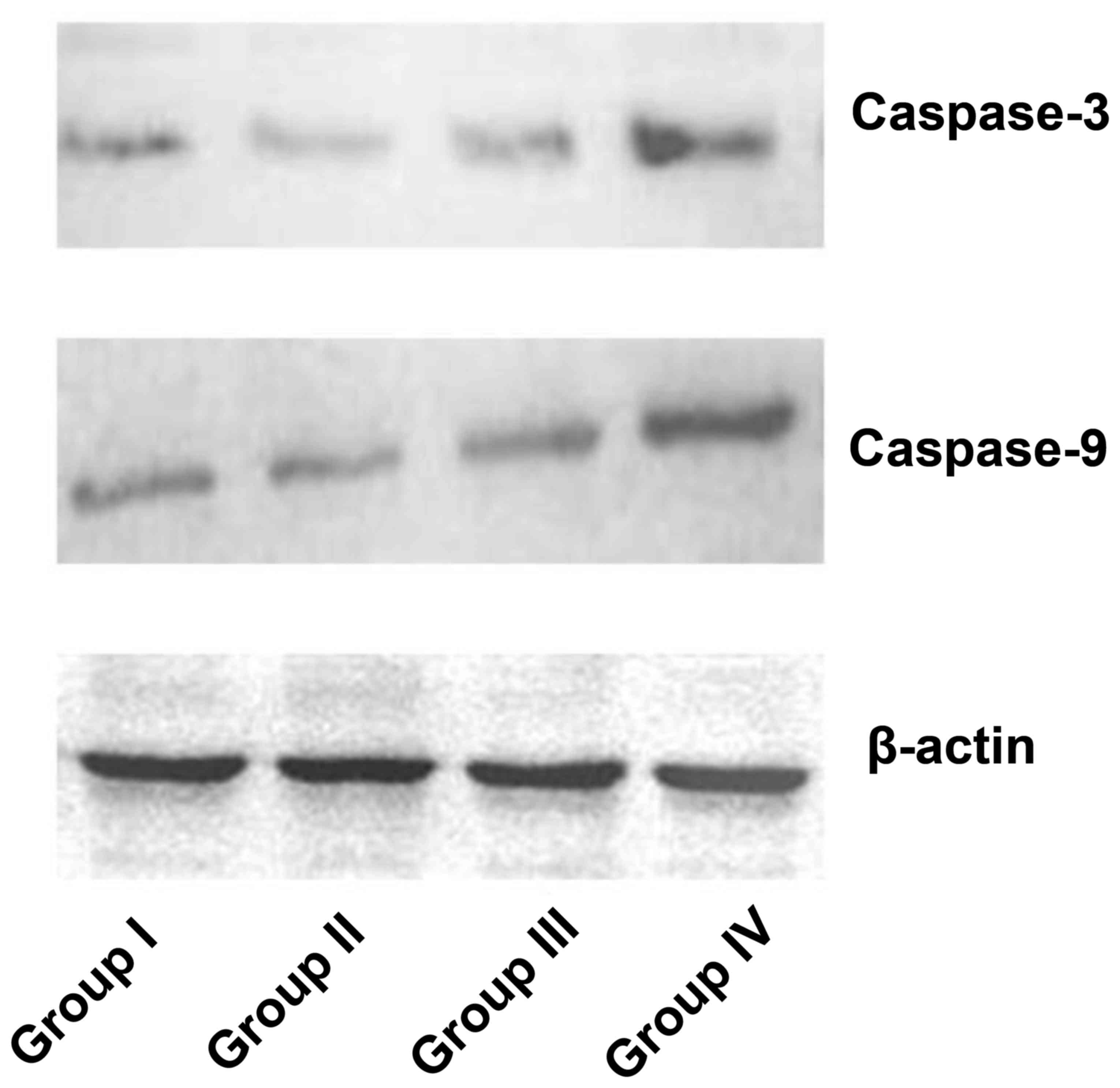
Caspase-3 and caspase-9 protein levels
increased in the AE-induced PDT group
Caspase-3 and caspase-9 protein levels of human
gastric cancer cells in all groups were detected by western blot
analysis. Caspase-3 and caspase-9 protein levels were visibly
increased in group IV compared with the other groups (Fig. 5).
Discussion
PDT has been demonstrated in experiments and trials
to be a novel effective treatment for various types of cancer,
including precancerous lesions. It was revealed that during PDT,
local hypoxia was produced directly by increasing oxygen
consumption or indirectly by destruction of tumor vasculature, as a
result of an effective therapy (7).
The photosensitizer serves an important function in
the PDT process. AE is a novel type of anthraquinone compound
present in aloe and rhubarb, which has demonstrated antitumor
activity (8–10). AE was observed to exhibit fluorescence
with a maximum excitation wavelength of 430 nm, and revealed an
inhibitory effect on tumor cells in previous studies (8,14,15). As a result, AE may be used as a
photosentisizer in PDT for cancer treatment. The present study
demonstrated that with an AE concentration >10 µM, the viability
of human gastric cancer cells significantly decreased and that the
effect was dose-dependent (Fig. 1).
Considering the antitumor activity of AE, in order to explore the
effect of AE-induced PDT, a 10 µM concentration of AE was selected
for further PDT experiments. AE-induced PDT inhibited human gastric
cancer cell viability d with an energy density >1.6
J/cm2, with the IC50 observed when the energy
density reached 12.8 J/cm2 in the present study. It was
revealed that AE demonstrated antitumor activity, and as a
photosentisizer, the AE-induced PDT treatment was also
effective.
Tumor cells usually undergo two types of cell death
following PDT treatment, apoptosis and necrosis. In general, it is
believed that a low dose of PDT induces an increased rate of
apoptosis, whereas a high dose of PDT primarily induces necrosis
(6,7).
However, necrosis is usually avoided in order to limit damage to
normal tissues. Therefore 12.8 J/cm2, a lower effective
energy density, was selected in the present study. Cell apoptosis
has been reported in numerous previous studies (1,2,4,14,16,17). In
the present study, cell apoptosis, including cell shrinkage,
nucleus fragmentation and apoptotic bodies, were observed in the
TUNEL assay (Fig. 3). Flow cytometry
analysis also revealed that the rate of cell apoptosis in the
AE-induced PDT group (7.0400±0.3318%) was significantly higher
(Fig. 4). It was demonstrated that
cell apoptosis serves a critical function in AE-induced PDT in
human gastric cancer cells.
The caspase family, which contains initiator
caspases (caspase-1, 2, 4, 5, 8, 9, 10 and 14) and effector
caspases (caspase-3, 6 and 7), has been revealed to contribute to
cell apoptosis (18). Cell apoptosis
may be initiated by three distinct pathways: The death receptor
pathway involving caspase-8 activation, the endoplasmic reticulum
stress pathway involving activation of caspase-12 and the
mitochondrial pathway involving caspase-9 activation and inducing
downstream caspase-3 activation. It has been demonstrated that PDT
induced tumor cell apoptosis by the mitochondrial pathway, in
previous studies (19,20). Thus, capcase-9 and caspase-3 were
selected in the present study. It was observed that caspase-9 and
caspase-3 expression levels increased in human gastric cancer cells
following AE-induced PDT treatment (Fig.
5). This revealed that the mitochondrial pathway for apoptosis
was involved in AE-induced PDT.
The upregulation of caspase-9 and caspase-3 was
observed in the present study. However, other important targets
which are also important in the mitochondrial pathway, including
mitochondrial membrane potential, were not detected. The mechanisms
underlying cell apoptosis are complicated and are regulated by pro-
and anti-apoptotic factors. A mechanism has been revealed in which
caspase-8 activated by the death receptor pathway cleaves Bid, and
pro-caspase-9 is activated as a result of releasing cytochrome c
from the mitochondrion, amplifying the apoptotic signal (21). According to the mechanism described
above, caspase-8 and Bid may participate in apoptosis induction.
However, caspase-8 and Bid were not detected in the present study.
In addition, AE is poorly soluble in water, and absorption of AE
into the cells may limit the PDT effect. Stronger apoptosis
induction may be observed when there is an increase in the
absorption of AE (22). Modification
to the form of AE should be considered in further studies. The
present study used human gastric cancer cells to investigate the
effect of AE-induced PDT, and the effect observed herein may be
different when another cell line is used. Further studies
investigating other types of cancer cells are also required.
In conclusion, the present study revealed that 10 µM
AE-induced PDT increased the apoptosis rate of SGC-7901 human
gastric cancer cells. An increase in caspase-9 and caspase-3
expression was also observed following PDT induced by 10 µM AE,
which indicated that the mitochondrial pathway for apoptosis may be
involved in the effect of AE-induced PDT. AE-induced PDT may have
the potential to be a novel therapy for the treatment of human
gastric cancer. However, further studies are required.
References
|
1
|
Nowak-Stępniowska A, Wiktorska K, Małecki
M, Milczarek M, Lubelska K and Padzik-Graczyk A: Cytotoxicity of
PP(Arg)2- and PP(Ala)2(Arg)2-based photodynamic therapy and early
stage of apoptosis induction in human breast cancers in vitro. Acta
Biochim Pol. 59:603–611. 2012.PubMed/NCBI
|
|
2
|
Li JH, Chen ZQ, Huang Z, Zhan Q, Ren FB,
Liu JY, Yue W and Wang Z: In vitro study of low intensity
ultrasound combined with different doses of PDT: Effects on C6
glioma cells. Oncol Lett. 5:702–706. 2013.PubMed/NCBI
|
|
3
|
Qumseya BJ, David W and Wolfsen HC:
Photodynamic therapy for barrett's esophagus and esophageal
carcinoma. Clin Endosc. 46:30–37. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Buytaert E, Dewaele M and Agostinis P:
Molecular effectors of multiple cell death pathways initiated by
photodynamic therapy. Biochim Biophys Acta. 1776:86–107.
2007.PubMed/NCBI
|
|
5
|
Verma S, Watt GM, Mai Z and Hasan T:
Strategies for enhanced photodynamic therapy effects. Photochem
Photobiol. 83:996–1005. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Robertson CA, Evans DH and Abrahamse H:
Photodynamic therapy (PDT): A short review on cellular mechanisms
and cancer research applications for PDT. J Photochem Photobiol B.
96:1–8. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kushibiki T, Hirasawa T, Okawa S and
Ishihara M: Responses of cancer cells induced by photodynamic
therapy. J Healthc Eng. 4:87–108. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pecere T, Gazzola MV, Mucignat C, Parolin
C, Vecchia FD, Cavaggioni A, Basso G, Diaspro A, Salvato B, Carli M
and Palù G: Aloe-emodin is a new type of anticancer agent with
selective activity against neuroectodermal tumors. Cancer Res.
60:2800–2804. 2000.PubMed/NCBI
|
|
9
|
Chiu TH, Lai WW, Hsia TC, Yang JS, Lai TY,
Wu PP, Ma CY, Yeh CC, Ho CC, Lu HF, et al: Aloe-emodin induces cell
death through S-phase arrest and caspase-dependent pathways in
human tongue squamous cancer SCC-4 cells. Anticancer Res.
29:4503–4511. 2009.PubMed/NCBI
|
|
10
|
Lin KY and Uen YH: Aloe-emodin, an
anthraquinone, in vitro inhibits proliferation and induces
apoptosis in human colon carcinoma cells. Oncol Lett. 1:541–547.
2010.PubMed/NCBI
|
|
11
|
Zhu S, Jin J, Wang Y, Ouyang Z, Xi C, Li
J, Qiu Y, Wan J, Huang M and Huang Z: The endoplasmic reticulum
stress response is involved in apoptosis induced by aloe-emodin in
HK-2 cells. Food Chem Toxicol. 50:1149–1158. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Vath P, Wamer WG and Falvey DE:
Photochemistry and phototoxicity of aloe emodin. Photochem
Photobiol. 75:346–352. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bai DQ, Yu LH, Xia XS, Yu HP, Jiang Q, Tan
Y, Zeng XB and Xu CS: Study on spectral properties of aloe-emodin
in different solvents. Laser J. 28:83–85. 2007.
|
|
14
|
Lee HZ, Yang WH, Hour MJ, Wu CY, Peng WH,
Bao BY, Han PH and Bau DT: Photodynamic activity of aloe-emodin
induces resensitization of lung cancer cells to anoikis. Eur J
Pharmacol. 648:50–58. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cardenas C, Quesada AR and Medina MA:
Evaluation of the anti-angiogenic effect of aloe-emodin. Cell Mol
Life Sci. 63:3083–3089. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Juzeniene A and Moan J: The history of PDT
in Norway Part II. Recent advances in general PDT and ALA-PDT.
Photodiagnosis Photodyn Ther. 4:80–87. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
MacCormack MA: Photodynamic therapy in
dermatology: An update on applications and outcomes. Semin Cutan
Med Surg. 27:52–62. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nicholson DW: Caspase structure,
proteolytic substrates, and function during apoptotic cell death.
Cell Death Differ. 6:1028–1042. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lam M, Oleinick NL and Nieminen AL:
Photodynamic therapy-induced apoptosis in epidermoid carcinoma
cells. Reactive oxygen species and mitochondrial inner membrane
permeabilization. J Biol Chem. 276:47379–47386. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hilf R: Mitochondria are targets of
photodynamic therapy. J Bioenerg Biomembr. 39:85–89. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Buggiani G, Troiano M, Rossi R and Lotti
T: Photodynamic therapy: Off-label and alternative use in
dermatological practice. Photodiagnosis Photodyn Ther. 5:134–138.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li KT, Duan QQ, Chen Q, He JW, Tian S, Lin
HD, Gao Q and Bai DQ: The effect of aloe emodin-encapsulated
nanoliposome-mediated r-caspase-3 gene transfection and
photodynamic therapy on human gastric cancer cells. Cancer Med.
5:361–369. 2015. View
Article : Google Scholar : PubMed/NCBI
|