Introduction
Glioblastoma constitutes the largest group of brain
tumors that respond poorly to present anti-neoplastic therapies
(1). Currently, microsurgery and
adjuvant concomitant chemoradiotherapy with temozolomide are the
recommended first-line treatments for patients with primary
glioblastoma (2). Despite advances in
the treatment of glioblastoma, the prognosis for patients with
newly diagnosed glioblastoma remains poor. The median survival of
these patients was 14.6 months when treated with chemoradiotherapy
with temozolomide, due to the high invasiveness and heterogeneity
of glioblastoma (3,4). Currently, there are no effective
therapeutic options to prevent temozolomide resistance. Therefore,
it is important to find novel therapeutic agents to overcome
treatment resistance to temozolomide.
The major metabolite 7-O-Succinyl macrolactin A
(SMA) is generated by Bacillus polyfermenticus KJS-2. SMA
has a range of activities including significant antiviral and
cancer cell cytotoxic properties (5,6). Recently,
SMA has been demonstrated to exhibit inhibitive effects against
intestinal inflammation in colon epithelial cells (7) and anti-cell invasion (8). Kang et al (9) suggested that SMA and macrolactin A (MA)
effectively inhibited angiogenesis activity in human umbilical vein
endothelial cells and emphasized that two agents may be explored
for treatment of cancer. Additionally, Regmi et al (10) suggested that SMA may function as a
potential monotherapy or combination therapy with 5-FU or cisplatin
to demonstrate the antitumor activity with numerous cell line in
vivo and in vitro.
To the best of our knowledge, the effect of SMA on
brain tumor especially glioblastoma have not been investigated.
Therefore, the anti-neoplastic effects of SMA tromethamine salt
(SMA salt) on glioblastoma were assessed in vitro and in
vivo in the present study. Accordingly, the possibility of
utilizing SMA salt as a novel antitumor agent for glioblastoma has
been evaluated.
Materials and methods
Cell culture
Human glioma U87MG, U251MG and LN229 cell lines were
originally purchased from the American Type Culture Collection
(Manassas, VA, USA). These cell lines were cultured in complete
Dulbecco's modified Eagle's medium (DMEM) that was supplemented
with 10% fetal bovine serum (FBS). All cells were incubated at 37°C
in a humidified atmosphere of 5% CO2.
Drug preparation
SMA salt was prepared in powder form by Daewoo
Pharmaceutical Ind. Co., Ltd. (Busan, Republic of Korea). SMA salt
was diluted in saline to a concentration of 10 mM. DMEM without FBS
was used for additional dilutions.
Cell viability assay
The cytotoxicity of the SMA salt from Daewoo
Pharmaceutical Ind. Co., Ltd. was measured using the Cell Counting
Kit-8 (CCK-8; Dojindo Molecular Technologies Inc., Kumamoto,
Japan). CCK-8 contains Dojindo's highly water-soluble tetrazolium
salt, which produces a water-soluble formazan dye upon reduction in
the presence of an electron mediator. The U87MG, U251MG and LN229
cell lines were seeded into 96-well plates at a density of
5×103 cells/well to allow for overnight adhesion at 37°C
and 5% CO2. Subsequent to adhesion, the cells were
treated with SMA salt at a concentration of 1, 10, 100 and 1,000 µM
at 37°C in 5% CO2. After 2 days, 10 µl of the CCK-8
solution was added to each well of the plate and the plate was
subsequently incubated for 2 h in an incubator (37°C; 5%
CO2). The optical density (OD) of the sample plate was
measured at 450 nm in a microplate reader. The viability of the
tumor cells was assessed by calculating the OD ratio of the
specific OD in each sample to the OD of the control sample.
Migration assay
The insert of a 24-well Transwell apparatus (Corning
Incorporated, Corning, NY, USA) was incubated at 37°C for 1–2 h to
adjust to room temperature. SMA salt-treated (1, 10, 100 and 1,000
µM) U87MG, U251MG and LN229 cells, and un-treated U87MG, U251MG and
LN229 cells as the control (2×105 cells/ml), were
prepared in serum-free medium. FBS-containing medium (750 µl) was
added to the lower chamber and 200 µl of prepared cell suspension
was added to the insert. After 24 h, cells that remained in the
insert (i.e., non-invading cells) were gently retrieved using a
cotton-tipped swab and allowed to air-dry for 20 min. A solution of
0.4% crystal violet (500 µl) was added to each well of the
apparatus. After 10 min, the migrated cells that traversed the
membrane separating the insert from the lower chamber were stained
by dipping the lower surface of the membrane into the stain. The
stained membranes were washed several times using water and allowed
to air-dry. The cells that adhered to the membrane were quantified
by dissolving the stained cells in 10% acetic acid and transferring
the mixture to a 96-well plate for colorimetric determination of
the optical density at 570 nm. These experiments were performed in
triplicate.
Invasion assay
Matrigel (BD Biosciences, Franklin Lakes, NJ, USA)
was thawed overnight at 4°C and diluted (1–5 mg/ml) in serum-free
cold DMEM. A total of 100 µl of the diluted Matrigel was added to
the upper chamber of a Transwell apparatus and incubated at 37°C
for 4–5 h to allow the gel to swell. SMA salt-treated U87MG, U251MG
and LN229 cells (2×105 cells/ml) were prepared in
serum-free medium. Medium containing FBS (750 µl) was added to the
lower chamber of the Transwell apparatus and 200 µl of prepared
cell suspension was added to each insert and incubated at 37°C in
5% CO2. After 24 h, non-invading cells were retrieved
and invasive cells were quantified as described above.
Mouse glioblastoma xenograft
model
A total of 28 6-week-old female BALB/c-nu mice were
purchased from Orient Bio, Inc. (Seongnam, Republic of Korea;
Charles River, Wilmington, MA, USA) and maintained at Seoul
National University Bundang Hospital Preclinical Research Center in
temperatures of 18–22°C and an atmosphere of supply air filtered
using a HEPA Filter Unit. A total of 10 to 15 fresh air changes per
hour were provided in the animal housing rooms with the 100%
Outdoor Air System in a positive pressure room. They were fed with
Purina irradiated Lab. Rodent chow 38057, and were maintained in a
12 h light/dark cycle. The mean weight of the mice was 18.7 g.
First, Mice were anesthetized with zoletil. Then the head was fixed
in a stereotactic frame and a midline scalp incision was made. A
small hole was made 0.5 mm anterior and 2 mm lateral to the exposed
bregma. A sterile 10 µl Hamilton syringe with a #26S needle was
inserted at a depth of 3.5 mm from the surface of the skull and
withdrawn by 0.5 mm to inject 2×105 U87MG cells within a volume of
2 µl. The injection rate was set at 0.5 ml/min. Following the
implantation of the tumor cells, the needle was kept in place for 3
min to prevent reflux. The needle was subsequently completely
withdrawn from the brain over the course of 3 min (1.0 mm/min) and
the skin was sutured. The present study was approved by the
Institutional Animal Care and Use Committee of the Medical Science
Research Institute, Seoul National University Bundang Hospital
(Gyeonggi-do, Republic of Korea).
Treatment protocol
Tumor-bearing mice were randomly assigned to four
groups: control (n=14) and SMA salt (n=14). SMA salt was
administered intraperitoneally at a dose of 50 mg/kg daily in the
SMA salt group. Animals in the control group were injected with
saline only. A single dose of the drugs was composed of a 5 sec
infusion of a volume equaling 5 ml/kg. The drug treatments began 7
days subsequent to the implantation of tumor cells. Half of the
animals were sacrificed 1 month subsequent to the implantation of
the tumor cells for tumor volume analysis; the remaining animals
were observed for another 2 months to analyze their survival. The
humane endpoint was defined as a weight reduction of >25% of the
initial weight. Weight loss in all mice remained above this
threshold, and all surviving mice were anaesthetized and humanely
sacrificed at the end of the study (11). The mice that did not reach a 25%
reduction in initial weight were humanely anaesthetized and
sacrificed (cervical dislocation) at the end of the survival
experiment.
Evaluation of tumor growth
Subsequent to being sacrificed, their brains of the
mice were removed following vascular perfusion with a solution
containing PBS, and fixed with 4% paraformaldehyde at 4°C for 2
weeks for paraffin embedding. The fixed brains were sectioned
coronally into slices of 10-µm thickness using a microtome. The
slices were mounted on individual slides and stained with
hematoxylin and eosin. The maximal length (L), width (W) and height
(H) of each tumor was measured by the Axio Imager A2 microscope and
AxioVision ×40 software (version 4.8.1.0; Carl Zeiss Microscopy
GmbH, Jena, LA, USA), and tumor volume was calculated using the
following formula: Tumor volume=4/3 × π × (L/2 × W/2 × H/2)
(12).
Statistical analysis
All data are presented as the mean ± standard
deviation or are expressed as a percentage of controls ± standard
deviation. The present study used an unpaired t-test and the
Kruskal-Wallis test for the statistical analysis of the data. The
Kaplan-Meier method was used for the survival analysis of the
experimental animals. Differences with regard to survival were
tested for significance using the two-sided log-rank test. All
analyses were performed using the SPSS statistical software package
(version 17.0; SPSS, Inc, Chicago, IL, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
Effect of SMA salt on cell
viability
Prior to examining the effects of SMA salt, the
present study confirmed its cytotoxicity in glioblastoma cell
lines. The glioblastoma U87MG, U251MG and LN229 cell lines were
cultured in the presence of SMA salt at a concentration that ranged
between 0 and 1,000 µM for different durations (24–48 h). The
experiment included a group of cells that were cultured without SMA
salt as a negative control group.
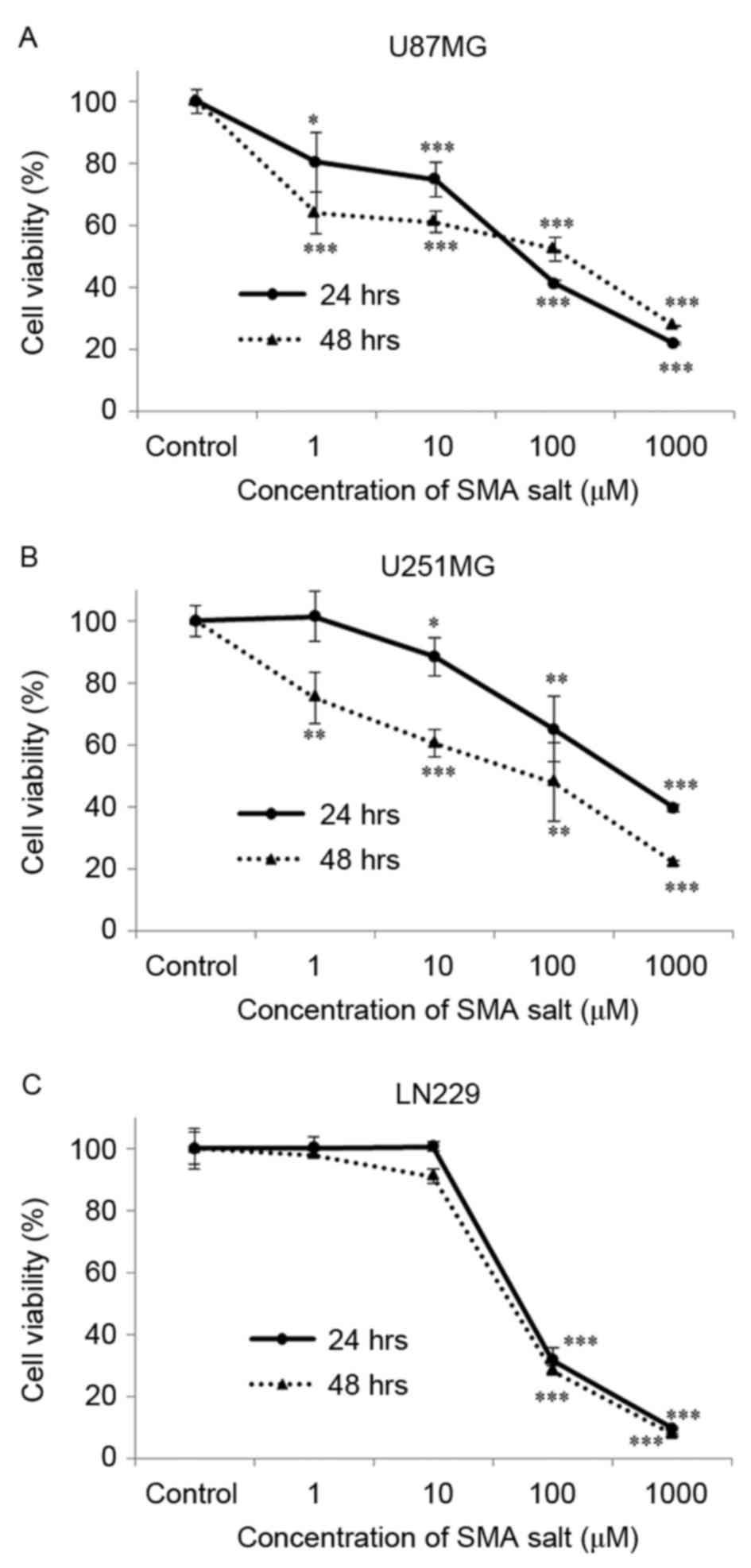
A concentration-dependent decrease in cell viability
subsequent to exposure to SMA salt was observed at concentration of
1 µM (80.4% at 24 h, P<0.05; 63.8% at 48 h, P<0.001) and 10
µM (88.4% at 24 h, P<0.05; 60.4% at 48 h, P<0.001) in the
U87MG and U251MG cell lines, respectively (Fig. 1A and B). At concentrations >10 µM,
SMA salt significantly inhibited cell viability of the LN229 cell
line (Fig. 1C). These results suggest
that SMA salt does affect the viability of glioblastoma cell
lines.
Effect of SMA salt on cell migration
and invasion
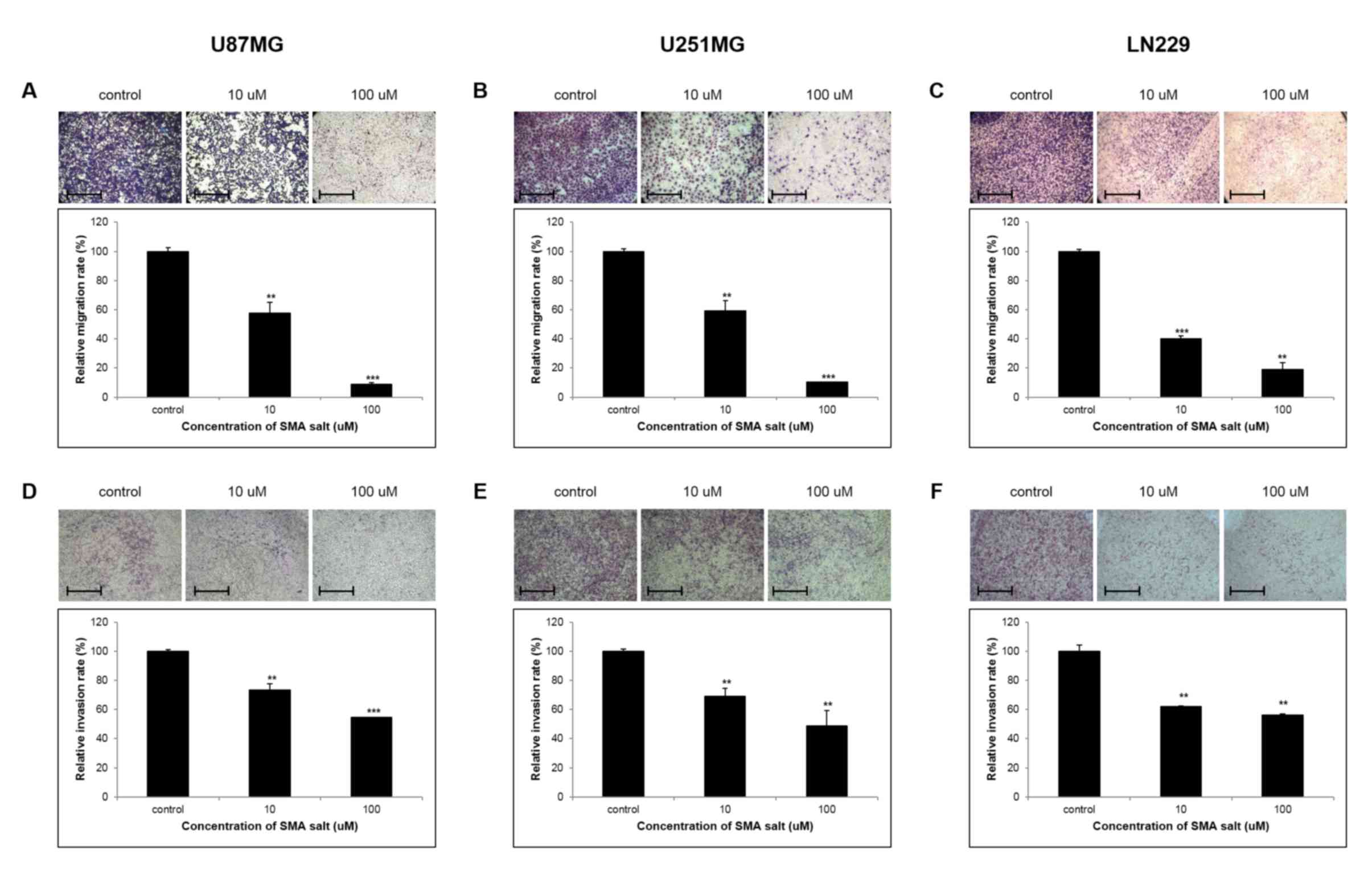
To ascertain the influence of SMA salt on the
migration of glioblastoma cell lines, Transwell apparatus was used
to detect cell migration through the membrane barrier between the
Transwell inserts and receptacles. At 48 h, SMA salt decreased the
migration ability of glioblastoma cells in concentration-dependent
manner (Fig. 2A and B). In the U87MG
and U251MG cell lines, SMA salt concentrations of 10 µM and 100 µM
resulted in a migration capacity that was ~2-fold and 10-fold
lower, respectively, compared with the control group. In the LN220
cell line, SMA salt also significantly inhibited the migration of
glioblastoma cells at concentrations >0 µM (Fig. 2C). In addition, a significant
inhibition of cell invasion was observed in each of the cell lines;
the percentage inhibition of invasion at a concentration of 100 µM
was ~50% (P<0.05) in all cell lines (Fig. 2D-F). SMA salt treatment significantly
decreased the migration ability and invasion potential of
glioblastoma cells in a concentration-dependent manner. These
results demonstrate that treatment with SMA salt clearly inhibits
cell migration and invasion.
Effect of SMA salt on glioma
growth
The in vivo antitumor effect of SMA salt was
estimated in tumor-bearing nude mice. A tumor volume analysis was
performed using mice that were euthanized 1 month following tumor
cell implantation (Fig. 3). The mean
tumor volume of the SMA salt group (3.81±1.91 mm3) was
significantly smaller compared with the control group (11.34±6.86
mm3; P=0.013).
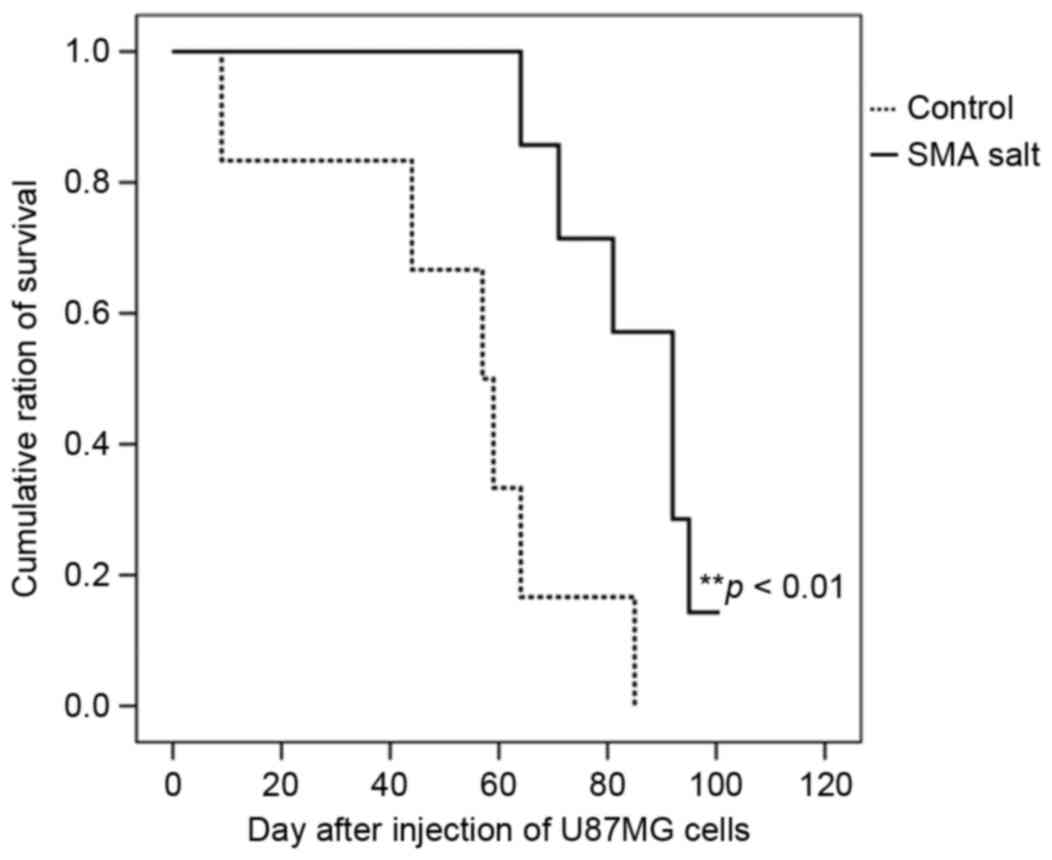
Effect on SMA salt on survival
Survival analyses of the control and SMA salt groups
were performed (Fig. 4). The median
survival of the control and SMA salt groups were 57 [95% confidence
interval (CI), 39–75] and 92 days (95% CI, 79–105), respectively.
The overall survival of the SMA salt group was significantly higher
compared with the overall survival of the control group
(P=0.006).
Discussion
Glioblastoma is the most malignant type of World
Health Organization Grade 4 infiltrative gliomas and is associated
with a median survival of less than two years (13,14).
Current standard therapies for glioblastoma include maximal safe
surgical resection, radiation and temozolomide chemotherapy
(2). Temozolomide induces apoptosis
by disrupting DNA transcription and inducing DNA damage (15). Despite aggressive treatment,
glioblastoma patients commonly exhibit resistance to temozolomide
treatment and generally survive no more than two years following
diagnosis (3–5,16–18). The migration and invasion of
glioblastoma cells is a possible explanation for this resistance.
The migration and invasion of glioblastoma cells are the primary
reasons for tumor recurrence and poor prognoses (19). The morbidity and high recurrence rate
of glioblastoma is largely attributable to the migration and
invasion of cells into adjacent brain structures (20,21).
Glioblastoma can be accompanied by a high proliferation rate and
invasion into surrounding normal brain tissue, which results in
tumor recurrence subsequent to the surgical resection of the
primary tumor (22). Therefore,
innovative therapeutic approaches for targeting these invasive
cells are required to enhance clinical outcomes (23).
Studies are now targeted at identifying new
therapeutic drugs and discovering novel potential combination
therapies to accompany temozolomide or other agents for
glioblastoma. In a previous study, we determined that the
combination of cilengitide with belotecan, which is a new synthetic
analog of camptotein, exhibited a significant antitumor effect on
glioblastoma (11). However, although
the combination of cilengitide with temozolomide chemoradiotherapy
demonstrated potential anticancer activity in phase I and II
studies in newly diagnosed glioblastoma (18,24–27), this
combination had negative outcomes in phase III trials (28). In addition, combination therapy with
bevacizumab and temozolomide chemoradiotherapy in patients with
glioblastoma did not improve survival time in phase 3 studies
(29,30). Although there have been numerous
trials using several antitumor agents, all outcomes have been
insufficient or yielded negative results. Thus, novel agents or
combination therapies are constantly being investigated and
developed for glioblastoma treatment.
SMA is a macrolide compound that is a derivative of
MA from Bacillus polyfermenticus KJS-2 (31). SMA has antibacterial and
immunosuppressive activities against vancomycin-resistant
enterococci and methicillin-resistant Staphylococcus aureus
(6,31). SMA also inhibits the proliferation of
B16-F10 cells and T-lymphoblast cells against human HIV viral
replication (31); additionally, it
inhibits the proliferation of neuronal cells against glutamate
toxicity (32). SMA is also known to
possess effective antibacterial properties (6). SMA also exhibits inhibitive effects on
cancer cell invasion and angiogenesis (8,9). In
addition, SMA markedly inhibits cell mobility in human fibrosarcoma
cells in a concentration-dependent manner (8). Currently, MA and SMA are being assessed
in preclinical studies as antitumor and anti-macular degeneration
agents at Daewoo Pharmaceutical Ind. Co., Ltd. (Gimhae, Korea)
(33). Therefore, the present study
investigated the possibility of utilizing SMA as new antitumor
agent for glioblastoma.
The present study, determined that SMA salt has
cytotoxic effects in glioblastoma cell lines in a dose- and
time-dependent manner. It was demonstrated that SMA salt also
exhibits an antitumor effect in an in vitro Transwell assay
and in a mouse glioma model using U87MG cells. In glioblastoma cell
lines, SMA salt clearly reduced cell migration and invasion in a
concentration-dependent manner in spite of its own cell mobility
character. In particular, at concentrations >10 µM, SMA salt
exhibited inhibition rates of migration and invasion that were
above 50% in all glioblastoma cell lines. In the in vivo
assays, a significant decrease in tumor volume was noted in the
group that was treated with SMA salt. Furthermore, this treatment
prolonged the survival time of tumor-bearing mice compared with
control group. Therefore, the combination therapy of SMA salt and
temozolomide can be expected to exhibit cytotoxic effects and may
become a successful treatment for patients with glioblastoma in the
future.
However, due to the limitations of the U87MG
xenograft animal model, the anti-migration and anti-invasion
activity of SMA salt was unable to be confirmed in vivo. The
human U87MG xenograft model is the standard method for glioblastoma
animal models (34–36); however, tumor invasion in this model
is not as extensive as spontaneous glioblastomas in humans
(37–43). To the best of our knowledge, it is
currently unknown how SMA salt is regulated in glioblastoma.
Additional studies should be conducted to demonstrate mechanism of
the anti-cancer effect of SMA salt and identify the anti-tumor
activity of SMA salt in the patient-derived xenograft cancer model.
Additional studies are planned to study the effects of SMA salt in
more detail to clarify the potential for SMA salt in
glioblastoma.
The present study demonstrated the in vitro
and in vivo effects of SMA salt in experimental
glioblastoma. These effects may be attributed to the inhibition of
migration and invasion by SMA salt as well as the cytotoxicity of
the drug. Thus, these results suggest that SMA salt has a
significant antitumor effect on glioblastoma and may be a promising
candidate for additional clinical studies.
Acknowledgements
The authors would like to thank the Daewoo
Pharmaceutical Ind. Co., Ltd. (Busan, Republic of Korea) for
donating the SMA salt. The present study was supported by grants
from the Seoul National University Bundang Hospital Research Fund
(grant nos. 03-2012-007 and 03-2013-007).
References
|
1
|
Jemal A, Murray T, Ward E, Samuels A,
Tiwari RC, Ghafoor A, Feuer EJ and Thun MJ: Cancer statistics,
2005. CA Cancer J Clin. 55:10–30. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Stupp R, Mason WP, van den Bent MJ, Weller
M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn
U, et al: Radiotherapy plus concomitant and adjuvant temozolomide
for glioblastoma. N Engl J Med. 352:987–996. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tate MC and Aghi MK: Biology of
angiogenesis and invasion in glioma. Neurotherapeutics. 6:447–457.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Grauer OM, Wesseling P and Adema GJ:
Immunotherapy of diffuse gliomas: Biological background, current
status and future developments. Brain Pathol. 19:674–693. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kim DH, Kim HK, Kim KM, Kim CK, Jeong MH,
Ko CY, Moon KH and Kang JS: Antibacterial activities of macrolactin
A and 7-O-succinyl macrolactin A from Bacillus
polyfermenticus KJS-2 against vancomycin-resistant
enterococciand methicillin-resistant Staphylococcus aureus. Arch
Pharm Res. 34:147–152. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Romero-Tabarez M, Jansen R, Sylla M,
Lünsdorf H, Häussler S, Santosa DA, Timmis KN and Molinari G:
7-O-Malonyl macrolactin A, a new macrolactin antibiotic form
Bacillus subtilis active against methicillin-resistant
Staphylococcus aureus, vancomycin-resistant Enterococci, and
a small-colony variant of Burkholderia cepacia. Antimicrob
Agents Chemother. 50:1701–1709. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Park S, Regmi SC, Park SY, Lee EK, Chang
JH, Ku SK, Kim DH and Kim JA: Protective effect of 7-O-succinyl
macrolactin A against intestinal inflammation is mediated through
PI3-kinase/Akt/mTOR and NF-κB signaling pathways. Eur J Pharmacol.
735:184–192. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chung SU, Hwang SW, Ji YH, Kang JS, Kang
KR, Kang UR, Kim DH and Kim JA: Anti-angiogenic composition
containing macrolactin a and a derivative thereof as active
ingredients. Patent WO2012008674 A1. Filed February 23, 2011;
issued January 19. 2012.
|
|
9
|
Kang Y, Regmi SC, Kim MY, Banskota S,
Gautam J, Kim DH and Kim J: Anti-angiogenic activity of macrolactin
A and its succinyl derivative is mediated through inhibition of
class I PI3K activity and its signaling. Arch Pharm Res.
38:249–260. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Regmi SC, Park SY, Kim SJ, Banskota S,
Shah S, Kim DH and Kim JA: The anti-tumor activity of succinyl
macrolactin A is mediated through the β-catenin destruction complex
via the suppression of tankyrase and PI3K/Akt. PLoS One.
10:e01417532015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kim YH, Lee JK, Kim B, DeWitt JP, Lee JE,
Han JH, Kim SK, Oh CW and Kim CY: Combination therapy of
cilengitide with belotecan against experimental glioblastoma. Int J
Cancer. 133:749–756. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Schmidt KF, Ziu M, Schmidt NO, Vaghasia P,
Cargioli TG, Doshi S, Albert MS, Black PM, Carroll RS and Sun Y:
Volume reconstruction techniques improve the correlation between
histological and in vivo tumor volume measurements in mouse models
of human gliomas. J Neurooncol. 68:207–215. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dirks PB: Brain tumor stem cells: The
cancer stem cell hypothesis writ large. Mol Oncol. 4:420–430. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bar EE, Lin A, Mahairaki V, Matsui W and
Eberhart CG: Hypoxia increases the expression of stem-cell markers
and promotes clonogenicity in glioblastoma neurospheres. Am J
Pathol. 177:1491–1502. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bei R, Marzocchella L and Turriziani M:
The use of temozolomide for the treatment of malignant tumors:
Clinical evidence and molecular mechanisms of action. Recent Pat
Anticancer Drug Discov. 5:172–187. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hegi ME, Diserens AC, Gorlia T, Hamou MF,
de Tribolet N, Weller M, Kros JM, Hainfellner JA, Mason W, Mariani
L, et al: MGMT gene silencing and benefit from temozolomide in
glioblastoma. N Engl J Med. 352:997–1003. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sher DJ, Henson JW, Avutu B, Hochberg FH,
Batchelor TT, Martuza RL, Barker FG II, Loeffler JS and Chakravarti
A: The added value of concurrently administered tomozolomide versus
adjuvant temozolomide alone in newly diagnosed glioblastoma. J
Neurooncol. 88:43–50. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Stupp R, Hegi ME, Neyns B, Goldbrunner R,
Schlegel U, Clement PM, Grabenbauer GG, Ochsenbein AF, Simon M,
Dietrich PY, et al: Phase I/IIa study of cilengitide and
temozolomide with concomitant radiotherapy followed by cilengitide
and temozolomide maintenance therapy in patients with newly
diagnosed giloblastoma. J Clin Oncol. 28:2712–2718. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chintala SK, Tonn JC and Rao JS: Matrix
metalloproteinases and their biological function in human gliomas.
Int J Dev Neurosci. 17:495–502. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cifarelli CP, Titus B and Yeoh HK:
Cadherin-dependent adhesion of human U373MG glioblastoma cells
promotes neurite outgrowth and increases migratory capacity:
Laboratory investigation. J Neurosurg. 114:663–669. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Konakondla S and Steven A: Toms: Cerebral
connectivity and high-grade gliomas: Evolving concepts of eloquent
brain in surgery for glioma. AIMS Med Sci. 4:52–70. 2017.
View Article : Google Scholar
|
|
22
|
Gilbert MR, Friedman HS, Kuttesch JF,
Prados MD, Olson JJ, Reaman GH and Zaknoen SL: A phase II study of
temozolomide in patients with newly diagnosed supratentorail
malignant glioma before radiation therapy. Neuro Oncol. 4:261–267.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Davis FG and McCarthy BJ: Current
epidemiological trends and surveillance issues in brain tumors.
Expert Rev Anticancer Ther. 1:395–401. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nabors LB, Mikkelsen T, Rosenfeld SS,
Hochberg F, Akella NS, Fisher JD, Cloud GA, Zhang Y, Carson K,
Wittemer SM, et al: Phase I and correlative biology study of
cilengitide in patients with recurrent malignant glioma. J Clin
Oncol. 25:1651–1657. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Reardon DA, Fink KL, Mikkelsen T,
Cloughesy TF, O'Neill A, Plotkin S, Glantz M, Ravin P, Raizer JJ,
Rich KM, et al: Randomized phase II study of cilengitide, an
integrin-targeting arginine-glycine-aspartic acid peptide, in
recurrent glioblastoma multiforme. J Clin Oncol. 26:5610–5617.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nabors LB, Mikkelsen T, Hegi ME, Ye X,
Batchelor T, Lesser G, Peereboom D, Rosenfeld MR, Olsen J, Brem S,
et al: A safety run-in and randomized phase 2 study of cilengitide
combined with chemoradiation for newly diagnosed glioblastoma
(NABTT 0306). Cancer. 118:5601–5607. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gilbert MR, Kuhn J, Lamborn KR, Lieberman
F, Wen PY, Mehta M, Cloughesy T, Lassman AB, Deangelis LM, Chang S
and Prados M: Cilengitide in patients with recurrent glioblastoma:
The results of NABTC 03–02, a phase II trial with measures of
treatment delivery. J Neurooncol. 106:147–153. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Stupp R, Hegi ME, Gorlia T, Erridge SC,
Perry J, Hong YK, Aldape KD, Lhermitte B, Pietsch T, Grujicic D, et
al: Cilengitide combined with standard treatment for patients with
newly diagnosed glioblastoma with methylated MGMT promoter (CENTRIC
EORTC 26071-22072 study): A multicentre, randomised, open-label,
phase 3 trial. Lancet Oncol. 15:1100–1108. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chinot OL, Wick W, Mason W, Henriksson R,
Saran F, Nishikawa R, Carpentier AF, Hoang-Xuan K, Kavan P, Cernea
D, et al: Bevacizumab plus radiotherapy-temozolomide for newly
diagnosed glioblastoma. N Engl J Med. 370:709–722. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gilbert MR, Dignam JJ, Armstrong TS, Wefel
JS, Blumenthal DT, Vogelbaum MA, Colman H, Chakravarti A, Pugh S,
Won M, et al: A randomized trial of bevacizumab for newly diagnosed
glioblastoma. N Engl J Med. 370:699–708. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gustafson K, Roman M and Fenical W: The
macrolactins, a novel class of antiviral and cytotoxic macrolides
from a deep-sea marine bacterium. J Am Chem Soc. 111:7519–7524.
1989. View Article : Google Scholar
|
|
32
|
Kim H, Kim W, Ryoo I, Kim C, Suk J, Han K,
Hwang S and Yoo I: Neuronal cell protection activity of macrolactin
A produced by Actinomadura sp. J Microbiol Biotechnol. 7:429–434.
1997.
|
|
33
|
Bae SH, Kwon MJ, Park JB, Kim D, Kim DH,
Kang JS, Kim CG, Oh E and Bae SK: Metabolic Drug-Drug Interaction
Potential of Macrolactin A and 7-O-Succinyl Macrolactin A Assessed
by Evaluating Cytochrome P450 Inhibition and Induction and
UDP-Glucuronosyltransferase inhibition in vitro. Antimicrob Agents
Chemother. 58:5036–5046. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Keffer J, Probert L, Cazlaris H,
Georgopoulos S, Kaslaris E, Kioussis D and Kollias G: Transgenic
mice expressing human tumour necrosis factor: A predictive genetic
model of arthritis. EMBO J. 10:4025–4031. 1991.PubMed/NCBI
|
|
35
|
Conrad C, Miller CR, Ji Y, Gomez-Manzano
C, Bharara S, McMurray JS, Lang FF, Wong F, Sawaya R, Yung WK and
Fueyo J: Delta24-hyCD adenovirus suppresses glioma growth in vivo
by combining oncolysis and chemosensitization. Cancer Gene Ther.
12:284–294. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Jiang H, Gomez-Manzano C, Alemany R,
Medrano D, Alonso M, Bekele BN, Lin E, Conrad CC, Yung WK and Fueyo
J: Comparative effect of oncolytic adenoviruses with E1A-55kDa or
E1B-55kDa deletions in malignant gliomas. Neoplasia. 7:48–56. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Samoto K, Ehtesham M, Perng GC, Hashizume
K, Wechsler SL, Nesburn AB, Black KL and Yu JS: A herpes simplex
virus type 1 mutant with gamma 34.5 and LAT deletions effectively
oncolyses human U87 glioblastomas in nude mice. Neurosurgery.
50:599–606. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kirsch M, Strasser J, Allende R, Bello L,
Zhang J and Black PM: Angiostatin suppresses malignant glioma
growth in vivo. Cancer Res. 58:4654–4659. 1998.PubMed/NCBI
|
|
39
|
Lund EL, Bastholm L and Kristjansen PE:
Therapeutic synergy of TNP-470 and ionizing radiation: Effects on
tumor growth, vessel morphology, and angiogenesis in human
glioblastoma multiforme xenografts. Clin Cancer Res. 6:971–978.
2002.
|
|
40
|
Schmidt NO, Ziu M, Carrabba G, Giussani C,
Bello L, Sun Y, Schmidt K, Albert M, Black PM and Carroll RS:
Antiangiogenic therapy by local intracerebral microinfusion
improves treatment efficiency and survival in an orthotopic human
glioblastoma model. Clin Cancer Res. 10:1255–1262. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Candolfi M, Curtin JF, Nichols WS,
Muhammad AG, King GD, Pluhar GE, McNiel EA, Ohlfest JR, Freese AB,
Moore PF, et al: Intracranial glioblastoma models in preclinical
neuro-oncology: Neuropathological characterization and tumor
progression. J Neurooncol. 85:133–148. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Bernstein JJ, Goldberg WJ, Laws ER Jr,
Conger D, Morreale V and Wood LR: C6 glioma cell invasion and
migration of rat brain after neural homografting: Ultrastructure.
Neurosurgery. 26:622–628. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Chicoine MR and Silbergeld DL: Invading C6
glioma cells maintaining tumorigenicity. J Neurosurg. 83:665–671.
1995. View Article : Google Scholar : PubMed/NCBI
|