Introduction
In previous studies, the anti-cytotoxic T lymphocyte
antigen (CTLA)-4 antibody was able to enhance the activity of
conventional T-cells through the blockade of CTLA-4, which competes
with the costimulatory molecule cluster of differentiation (CD) 28
to bind to the ligands CD80 and CD86 on dendritic cells (DCs)
(1,2).
However, the mechanism by which CTLA-4 influences the suppressive
effect of regulatory T-cells (Tregs) in vivo remains
unclear. Although studies have reported that transgenic CTLA-4
enhanced Treg function and anti-CTLA-4 antibodies induced
autoimmune colitis through the suppression of Tregs in a mouse
model, the presence of anti-CTLA4 antibodies did not affect the
suppressive effect of Treg on CD4+CD25−
T-cells in mouse gastritis (3,4).
Therefore, investigation into the role of CTLA-4 in cancer
immunotherapy is required. The present study assessed the
inhibition of CTLA-4 using an anti-CTLA-4 monoclonal antibody
(mAb), in order to determine whether this inhibition was able to
increase the level of antitumor immunity induced by dendritic
cell-mediated radioimmunotherapy (IR/DC) in a mouse model in
vivo. Since malignant cells may exhibit acquired tolerance to
tumor-specific antigens and Tregs may be involved in the survival
of tumor cells through the suppression of antitumor immunity
(5,6),
the present study hypothesized that blocking CTLA-4 may break the
tolerance and induce antitumor immunity through the inhibition of
Tregs.
Materials and methods
Mice and cancer cell lines
A total of seventy-five male C57BL/6 mice aged 6
weeks and weighing 18–22 g were purchased from Central Lab., Animal
Inc. (Seoul, Korea) and the animal experiments were approved by the
Dongnam Institute of Radiological and Medical Sciences
Institutional Animal Care and Use Committee (Busan, Korea). All
mice were maintained in a specific pathogen-free state under a
strict light cycle (lights on at 08:00 h and off at 20:00 h) at
21±2°C and 45±10% relative humidity. The Lewis lung carcinoma (LLC)
CRL-1642 cells, derived from C57BL/6 mice, were obtained from
American Type Culture Collection (Manassas, VA, USA). The cells
were cultured for 7–10 days in Dulbecco's modified Eagle's medium
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 10% fetal bovine serum (FBS; Biowest, Miami, FL,
USA), 100 U/ml penicillin and 100 µg/ml streptomycin at 37°C in a
humidified atmosphere containing 5% CO2.
Isolation of bone marrow-derived
DCs
The C57BL/6 mice (n=5) were sacrificed using
CO2 gas and bone marrow cells were isolated from the
tibias and femurs. Red blood cells (RBCs) were lysed by treatment
with ammonium-chloride-potassium (ACK) lysing buffer (Gibco; Thermo
Fisher Scientific, Inc.) and washed with PBS. The 1×106 cells/ml
cells were cultured in 100-mm culture dishes filled with 10 ml
RPMI-1640 medium supplemented with 10% FBS, 50 mM 2-mercaptoethanol
(Sigma-Aldrich; Merck Millipore, Darmstadt, Germany), 100 U/ml
penicillin, 100 µg/ml streptomycin, 20 ng/ml recombinant mouse
granulocyte macrophage colony-stimulating factor (R&D Systems,
Inc., Minneapolis, MN, USA) and 10 ng/ml recombinant mouse
interleukin-4 (R&D Systems, Inc.) for 3 days. Half of the
medium was replaced with fresh medium every day. On the 6th day,
the non-adherent cells were collected by pipetting and the surface
markers on the cells, such as CD80 (0.2 mg/ml; cat. no., 553769; BD
Pharmingen, San Diego, CA, USA), CD86 (0.2 mg/ml; cat. no., 553692;
BD Pharmingen), I-A-I-E (0.2 mg/ml; cat. no., 557000; BD
Pharmingen), CD11c (0.5 mg/ml; cat. no., 553801; BD Pharmingen)
were analyzed using a FC500 flow cytometer (Beckman Coulter, Inc.,
Miami, FL, USA).
Ionizing radiation (IR) and
immunization procedure
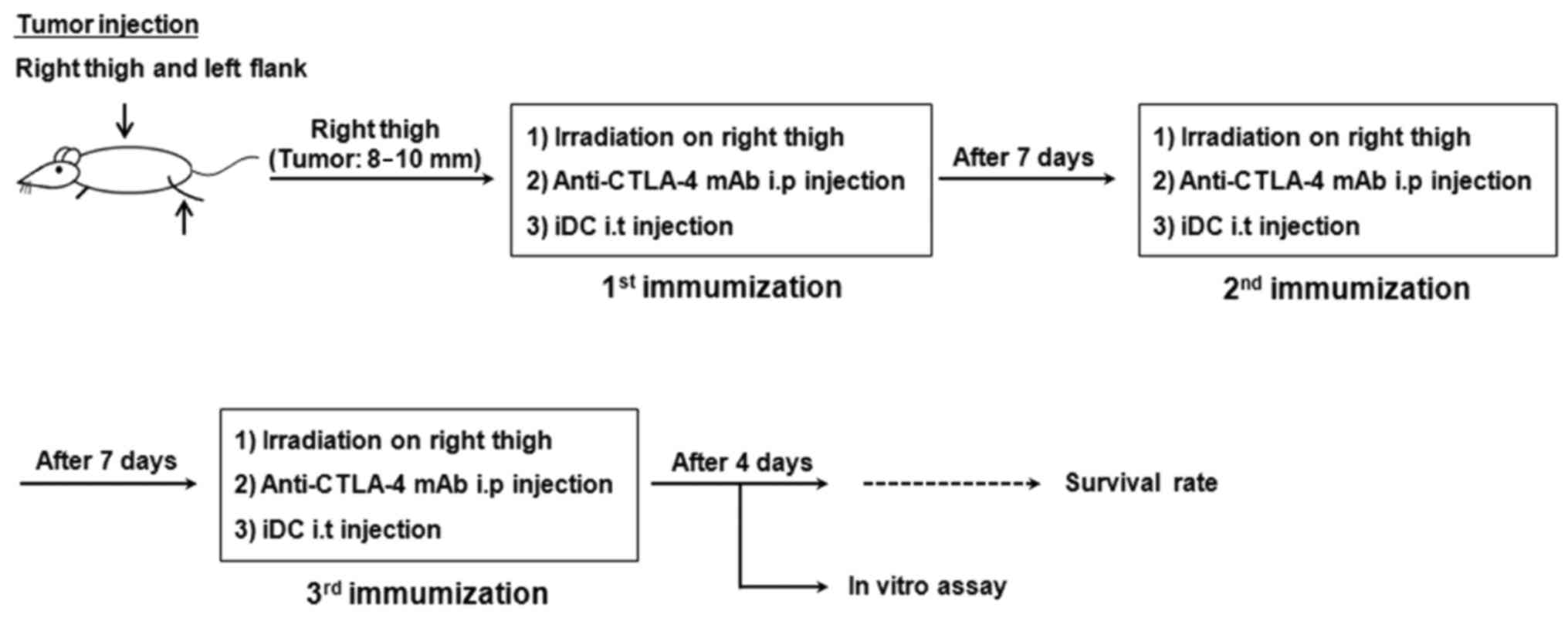
The IR and immunization procedures are shown in
Fig. 1. Briefly, LLC cells were
transplanted subcutaneously into the right thigh (3×105 cells) and
left flank (1.5×105 cells) of the mice (12 mice/group). When the
diameter of tumor masses reached ~10 mm, IR was applied at a dose
of 12 Gy (6 MV photon beam, dose rate of 6.1 Gy/min) to the tumors
on the right thighs of the mice using a linear accelerator
(Infinity; Elekta Limited, Crawley, UK). Dosimetry was evaluated
using an ionization chamber connected to an electrometer system,
according to the International Atomic Energy Agency (IAEA)
guideline (IAEA TRS-398) (7). Prior
to irradiation, the mice were anesthetized with an intraperitoneal
injection of 50 mg zoletile (Virbac, Nice, France) plus 5 mg rompun
(Bayer Korea, Ansan, Korea) per kg body weight and placed in a
customized restraining device and positioned. The irradiation field
square was set as 20×20 cm and the radiation was focused on the
legs of the mice to minimize whole body exposure. After 24 h, 1×106
cells/100 µl immature DCs were injected into the irradiated tumor
for immunization. Immunization was performed 3 times at 1-week
intervals. Tumor size was measured twice per week using the
following formula: Tumor size (mm2) = length (mm) ×
width (mm). The 100 µl anti-CTLA-4 mAb (diluted to 2 mg/ml with
PBS; catalog no., BE0103; clone 9H10; Bio X Cell, West Lebanon, NH,
USA) was administered intraperitoneally to the mice on the same day
as every iDC injection.
Flow cytometric analysis
The proportion and number of effector T-cells and
CD4+CD25+ forkhead box P3 (FOXP3)+ cells within the total CD4+ cell
population was evaluated following IR/iDC combined with anti-CTLA-4
mAb treatment. The C57BL/6 mice (5 mice/group) were sacrificed
using CO2 gas to harvest their tumor tissue and spleens,
after 4 days following the final immunization. Tumor infiltrating
leukocytes (1×105 cells/ml) were prepared from individual tumor
tissues cut with scissors and dissociated using 0.04 mg/ml Liberase
(Roche Applied Science, Penzberg, Germany) in RPMI-1640 medium
(Welgene, Daegu, Korea) at 37°C for 90 min, followed by passage
through a 0.45-µm nylon mesh (BD Pharmingen). Single cell
suspensions of splenocytes (1×107 cells/ml) were obtained by
grinding the spleens, followed by passage through a 0.45-µm nylon
mesh. RBCs were lysed using 1X ACK lysing buffer at room
temperature for 2 min. The separated cells were washed with PBS and
immunostained with phycoerythrin (PE)-conjugated anti-mouse CD4 (BD
Pharmingen, catalog no., 553652) and PE-cyanin7 (Cy7)-conjugated
anti-mouse CD25 (BD Pharmingen, catalog no., 552880), respectively,
at a concentration of 20 µl/106 cells, in the dark at 4°C for 30
min. The CD4/CD25 double stained cells were resuspended in a 1:4
dilution (total volume 1 ml) of TF Fix/Perm Buffer (transcription
factor buffer set; BD Pharmingen; catalog no., 562725) and
incubated at 4°C for 40 min in the dark. The permeabilized cells
were stained with PE-Cy5-conjugated anti-mouse FOXP3 (eBioscience,
CA, USA; catalog no., 15-5773-82) at a concentration of 20 µl/106
cells and were incubated at 4°C for 30 min in the dark.
Subsequently, the cells were washed in a 1:5 dilution (total volume
2 ml) of TF Perm/Wash Buffer (transcription factor buffer set; BD
Pharmingen; catalog no., 562725). Flow cytometry analysis was
performed on a FC500 flow cytometer. Results were generated using
CXP v1.0 Software (Beckman Coulter, Inc.).
Enzyme-linked immunospot (ELISpot)
assay
ImmunoSpot plates for ELISpot (Merck Millipore) were
pretreated with 35% ethanol for 1 min at room temperature.
Following the removal of the ethanol, the plates were coated with
capture antibodies (10 µg/ml; cat. no., 51-1818KA; BD Pharmingen)
overnight at 4°C. The plates were blocked with 10 g/l bovine serum
albumin (Sigma-Aldrich; Merck Millipore) for 2 h and washed three
times with PBS. Splenocytes (0.5×106 cells/well) and 50 µg/ml LLC
cell lysates were added to each well and incubated at 37°C for 24
h. The plate was washed three times with PBS and an additional
three times with PBS-Tween buffer, and biotinylated detection
antibodies (diluted to 2 µg/ml with PBS; catalog no., 51-1818KA; BD
Pharmingen) were added and the plate was incubated for 2 h at room
temperature. The plates were washed >3 times with PBS-Tween
buffer. Streptavidin-horseradish peroxidase (100 µl per well) was
then added, and the plates were incubated for 2 h at room
temperature. Subsequent to being washed twice with PBS, a
chromogenic substrate and H2O2 (AEC
substrate; catalog no., 551951; BD Pharmingen) were added to each
well to produce visible spots. When adequate spots were developed,
distilled water was added to stop the reaction and the plates were
air-dried overnight. The number of spots were calculated and the
images were analyzed using an EliSpot Reader System (Autoimmun
Diagnostika GmbH, Strassberg, Germany).
Cytotoxicity assay
Splenoctyes (3×107) isolated from the mice of each
group were stimulated by co-culture with mitomycin C
(Sigma-Aldrich; Merck Millipore) and 10 µg/ml treated LLC cells
(3×106 cells) for 5 days. The LLC cells were labeled with
5-carboxyfluorescein diacetate succinmidyl ester (CFSE;
eBioscience, Inc., San Diego, CA, USA) at a concentration of 5 µM
for 10 min at 37°C in a humidified incubator with 5%
CO2. Subsequent to labeling, the cells were washed with
RPMI-1640 medium containing 10% FBS. Stimulated splenocytes
(effector cells) were co-cultured with CFSE-labeled LLC cells
(target cells; 2×104/well) at the appropriate effector-to-target
cell count ratios (target cell: effect cell, 40:1; 20:1; 10:1) in
round-bottomed plates at 37°C in a humidified atmosphere containing
5% CO2 for 6 h. Subsequent to incubation, the cells were
transferred to tubes and placed in an ice water bath. Propidium
iodide (50 µg/ml) was added for DNA labeling of dead cells. The
dead cells were analyzed using a FC500 flow cytometer. Results were
generated using CXP v1.0 Software (Beckman Coulter, Inc, Brea, CA,
USA).
Statistical analysis
Statistical analysis was performed using one-way
analysis of variance followed by Tukey's multiple comparison test
and log-rank tests. P<0.05 was considered to indicate a
statistically significant difference. The software used for
statistical analysis was SPSS-18 software (SPSS, Inc., Chicago, IL,
USA).
Results
Anti-CTLA-4 mAb enhances the antitumor
effect of IR/DC in a mouse model of lung cancer
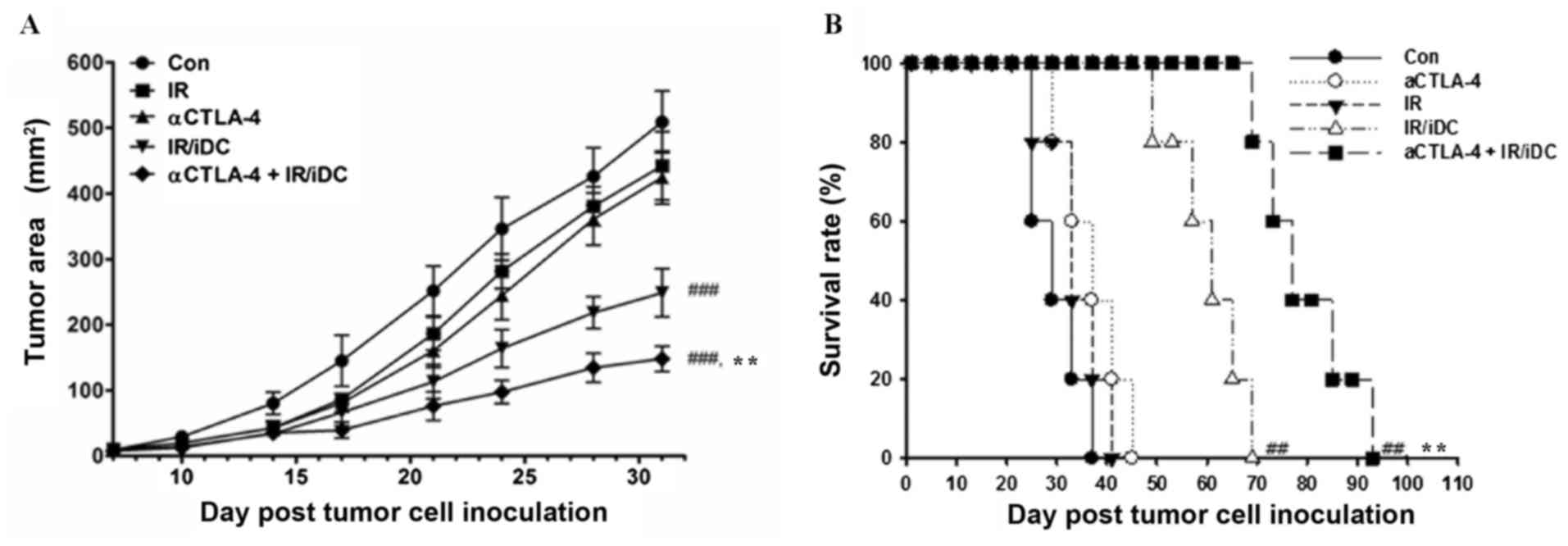
To evaluate the systemic antitumor effects of
anti-CTLA-4 mAb and IR/DC, LLC transplanted mice were treated with
single agents or the combination therapy 3 times at 1-week
intervals. The growth of distant tumors was significantly
suppressed by treatment with IR/DC and combined therapy (Fig. 2A). Single treatment with IR or
anti-CTLA-4 mAb did not significantly inhibit distant tumor growth.
Similar results were shown in the survival test; combination
therapy significantly increased the survival of the mice (Fig. 2B). Although single treatment with
anti-CTLA-4 mAb exhibited only a small effect when used alone, this
treatment markedly enhanced the antitumor effect of IR/DC in terms
of the tumor growth rate and survival time (Fig. 2). These results suggest that, although
the anti-CTLA-4 mAb may be ineffective with respect to inducing
antitumor immunity, it may be a good enhancer of immune reponse in
other types of immunotherapy.
Ratio of Tregs to CD4+
T-cells was decreased in the spleen and tumors following combined
therapy
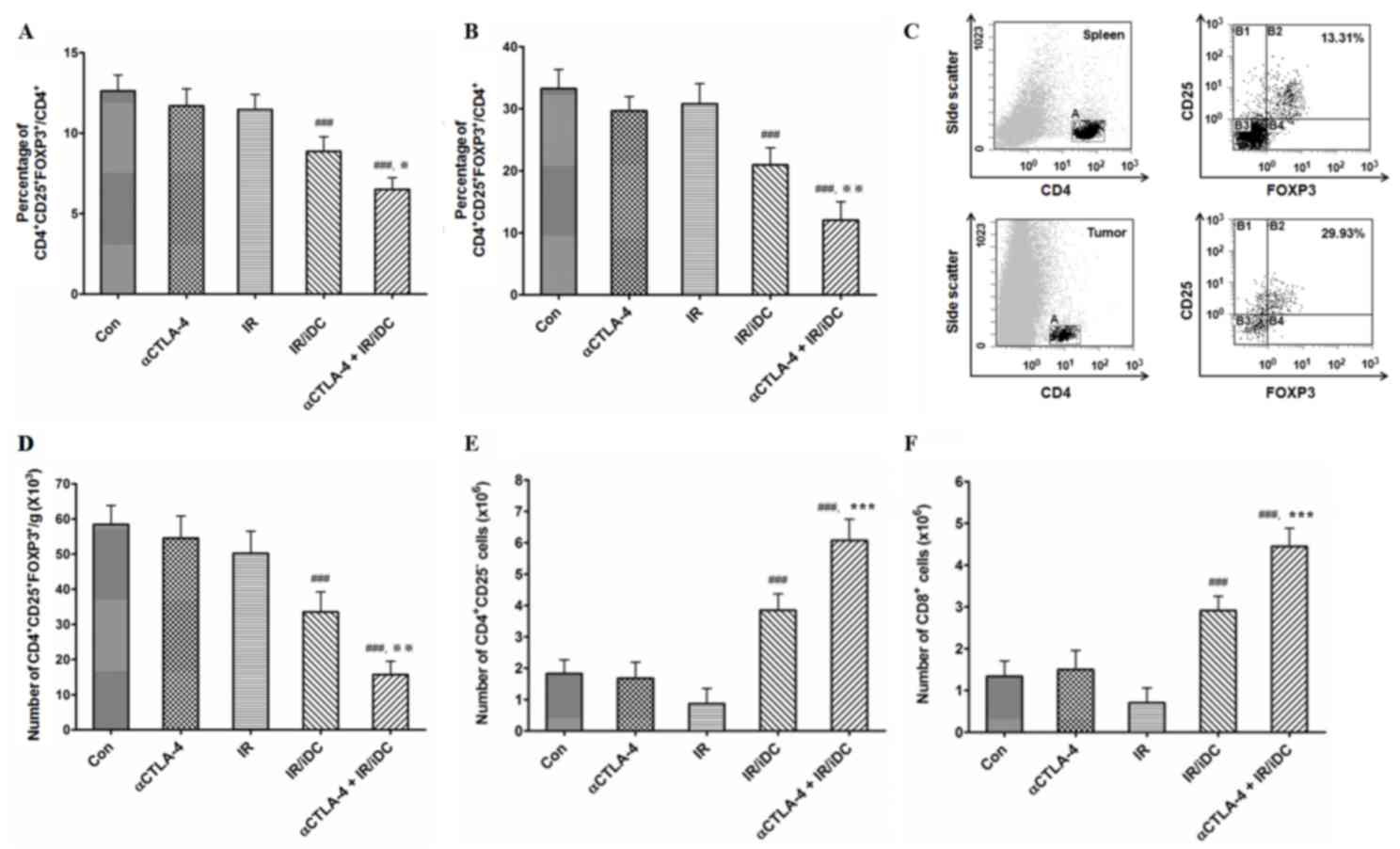
Since it was thought that the function of Tregs was
affected by treatment with an anti-CTLA-4 mAb and IR/DC, the ratios
of Tregs to CD4+ T-cells in the spleens (Fig. 3A) and tumors (Fig. 3B) were measured. In the present study,
CD4+ T-cells and Tregs were detected using fluorescence-conjugated
anti-CD4, anti-CD25 and anti-FOXP3 mAbs (Fig. 3C). The ratios of Tregs to CD4+ T-cells
were significantly decreased by treatment with IR/DC. Although
single treatment with the anti-CTLA-4 mAb did not affect the
proportion of Tregs, the Treg proportion was reduced by combination
treatment with the anti-CTLA-4 mAb and IR/DC. Decreased ratio of
Treg could derived from the decreased number of Treg cells and
decreased proliferation of conventional T-cells. Although the
decreased ratio of Tregs to CD4+ T-cells did not distinguish a
reduced number of Tregs from the proliferation of conventional CD4+
T-cells, it was certain that decreased ratio of Treg contributed to
the induction of antitumor immunity.
Combined therapy decreases the number
of Tregs and increases the number of conventional T-cells in the
mouse spleen
Following dissection and weighing, the spleens were
mashed and the total splenocytes were counted. The number of Tregs
per g/spleen was calculated, and the number was significantly
decreased following treatment with IR/DC and combined therapy
compared to untreated control (Fig.
3D). Single treatment with the anti-CTLA-4 mAb did not
significantly alter the number of Tregs. To evaluate the influence
of IR/DCand the anti-CTLA-4 mAb on two types of T-cells, total
numbers of conventional CD4+/CD25- and CD8+ T-cells were also
calculated. The numbers of CD4+/CD25- and CD8+ T-cells were
significantly increased in the mice following treatment with IR/DC,
and the numbers of cells were further increased by combined therapy
with the anti-CTLA-4 mAb and IR/DC (Fig.
3E and F).
Number of tumor-specific T cells is
increased following treatment with the anti-CTLA-4 mAb and/or
IR/DC, but not subsequent to IR
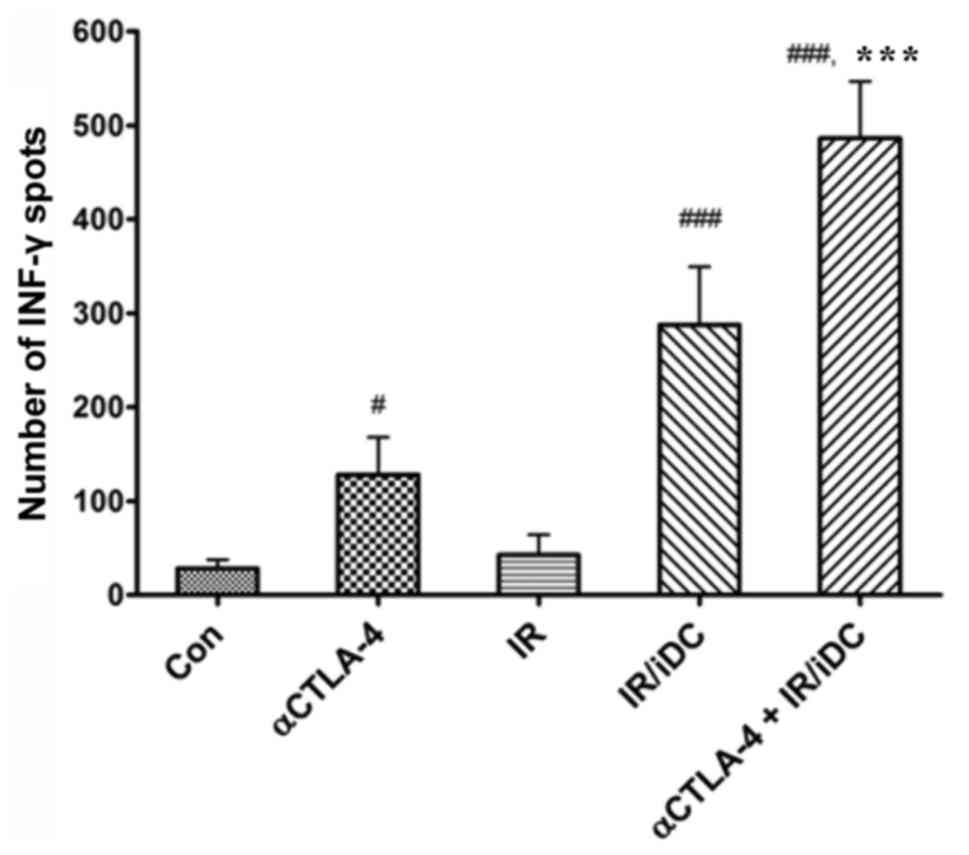
Since the ratio of reactive T-cells to tumor antigen
is small, it is important the absolute quantity of T-cells respond
to specific tumor antigens and secrete the tumor protective
cytokine, IFN-γ. When tumor antigens reactive T-cells were counted
using an EliSpot assay, the number of IFN-γ spots secreted by
activated T-cells was significantly increased following treatment
with the anti-CTLA-4 mAb alone, IR/DC alone and the combined
therapy (Fig. 4). This result
suggests that the anti-CTLA-4 mAb and IR/DC enhance antitumor
immunity through an increased number of general immune cells and
the clonal expansion of specific T-cells against tumor
antigens.
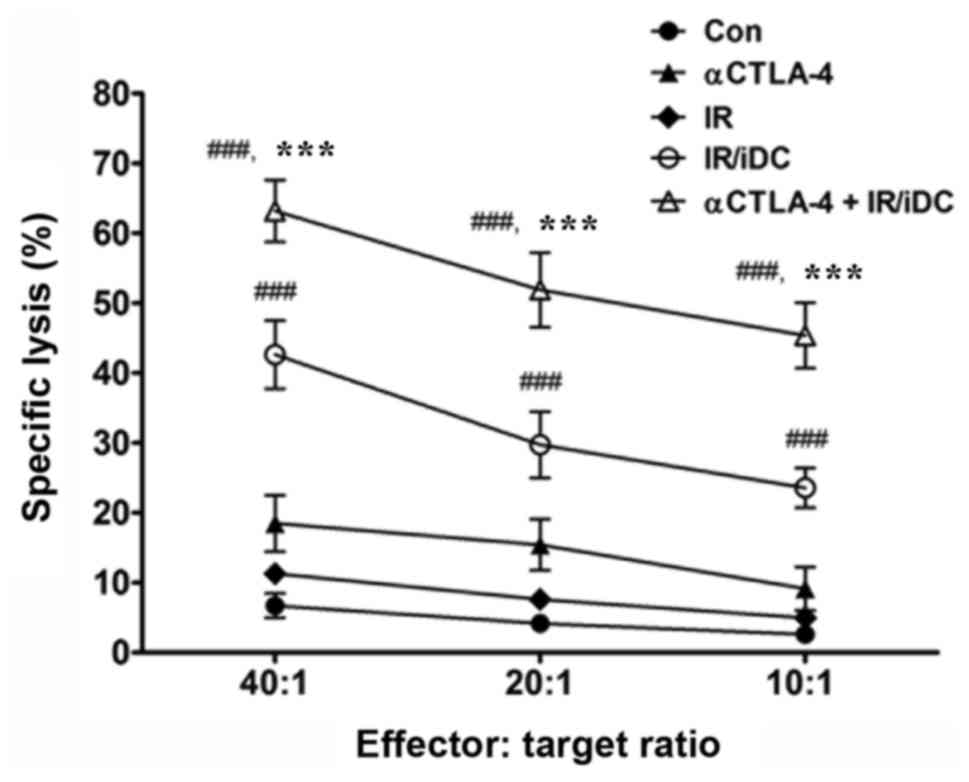
Splenocytes activated by combination
therapy are more cytotoxic to tumor cells than splenocytes
activated by single agent therapy
In the present study, effective immune responses
were revealed to be induced by combination therapy with the
anti-CTLA-4 mAb and IR/DC, and immunized splenocytes were shown to
effectively destroy tumor cells (Fig.
5). The specific lysis of LLC cells was increased by
splenocytes activated by IR/DC compared with inactivated
splenocytes, and it was further increased when IR/DC was combined
with the anti-CTLA-4 mAb. It was previously suggested that IR/DC is
a potent strategy to induce antitumor immunity (8,9). However,
the combination of IR/DC with the anti-CTLA-4 mAb may be a more
effective for immunotherapy by overcoming an immune checkpoint,
CTLA-4.
Discussion
Tregs serve a central role in the maintenance of
immune homeostasis and self-tolerance. However, due to their highly
suppressive functions, elevated Treg numbers assist the survival of
tumors by promoting evasion of immune surveillance (10) and, therefore, represent a major
obstacle to successful immunotherapy (11). Following the discovery of the
expression of CTLA-4 on the surface of T-cells, certain functions
of CTLA-4 have been hypothesized, including competition with the
costimulatory molecule CD28 on CD4+/CD25−
T-cells for binding to the CD80 and CD86 ligands on antigen
presenting cells (APCs), as well as the direct inhibition of APCs
and CD4+/CD25− T-cells by CTLA-4 and CD80/86
interactions (12). Currently, two
types of human anti-CTLA-4 mAbs, ipilimumab and tremelimumab, are
being used in the treatment of metastatic melanoma and metastatic
mesothelioma (13,14). However, the precise role of CTLA-4 in
Tregs during cancer immunotherapy remains unclear. Therefore, an
investigation into the role of CTLA-4 on Tregs in cancer
immunotherapy was required. The present study revealed that
blocking CTLA-4 using an anti-CTLA-4 mAb increased the level of
antitumor immunity that was induced by IR/DC, and consequently
inhibited tumor growth in vivo. However, single treatment
with the anti-CTLA-4 mAb was not sufficient to evoke antitumor
immunity or a reduction in the number of Tregs despite a slight
increase in the number of tumor-specific T-cells.
Since malignant cells may evade early immune
surveillance and acquire tolerance to tumor-specific antigens,
breaking the acquired tolerance and enhancing antitumor immunity is
required for successful immunotherapy (15). Immune check point proteins such as
CTLA-4 and programmed cell death protein-1 have been suggested to
contribute to the evasion of immune surveillance by tumor cells;
thus inhibiting these proteins may enhance antitumor immunity
(16,17). The competitive influence of CTLA-4 on
conventional T-cells and Tregs have previously been reported
(1,3).
In addition, an increased number of Tregs has been associated with
treatment failure and a poor prognosis of cancer patients. The
present study demonstrated that an anti-CTLA-4 mAb inhibited tumor
growth in vivo through altered Treg function or
proliferation.
Although it is known that anti-CTLA-4 mAbs exhibit
antitumor effects on several types of cancer (18–20), the
benefit of this treatment alone was limited until now. However, it
was found that anti-CTLA-4 combined treatment with IR/DC
immunotherapy may provide a more powerful and effective modality to
treat patients with cancer through the efficient reduction of Treg
function.
Acknowledgements
The present study was supported by a National
Research Foundation of Korea grant funded by the Korean government
(grant no. 50595-2015).
References
|
1
|
Birebent B, Lorho R, Lechartier H, de
Guibert S, Alizadeh M, Vu N, Beauplet A, Robillard N and Semana G:
Suppressive properties of human CD4+CD25+
regulatory T cells are dependent on CTLA-4 expression. Eur J
Immunol. 34:3485–3496. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pedicord VA, Montalvo W, Leiner IM and
Allison JP: Single dose of anti-CTLA-4 enhances CD8+
T-cell memory formation, function, and maintenance. Proc Natl Acad
Sci USA. 108:266–271. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Takahashi T, Tagami T, Yamazaki S, Uede T,
Shimizu J, Sakaguchi N, Mak TW and Sakaguchi S: Immunologic
self-tolerance maintained by CD25(+)CD4(+) regulatory T cells
constitutively expressing cytotoxic T lymphocyte-associated antigen
4. J Exp Med. 192:303–310. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu H, Hu B, Xu D and Liew FY:
CD4+CD25+ regulatory T cells cure murine
colitis: The role of IL-10, TGF-beta and CTLA4. J Immunol.
171:5012–5017. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Houghton AN and Guevara-Patino JA: Immune
recognition of self in immunity against cancer. J Clin Invest.
114:468–471. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nishikawa H and Sakaguchi S: Regulatory T
cells in tumor immunity. Int J Cancer. 127:759–767. 2010.PubMed/NCBI
|
|
7
|
Andreo P, Burns DT, Hohlfeld K, Huq MS,
Kanai T, Laitano F, Smyth VG and Vynckier S: Absorbed dose
determination in external beam radiotherapy: An international code
of practice for dosimetry based on standards of absorbed dose to
waterTech Report Series No. 398. International Atomic Energy
Agency; Vienna: pp. 1–183. 2006
|
|
8
|
Son CH, Bae JH, Shin DY, Lee HR, Yang K
and Park YS: Antitumor effect of dendritic cell loaded ex vivo and
in vivo with tumor-associated antigens in lung cancer model.
Immunol Invest. 43:447–462. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim KW, Kim SH, Shin JG, Kim GS, Son YO,
Park SW, Kwon BH, Kim DW, Lee CH, Sol MY, et al: Direct injection
of immature dendritic cells into irradiated tumor induces efficient
antitumor immunity. Int J Cancer. 109:685–690. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Facciabene A, Motz GT and Coukos G:
T-regulatory cells: Key players in tumor immune escape and
angiogenesis. Cancer Res. 72:2162–2171. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Voena C and Chiarle R: Advances in cancer
immunology and cancer immunotherapy. Discov Med. 21:125–133.
2016.PubMed/NCBI
|
|
12
|
Sansom DM and Walker LS: The role of CD28
and cytotoxic T-lymphocyte antigen-4 (CTLA-4) in regulatory T-cell
biology. Immunol Rev. 212:131–148. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Niezgoda A, Niezgoda P and Czajkowski R:
Novel approaches to treatment of advanced melanoma: A review on
targeted therapy and immunotherapy. BioMed Res Int.
2015:8513872015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Calabrò L, Ceresoli GL, di Pietro A,
Cutaia O, Morra A, Ibrahim R and Maio M: CTLA4 blockade in
mesothelioma: Finally a competing strategy over cytotoxic/target
therapy? Cancer Immunol Immunother. 64:105–112. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Staveley-O'Carroll K, Sotomayor E,
Montgomery J, Borrello I, Hwang L, Fein S, Pardoll D and Levitsky
H: Induction of antigen-specific T cell anergy: An early event in
the course of tumor progression. Proc Natl Acad Sci USA.
95:1178–1183. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
van Elsas A, Hurwitz AA and Allison JP:
Combination immunotherapy of B16 melanoma using anti-cytotoxic T
lymphocyte-associated antigen 4 (CTLA-4) and granulocyte/macrophage
colony-stimulating factor (GM-CSF)-producing vaccines induces
rejection of subcutaneous and metastatic tumors accompanied by
autoimmune depigmentation. J Exp Med. 190:355–366. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dong H, Strome SE, Salomao DR, Tamura H,
Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, et al:
Tumor-associated B7-H1 promotes T-cell apoptosis: A potential
mechanism of immune evasion. Nat Med. 8:793–800. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yang YF, Zou JP, Mu J, Wijesuriya R, Ono
S, Walunas T, Bluestone J, Fujiwara H and Hamaoka T: Enhanced
induction of antitumor T-cell responses by cytotoxic T
lymphocyte-associated molecule-4 blockade: The effect is manifested
only at the restricted tumor-bearing stages. Cancer Res.
57:4036–4041. 1997.PubMed/NCBI
|
|
19
|
Paradis TJ, Floyd E, Burkwit J, Cole SH,
Brunson B, Elliott E, Gilman S and Gladue RP: The anti-tumor
activity of anti-CTLA-4 is mediated through its induction of IFN
gamma. Cancer Immunol Immunother. 50:125–133. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fecci PE, Ochiai H, Mitchell DA, Grossi
PM, Sweeney AE, Archer GE, Cummings T, Allison JP, Bigner DD and
Sampson JH: Systemic CTLA-4 blockade ameliorates glioma-induced
changes to the CD4+ T cell compartment without affecting
regulatory T-cell function. Clin Cancer Res. 13:2158–2167. 2007.
View Article : Google Scholar : PubMed/NCBI
|