Introduction
Pancreatic cancer, which has a 5-year patient
survival rate of <5%, is a major cause of cancer-associated
mortality worldwide (1,2). Complete resection is an essential part
of treatment for patients with pancreatic cancer. However, only
10–20% of patients are candidates for curative resection.
Furthermore, due to the high rate of recurrence, the postoperative
5-year survival rate is 15–25% when curative resection is performed
(3–5).
Several studies have conducted randomized controlled studies on
adjuvant chemotherapy following pancreatic cancer resection
(6–8).
The European Study Group for Pancreatic Cancer 1 and 3 trials, and
the Charite Onkologic 001 trial demonstrated that the
administration of gemcitabine or fluorouracil plus folinic acid
significantly improves overall survival following surgical
resection in patients with pancreatic cancer in comparison to
surgery alone (6–8). Based on these results, adjuvant
chemotherapy with gemcitabine is now considered to be the standard
treatment and is routinely recommended following curative resection
for pancreatic cancer. However, adjuvant chemotherapy with
gemcitabine is unable to completely prevent the development of
recurrence. The selection of patients who would benefit most from
gemcitabine treatment may be an important step towards improving
the clinical outcomes associated with pancreatic cancer.
Ribonucleotide reductase subunit M1 (RRM1) is a
multimeric enzyme that converts ribonucleotides to
deoxyribonucleosides, both of which are required for DNA
polymerization and repair (9,10). It has previously been reported that
the overexpression of the RRM1 gene is associated with gemcitabine
resistance. Patients with advanced pancreatic carcinoma who
exhibited high levels of RRM1 expression were demonstrated to have
poor survival rates following gemcitabine treatment, while patients
with non-small cell lung cancer who had low levels of RRM1
expression were revealed to benefit significantly from
gemcitabine/cisplatin neoadjuvant chemotherapy (11,12).
However, few published studies have evaluated the prognostic value
of RRM1 expression in patients with pancreatic cancer who undergo
resection followed by adjuvant chemotherapy with gemcitabine, and
no definite conclusions have been made regarding the prognostic
value of RRM1 in such patients (13,14). Using
cancer tissue samples from individuals, the characterization of the
genes that are associated with tumor sensitivity or resistance and
antitumor agents serves an essential role in the development and
provision of individualized adjuvant chemotherapy treatments.
In the present study, RRM1 expression was
investigated in consecutive patients who underwent curative
resection followed by adjuvant chemotherapy with gemcitabine. In
addition, the association between RRM1 expression and the
clinicopathological parameters and survival rates of patients were
evaluated.
Patients and methods
Patients
Consecutive patients were selected from the medical
records of those who underwent pancreatic surgery at the Kanagawa
Cancer Centre (Yokohama, Kanagawa, Japan) between April 2005 and
December 2014. The following inclusion criteria were applied: i) A
pathologically common type of pancreatic adenocarcinoma according
to the definitions of the International Union Against Cancer (UICC)
tumor-node-metastasis (TNM) 6th edition (15); ii) the patient had initially undergone
curative resection, with the resected specimen available from the
archive; and iii) the patient had received adjuvant chemotherapy
with gemcitabine. The resected specimens were histopathologically
examined and were staged according to the UICC TNM 6th edition
(15). Patients with other pancreatic
and periampullary neoplasms, including intraductal papillary
mucinous neoplasm, cystadenocarcinoma and endocrine tumors, and
patients who had undergone R2 resection were excluded from the
present study. The present study was approved by the Institutional
Review Board Committee of the Kanagawa Cancer Center.
Surgical procedure
All pancreatic surgeries were performed in
accordance with standardized procedures that have been previously
described (16–19). Briefly, for distal pancreatectomy
cases, lymph node dissection was performed in the region of the
celiac trunk, and the superior mesenteric artery and vein, in
addition to behind the pancreas along the left side of the renal
vein and the left adrenal gland. In each case, intraperitoneal
drains were placed close to the pancreatic anastomosis and stump.
For pancreaticoduodenectomy cases, pylorus-preserving
pancreaticoduodenectomy was performed as the standard procedure.
Lymph node dissection along the hepatoduodenal ligament, common
hepatic artery, vena cava, superior mesenteric vein and the right
side of the superior mesenteric artery was performed as part of the
standard procedure. Multiple intraperitoneal drains were placed,
with the first being posterior to the hepaticojejunostomy and the
second on the anterior surface of the pancreaticojejunostomy or the
closed remnant of the pancreas.
Adjuvant chemotherapy
Gemcitabine treatment was initiated within 8 weeks
of surgery. The patients received a weekly dose via intravenously
of 1,000 mg/m2 for 3 weeks, followed by 1 week of rest. Gemcitabine
treatment was continued for 6 months.
Follow-up
Patients were followed up at outpatient clinics.
Hematological tests and physical examinations were performed at
least every 2 weeks during adjuvant chemotherapy treatment, and at
least every 3 months for 5 years following the end of the adjuvant
chemotherapy course. The carcinoembryonic antigen and cancer
antigen 19–9 tumor marker levels were measured at least every 3
months for 5 years. Patients underwent a computed tomography
examination every 3 months during the first 3 years after surgery,
and then every 6 months until 5 years after surgery. Peritoneal
recurrence was defined as positive when imaging results revealed at
least one of the following findings: Massive ascites, ascites
confirmed by cytology, enhanced abdominal nodules, abnormal
intestinal wall thickness, increased fat density of the intestinal
mesentery, diffuse hydronephrosis or an intraabdominal mass.
Imaging results were assessed by a radiologist and two staff
physicians at Kanagawa Cancer Center (Kanagawa, Japan). When liver
metastasis was suspected based on the imaging results,
gadolinium-ethoxybenzyl-diethylentriaminepenta-acetate-enhanced
magnetic resonance imaging or contrast-enhanced ultrasonography was
performed to confirm the diagnosis.
Immunohistochemical analysis of RRM1
expression
Hematoxylin and eosin-stained 5-µm slides containing
specimens from each pancreatic adenocarcinoma sample were reviewed,
and a representative tumor region and the corresponding
formalin-fixed paraffin-embedded tissue block was selected for use
in a tissue microarray. RRM1 expression was evaluated using human
mouse monoclonal antibody directed against RRM1 (dilution, 1:100;
#60073-2; Proteintech Group, Inc., Chicago, IL, USA) and the
horseradish peroxidase secondary antibody was Histofine®
Simple Stain MAX-PO (#424151; Nichirei Biosciences, Inc., Tsukiji,
Japan). The immunohistochemical staining procedure was performed as
described previously (14,20). Images were captured using light
microscopy. The intensity of the RRM1 staining was scored as
follows: Grade 0, unstained; grade 1, slightly stained; grade 2,
weakly stained in comparison to plasma and stroma cells; and grade
3, stained as strongly as plasma and stroma cells. For the
evaluation of intratumoral RRM1 expression, if grade 2 or 3
staining was observed in >50% of the neoplasm, the sample was
considered to have high RRM1 expression, whereas if grade 0 or 1
staining was observed in >50% of tumor cells, the sample was
considered to have low RRM1 expression (Fig. 1). The cut-off value used was
determined on the basis of previous study results (14,20). The
immunohistochemical evaluation of RRM1 expression was confirmed
independently by two observers and a consensus was reached by joint
review.
Statistical analysis
The significance of the correlations between RRM1
expression and clinicopathological parameters was determined using
Fisher's exact or χ2 tests. Overall survival (OS) rate was defined
as the period between surgery and mortality. Recurrence-free
survival (RFS) was defined as the period between surgery and
recurrence or mortality. The data of the patients who had not
experienced an event were censored at the date of the final
observation. The OS and RFS rates were evaluated using univariate
and multivariate analyses. OS and RFS curves were calculated using
the Kaplan-Meier estimator method and compared using the log-rank
test. The univariate and multivariate survival analyses were
performed using Cox's proportional hazards model. P<0.05 was
considered to indicate a statistically significant difference. The
survival data were obtained from hospital records or from the city
registry system. All statistical analyses were performed using SPSS
software (version 11.0; SPSS, Inc., Chicago, IL, USA).
Results
Patients
A total of 201 patients underwent surgical resection
between April 2005 and December 2014. Of these patients, 101 were
eligible for inclusion in the present study. The patients were aged
between 40 and 78 years (median, 66 years), with 57 men and 44
women. In total, 28 patients underwent distal pancreatomy, 70
underwent pancreaticoduodenectomy and 3 underwent total pancreatic
resection. The median follow-up period was 67.3 months (range,
22.2–122.7 months).
Association between
clinicopathological factors and RRM1 expression
High RRM1 expression was observed in 41 (40.6%)
patients (Table I). The
clinicopathological factors were compared between patients with
high and low RRM1 expression. In total, 9 clinicopathological
factors were evaluated. The incidence of lymphatic invasion was
significantly higher in the patients with high RRM1 expression
compared with that in the low RRM1 expression group (P=0.021;
Table I).
 | Table I.Association between the
clinicopathological characteristics of patients with pancreatic
cancer and high (n=41) or low (n=60) ribonucleotide reductase
M1. |
Table I.
Association between the
clinicopathological characteristics of patients with pancreatic
cancer and high (n=41) or low (n=60) ribonucleotide reductase
M1.
| Clinicopathological
characteristic | Low RRM1 group, n
(%) | High RRM1 group, n
(%) | P-value |
|---|
| Gender |
|
| 0.725 |
| Male | 33 (55.0) | 24 (58.5) |
|
|
Female | 27 (45.0) | 17 (41.5) |
|
| Age, years |
|
| 0.955 |
|
<65 | 26 (43.3) | 18 (43.9) |
|
| ≥65 | 34 (56.7) | 23 (56.1) |
|
| R status |
|
| 0.404 |
| R0 | 52 (86.7) | 33 (80.5) |
|
| R1 | 8 (13.3) | 8 (19.5) |
|
| Tumor location |
|
| 0.233 |
|
Head | 46 (76.7) | 27 (65.9) |
|
|
Body/tail | 14 (23.3) | 14 (34.1) |
|
| Pathological
differentiation |
|
| 0.154 |
|
Well | 52 (86.7) | 31 (75.6) |
|
|
Moderate/poor | 8 (13.3) | 10 (24.4) |
|
| UICC pT factor |
|
| 0.142 |
|
T1/T2 | 6 (10.0) | 1 (2.4) |
|
| T3 | 54 (90.0) | 40 (97.6) |
|
| Lymph node
metastasis |
|
| 0.259 |
| N0 | 16 (26.7) | 7 (17.1) |
|
| N1 | 44 (73.3) | 34 (82.9) |
|
| Lymphatic
invasion |
|
| 0.021 |
| No | 33 (55.0) | 13 (31.7) |
|
|
Yes | 27 (45.0) | 28 (68.3) |
|
| Vascular
invasion |
|
| 0.551 |
| No | 24 (40.0) | 14 (34.1) |
|
|
Yes | 36 (60.0) | 27 (65.9) |
|
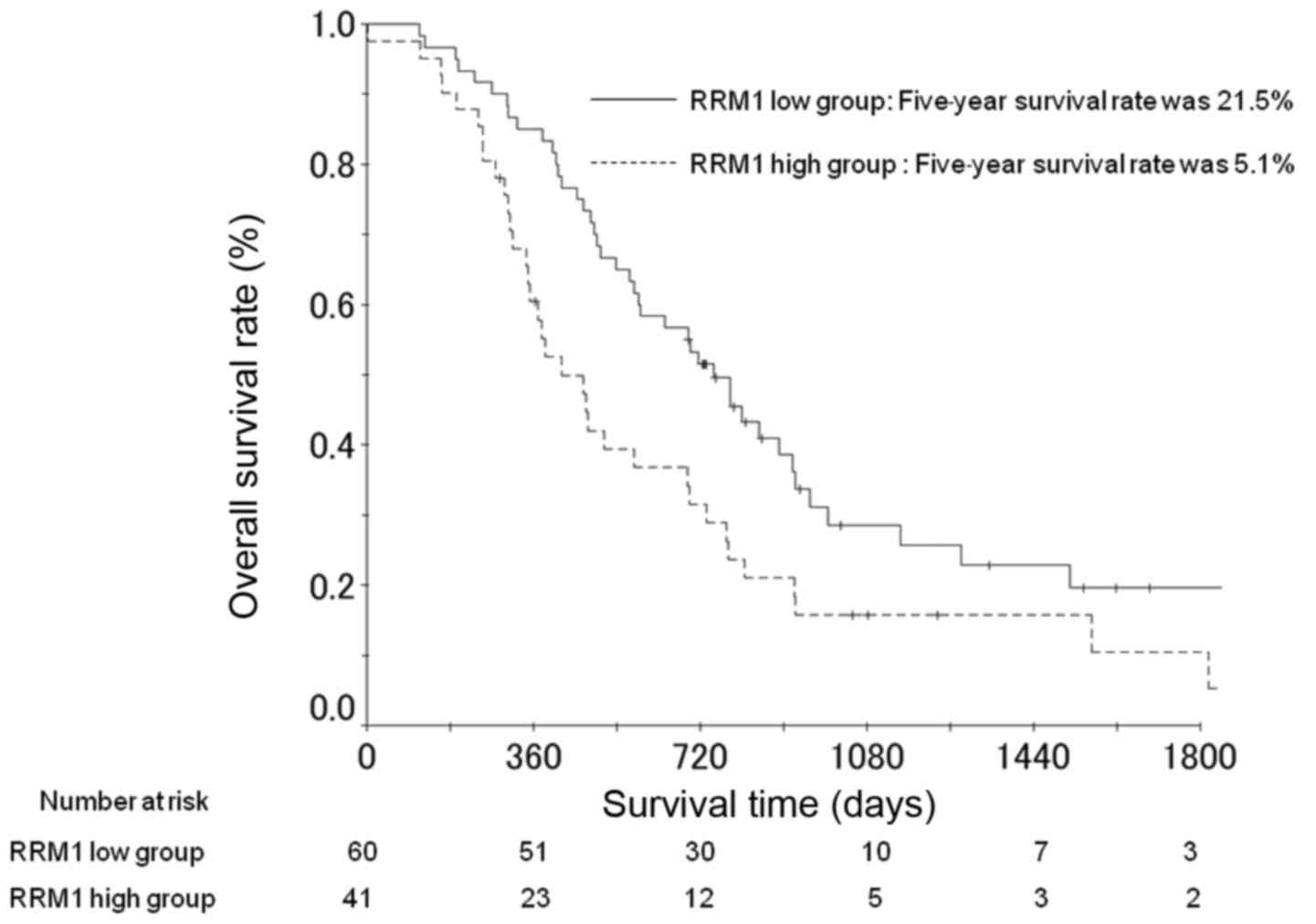
Survival analysis
The OS rates at 3 and 5 years post-surgery in the
patients with high RRM1 expression were 10.5 and 5.1%,
respectively; and 25.7 and 21.5% in the patients with low RRM1
expression (Fig. 2). The difference
between OS rates for patients with high and low RRM1 expression was
identified to be significant following multivariate analysis
(P=0.015; Table II). Multivariate
analysis also demonstrated that tumor location and lymphatic
invasion were significant risk factors for OS (Table II).
 | Table II.Univariate and multivariate analyses
of risk factors for the overall survival of patients with
pancreatic cancer. |
Table II.
Univariate and multivariate analyses
of risk factors for the overall survival of patients with
pancreatic cancer.
|
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|
|---|
| Factor | n | OR | 95% CI | P-value | OR | 95% CI | P-value |
|---|
| Gender |
|
|
| 0.561 |
|
| 0.900 |
|
Female | 44 | 1.000 |
|
| 1.000 |
|
|
|
Male | 57 | 1.143 | 0.728–1.728 |
| 1.033 | 0.620–1.620 |
|
| Age, years |
|
|
| 0.740 |
|
| 0.626 |
|
<65 | 44 | 1.000 |
|
| 1.000 |
|
|
|
≥65 | 57 | 1.081 | 0.683–1.683 |
| 1.123 | 0.703–1.703 |
|
| R status |
|
|
| 0.041 |
|
| 0.197 |
| R0 | 85 | 1.000 |
|
| 1.000 |
|
|
| R1 | 16 | 1.850 | 1.026–3.026 |
| 1.555 | 0.795–3.795 |
|
| Tumor location |
|
|
| 0.024 |
|
| 0.013 |
|
Body/tail | 28 | 1.000 |
|
| 1.000 |
|
|
|
Head | 73 | 1.840 | 1.085–3.085 |
| 1.980 | 1.153–3.153 |
|
| Pathological |
|
|
| 0.892 |
|
| 0.932 |
|
differentiation |
|
Well | 83 | 1.000 |
|
| 1.000 |
|
|
|
Moderate/poor | 18 | 1.042 | 0.572–1.572 |
| 1.029 | 0.533–1.533 |
|
| UICC pT factor |
|
|
| 0.035 |
|
| 0.273 |
|
T1/T2 | 7 | 1.000 |
|
| 1.000 |
|
|
| T3 | 94 | 4.545 | 1.113–18.113 |
| 2.284 | 0.522–9.522 |
|
| Lymph node |
|
|
| 0.038 |
|
| 0.704 |
|
metastasis |
|
|
|
|
|
|
|
| N0 | 23 | 1.000 |
|
| 1.000 |
|
|
| N1 | 78 | 1.802 | 1.034–3.034 |
| 1.131 | 0.599–2.599 |
|
| Lymphatic
invasion |
|
|
| 0.001 |
|
| 0.009 |
| No | 46 | 1.000 |
|
| 1.000 |
|
|
|
Yes | 55 | 2.192 | 1.374–3.374 |
| 1.898 | 1.174–3.174 |
|
| Vascular
invasion |
|
|
| 0.032 |
|
| 0.283 |
| No | 38 | 1.000 |
|
| 1.000 |
|
|
|
Yes | 63 | 1.678 | 1.044–2.044 |
| 1.358 | 0.776–2.776 |
|
| RRM1 status |
|
|
| 0.009 |
|
| 0.015 |
|
Low | 60 | 1.000 |
|
| 1.000 |
|
|
|
High | 41 | 1.814 | 1.160–2.160 |
| 1.777 | 1.116–2.116 |
|
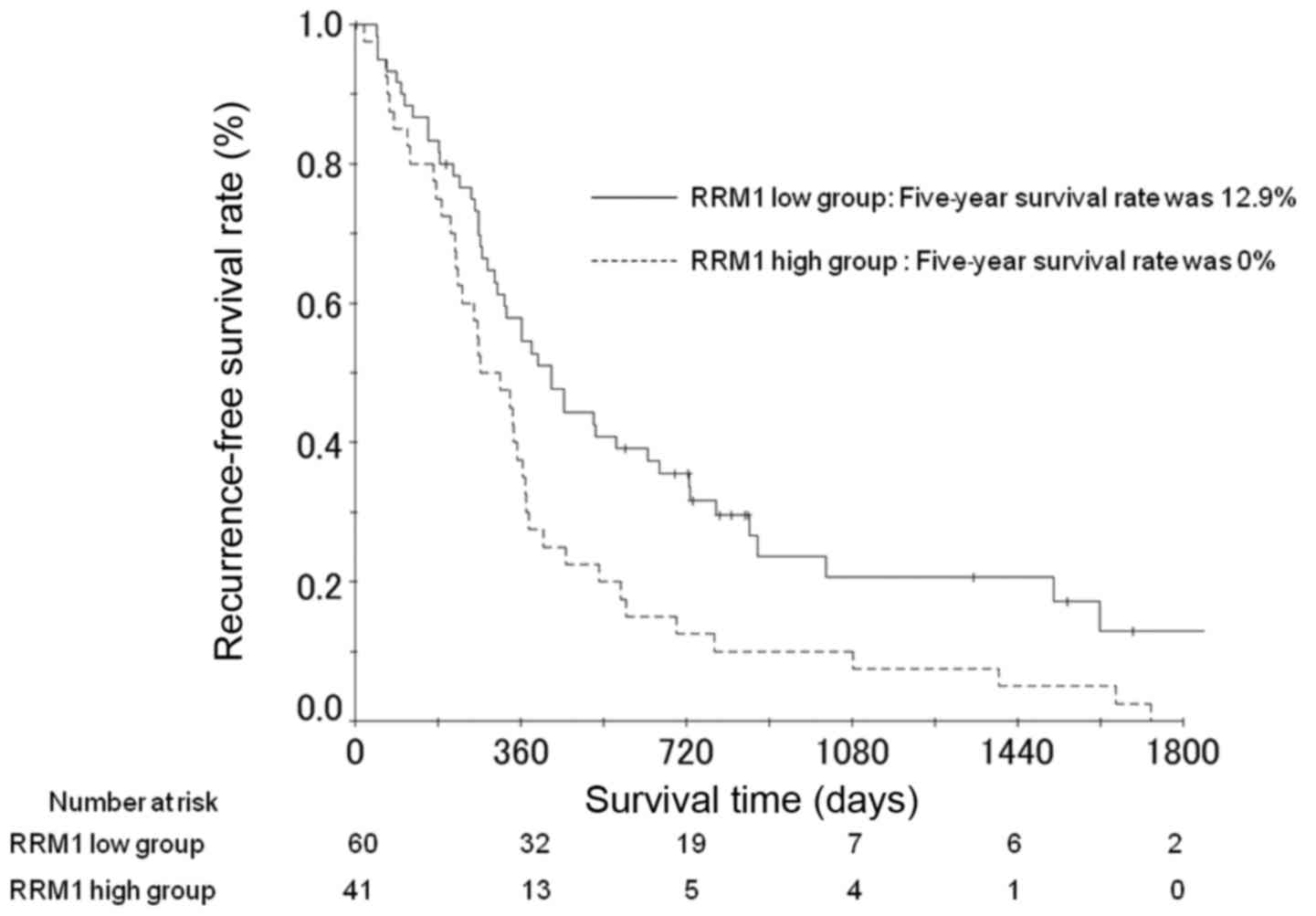
The RFS rates at 3 and 5 years post-surgery in the
patients with high RRM1 expression were 7.8 and 0%, respectively
(Fig. 3). For patients with low RRM1
expression, the RFS rates were 20.7 and 12.9%, respectively
(Fig. 3). The difference between RFS
rates for patients with low and high expression was significant
(P=0.042; Table III). Multivariate
analysis also demonstrated that tumor location, lymphatic invasion
and resection status were significant risk factors for RFS
(Table III).
 | Table III.Univariate and multivariate analyses
of risk factors for the recurrence-free survival of patients with
pancreatic cancer. |
Table III.
Univariate and multivariate analyses
of risk factors for the recurrence-free survival of patients with
pancreatic cancer.
|
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|
|---|
| Factor | n | OR | 95% CI | P-value | OR | 95% CI | P-value |
|---|
| Gender |
|
|
| 0.874 |
|
| 0.380 |
|
Female | 44 | 1.000 |
|
| 1.000 |
|
|
|
Male | 57 | 1.035 | 0.674–1.674 |
| 1.239 | 0.768–1.768 |
|
| Age, years |
|
|
| 0.293 |
|
| 0.272 |
|
<65 | 44 | 1.000 |
|
| 1.000 |
|
|
|
≥65 | 57 | 1.264 | 0.817–1.817 |
| 1.294 | 0.817–2.817 |
|
| R status |
|
|
| 0.001 |
|
| 0.007 |
| R0 | 85 | 1.000 |
|
| 1.000 |
|
|
| R1 | 16 | 2.668 | 1.469–4.469 |
| 2.322 | 1.261–4.261 |
|
| Tumor location |
|
|
| 0.016 |
|
| 0.014 |
|
Body/tail | 28 | 1.000 |
|
| 1.000 |
|
|
|
Head | 73 | 1.816 | 1.118–2.118 |
| 1.850 | 1.132–3.132 |
|
| Pathological
differentiation |
|
|
| 0.775 |
|
| 0.747 |
|
Well | 83 | 1.000 |
|
| 1.000 |
|
|
|
Moderate/poor | 18 | 1.083 | 0.627–1.627 |
| 1.099 | 0.620–1.620 |
|
| UICC pT factor |
|
|
| 0.148 |
|
| 0.730 |
|
T1/T2 | 7 | 1.000 |
|
| 1.000 |
|
|
| T3 | 94 | 1.778 | 0.814–3.814 |
| 1.167 | 0.484–2.484 |
|
| Lymph node
metastasis |
|
|
| 0.074 |
|
| 0.715 |
| N0 | 23 | 1.000 |
|
| 1.000 |
|
|
| N1 | 78 | 1.597 | 0.956–2.956 |
| 1.122 | 0.606–2.606 |
|
| Lymphatic
invasion |
|
|
| 0.001 |
|
| 0.031 |
| No | 46 | 1.000 |
|
| 1.000 |
|
|
|
Yes | 55 | 2.238 | 1.438–3.438 |
| 1.704 | 1.049–2.049 |
|
| Vascular
invasion |
|
|
| 0.204 |
|
| 0.818 |
| No | 38 | 1.000 |
|
| 1.000 |
|
|
|
Yes | 63 | 1.330 | 0.856–2.856 |
| 1.066 | 0.618–1.618 |
|
| RRM1 status |
|
|
| 0.008 |
|
| 0.042 |
|
Low | 60 | 1.000 |
|
| 1.000 |
|
|
|
High | 41 | 1.784 | 1.164–2.164 |
| 1.610 | 1.017–2.017 |
|
Discussion
The present study evaluated the RRM1 status in
patients with pancreatic adenocarcinoma who underwent curative
resection followed by adjuvant chemotherapy with gemcitabine, and
found that 40% of these patients exhibited high RRM1 expression.
Furthermore, the OS and RFS rates of the patients differed
significantly based on their RRM1 status. These results suggest
that gemcitabine alone was insufficient as an adjuvant therapy,
particularly in the patients with high RRM1 expression. Thus, these
patients should be a target group for future clinical trials using
novel treatments for pancreatic cancer.
Numerous studies have examined the presence and
effect of RRM1 protein overexpression or gene amplification in
patients with pancreatic adenocarcinoma. These studies reported
that RRM1 is highly expressed in 20.4–87.3% of patients (13,14,20–22).
However, the measurement of RRM1 expression was not standardized
and the background of the patients with pancreatic cancer was
heterogeneous, as it included patients with stage I–IV tumors.
Nakagawa et al (14) evaluated
the incidence of RRM1 in resectable pancreatic cancer cases using
immunohistochemical methods in 109 Japanese patients with
pancreatic carcinoma who were treated with adjuvant
gemcitabine-based chemotherapy following operative resection. It
was demonstrated that RRM1 expression was observed in 44 (40.4%)
patients. In addition, Xie et al (22) measured RRM1 expression using reverse
transcriptase-quantitative polymerase chain reaction analysis in
122 patients with resectable pancreatic adenocarcinoma. It was
revealed that high RRM1 expression was observed in 44 (36.1%)
patients. These results were similar to the results of the present
study. Thus, the incidence of high RRM1 expression is ~40% in
patients with resectable pancreatic cancer.
Regarding the association between RRM1 expression
and clinicopathological factors, Akita et al (23) reported that in an analysis of 64
patients with resected pancreatic carcinoma, there were no
significant differences in clinicopathological factors, including
UICC pT factor, and lymph node status, between patients with high
and low RRM1 expression. Nakagawa et al (14) reported similar results. In the current
study, a significant difference was only observed in lymphatic
invasion. However, there was no difference between the two groups
in any of the other clinicopathological parameters of the patients
with high and low RRM1 expression, including UICC pT factor and
lymph node status. Thus, RRM1 expression appears to be independent
from the other clinicopathological factors.
In the present study, the OS and RFS rates differed
significantly based on the patients' RRM1 status. It is
hypothesized that RRM1 is an essential enzyme that encodes the
regulatory subunit of ribonucleotide reductase and catalyzes the
reduction of ribonucleoside diphosphates to the corresponding
deoxyribonucleotides for use in de novo DNA synthesis
(24,25). There is a good rationale for this as
gemcitabine is converted into gemcitabine diphosphate, an active
metabolite capable of inhibiting ribonucleoside reductase, and RRM1
has been demonstrated to be a determinant of gemcitabine resistance
in pancreatic cancer cells under in vitro conditions
(11). Nakagawa et al
(14) evaluated 109 patients with
resected pancreatic cancer who underwent adjuvant chemotherapy with
gemcitabine and were divided into 2 groups based on their RRM1
levels. A significant association was identified between
disease-free survival and RRM1 expression (P=0.009). Furthermore,
the patients with high RRM1 levels experienced poorer overall
survival following gemcitabine treatment compared with those with
low RRM1 levels (P=0.019). In addition, Akita et al
(23) reported that patients with low
RRM1 expression experienced significantly improved OS rate compared
with patients with high RRM1 expression in an analysis of 68
patients with pancreatic carcinoma who underwent resection and
received gemcitabine chemotherapy. A similar result was observed in
a study of patients with advanced pancreatic cancer (11). Nakahira et al (11) evaluated 18 patients with recurrent
pancreatic cancer who were treated with gemcitabine and who were
divided into 2 groups based on RRM1 levels. A significant
association was observed between gemcitabine response and RRM1
expression (P=0.018). Additionally, patients with high RRM1 levels
exhibited poorer survival times following gemcitabine treatment
compared with those patients with low RRM1 levels (P=0.016). The
median survival time following gemcitabine treatment was 6.0 months
in the patients with high RRM1 levels, while it was 14.6 months in
the patients with low RRM1 levels. However, Giovannetti et
al (26) demonstrated that there
was no correlation between RRM1 expression and the clinical outcome
of patients with pancreatic cancer. These controversial findings
are probably associated with a range of factors, including the
interaction with other genes, environmental effects on gene
expression and differences in the detection methods, sample sizes
and study design.
Particular attention is required when interpreting
the results of the current study as there are several associated
potential limitations. Firstly, the present study was a
retrospective analysis and was performed at a single institution.
Thus, the possibility that these findings were observed by chance
cannot be excluded. Secondly, there was a selection bias in the
patients in this series. Surgeons often avoid performing
pancreatomy in certain patients, as the procedure is associated
with high rates of morbidity (40–60%) and mortality (1–1.5%)
(27–31). Thus, the fact that certain patients in
this study received pancreatectomy could be considered a potential
bias. In addition, the hospital is a specialized cancer center.
Finally, the evaluation of RRM1 expression was not standardized.
The appropriate RRM1 cutoff value remains unclear. Considering
these limitations, the results must be confirmed in another cohort
or in a prospective multicenter-study.
In conclusion, the OS and RFS rates of patients with
pancreatic cancer who underwent curative resection followed by
adjuvant chemotherapy with gemcitabine differed significantly based
on their RRM1 expression. These results suggest that gemcitabine
was insufficient, particularly for the patients with high RRM1
expression. Thus, these patients should be a target group for
future clinical trials using novel treatments for pancreatic
cancer.
Acknowledgements
The present study was supported by the Kanagawa
Prefectural Hospitals Cancer Fund (grant no., KCCH26-2), the
Yokohama Foundation for Advancement of Medical Science and the
Takeda Science Foundation.
References
|
1
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nakao A, Fujii T, Sugimoto H, Kanazumi N,
Nomoto S, Kodera Y, Inoue S and Takeda S: Oncological problems in
pancreatic cancer surgery. World J Gastroenterol. 12:4466–4472.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Matsuno S, Egawa S, Fukuyama S, Motoi F,
Sunamura M, Isaji S, Imaizumi T, Okada S, Kato H, Suda K, et al:
Pancreatic cancer registry in Japan: 20 years of experience.
Pancreas. 28:219–230. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Carpelan-Holmström M, Nordling S, Pukkala
E, Sankila R, Lüttges J, Klöppel G and Haglund C: Does anyone
survive pancreatic ductal adenocarcinoma? A nationwide study
re-evaluating the data of the finnish cancer registry. Gut.
54:385–387. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wagner M, Redaelli C, Lietz M, Seiler CA,
Friess H and Büchler MW: Curative resection is the single most
important factor determining outcome in patients with pancreatic
adenocarcinoma. Br J Surg. 91:586–594. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Neoptolemos JP, Stocken DD, Friess H,
Bassi C, Dunn JA, Hickey H, Beger H, Fernandez-Cruz L, Dervenis C,
Lacaine F, et al: A randomized trial of chemoradiotherapy and
chemotherapy after resection of pancreatic cancer. N Engl J Med.
350:1200–1210. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Neoptolemos JP, Stocken DD, Bassi C,
Ghaneh P, Cunningham D, Goldstein D, Padbury R, Moore MJ, Gallinger
S, Mariette C, et al: Adjuvant chemotherapy with fluorouracil plus
folinic acid vs. gemcitabine following pancreatic cancer resection:
A randomized controlled trial. JAMA. 304:1073–1081. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Oettle H, Post S, Neuhaus P, Gellert K,
Langrehr J, Ridwelski K, Schramm H, Fahlke J, Zuelke C, Burkart C,
et al: Adjuvant chemotherapy with gemcitabine vs observation in
patients undergoing curative-intent resection of pancreatic cancer:
A randomized controlled trial. JAMA. 297:267–277. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kwon WS, Rha SY, Choi YH, Lee JO, Park KH,
Jung JJ, Kim TS, Jeung HC and Chung HC: Ribonucleotide reductase M1
(RRM1) 2464G>A polymorphism shows an association with
gemcitabine chemosensitivity in cancer cell lines. Pharmacogenet
Genomics. 16:429–438. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jordheim LP, Sève P, Trédan O and Dumontet
C: The ribonucleotide reductase large subunit (RRM1) as a
predictive factor in patients with cancer. Lancet Oncol.
12:693–702. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nakahira S, Nakamori S, Tsujie M,
Takahashi Y, Okami J, Yoshioka S, Yamasaki M, Marubashi S, Takemasa
I, Miyamoto A, et al: Involvement of ribonucleotide reductase M1
subunit overexpression in gemcitabine resistance of human
pancreatic cancer. Int J Cancer. 120:1355–1363. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rosell R, Felip E, Taron M, Majo J, Mendez
P, Sanchez-Ronco M, Queralt C, Sanchez JJ and Maestre J: Gene
expression as a predictive marker of outcome in stage IIB-IIIA-IIIB
non-small cell lung cancer after induction gemcitabine-based
chemotherapy followed by resectional surgery. Clin Cancer Res.
10:4215s–4219s. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kim R, Tan A, Lai KK, Jiang J, Wang Y,
Rybicki LA and Liu X: Prognostic roles of human equilibrative
transporter 1 (hENT-1) and ribonucleoside reductase subunit M1
(RRM1) in resected pancreatic cancer. Cancer. 117:3126–3134. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nakagawa N, Murakami Y, Uemura K, Sudo T,
Hashimoto Y, Kondo N and Sueda T: Combined analysis of intratumoral
human equilibrative nucleoside transporter 1 (hENT1) and
ribonucleotide reductase regulatory subunit M1 (RRM1) expression is
a powerful predictor of survival in patients with pancreatic
carcinoma treated with adjuvant gemcitabine-based chemotherapy
after operative resection. Surgery. 153:565–575. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sobin LH and Wittekind CH: TNM
Classification of Malignant Tumors. 6th. John Wiley & Sons; New
York, NY: 2002
|
|
16
|
Büchler MW, Friess H, Wagner M, Kulli C,
Wagener V and Z'Graggen K: Pancreatic fistula after pancreatic head
resection. Br J Surg. 87:883–889. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wagner M, Z'graggen K, Vagianos CE,
Redaelli CA, Holzinger F, Sadowski C, Kulli C, Zimmermann H, Baer
HU and Büchler MW: Pylorus-preserving total pancreatectomy. Early
and late results. Dig Surg. 18:188–195. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Andrén-Sandberg A, Wagner M, Tihanyi T,
Löfgren P and Friess H: Technical aspects of left-sided pancreatic
surgery for cancer. Dig Surg. 16:305–312. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Seiler CA, Wagner M, Sadowski C, Kulli C
and Büchler MW: Randomized prospective trial of pylorus-preserving
vs. Classic duodenopancreatectomy (Whipple procedure): Initial
clinical results. J Gastrointest Surg. 4:443–452. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Valsecchi ME, Holdbrook T, Leiby BE,
Pequignot E, Littman SJ, Yeo CJ, Brody JR and Witkiewicz AK: Is
there a role for the quantification of RRM1 and ERCC1 expression in
pancreatic ductal adenocarcinoma? BMC Cancer. 12:1042012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Maréchal R, Bachet JB, Mackey JR, Dalban
C, Demetter P, Graham K, Couvelard A, Svrcek M, Bardier-Dupas A,
Hammel P, et al: Levels of gemcitabine transport and metabolism
proteins predict survival times of patients treated with
gemcitabine for pancreatic adenocarcinoma. Gastroenterology.
143:664–674, e1-e6. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xie H, Jiang W, Jiang J, Wang Y, Kim R and
Liu X and Liu X: Predictive and prognostic roles of ribonucleotide
reductase M1 in resectable pancreatic adenocarcinoma. Cancer.
119:173–181. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Akita H, Zheng Z, Takeda Y, Kim C, Kittaka
N, Kobayashi S, Marubashi S, Takemasa I, Nagano H, Dono K, et al:
Significance of RRM1 and ERCC1 expression in resectable pancreatic
adenocarcinoma. Oncogene. 28:2903–2909. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Elledge SJ, Zhou Z and Allen JB:
Ribonucleotide reductase: Regulation, regulation, regulation.
Trends Biochem Sci. 17:119–123. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Reichard P: From RNA to DNA, why so many
Ribonucleotide reductases? Science. 260:1773–1777. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Giovannetti E, Mey V, Nannizzi S,
Pasqualetti G, Del Tacca M and Danesi R: Pharmacogenetics of
anticancer drug sensitivity in pancreatic cancer. Mol Cancer Ther.
5:1387–1395. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Povoski SP, Karpeh MS Jr, Conlon KC,
Blumgart LH and Brennan MF: Association of preoperative biliary
drainage with postoperative outcome following
pancreaticoduodenectomy. Ann Surg. 230:131–142. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yeo CJ, Cameron JL, Lillemoe KD, Sohn TA,
Campbell KA, Sauter PK, Coleman J, Abrams RA and Hruban RH:
Pancreaticoduodenectomy with or without distal gastrectomy and
extended retroperitoneal lymphadenectomy for periampullary
adenocarcinoma, part 2: Randomized controlled trial evaluating
survival, morbidity, and mortality. Ann Surg. 236:355–368. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kawai M, Tani M, Terasawa H, Ina S, Hirono
S, Nishioka R, Miyazawa M, Uchiyama K and Yamaue H: Early removal
of prophylactic drains reduces the risk of intra-abdominal
infections in patients with pancreatic head resection: Prospective
study for 104 consecutive patients. Ann Surg. 244:1–7. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Munoz-Bongrand N, Sauvanet A, Denys A,
Sibert A, Vilgrain V and Belghiti J: Conservative management of
pancreatic fistula after pancreaticoduodenectomy with
pancreaticogastrostomy. J Am Coll Surg. 199:198–203. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tran KT, Smeenk HG, van Eijck CH, Kazemier
G, Hop WC, Greve JW, Terpstra OT, Zijlstra JA, Klinkert P and
Jeekel H: Pylorus preserving pancreaticoduodenectomy versus
standard Whipple procedure: A prospective, randomized, multicenter
analysis of 170 patients with pancreatic and periampullary tumors.
Ann Surg. 240:738–745. 2004. View Article : Google Scholar : PubMed/NCBI
|