Introduction
Colorectal cancer (CRC) is one of the most
frequently diagnosed types of cancer. Previous studies have
suggested that an increased risk of CRC may be associated with
dietary factors, blood insulin levels and the bioavailability of
insulin-like growth factors (IGFs) (1–3).
The IGF system is a complex network consisting of
two ligands (IGF-1 and IGF-2), two cell-surface receptors (IGF-1R
and IGF-2R), a family of six high-affinity IGF-binding proteins
(IGFBPs-1 to −6) and ≥4 additional low-affinity binding proteins
(IGFBP-related proteins). This system is involved in normal cell
growth, neoplastic transformation and tumor development. Imbalance
of the IGF system has been implicated in the pathogenesis and
progression of breast and other malignancies (3,4).
Abnormal expression of IGFs, as well as their
receptors and binding proteins, has been identified in several
malignancies, including CRC (5).
IGF-1 may be able to increase the risk of cancer development
(6). IGF-2 is also involved in tumor
progression and patient survival and has been suggested to function
as an autocrine growth factor in CRC (7). Overexpressed IGF-1R may promote
invasion, tumor growth, metastasis and progression (8). Furthermore, ≥6 types of IGFBPs are
expressed in most tissues and are present in the circulation in
normal patients (9). These IGFBPs
bind to IGFs with high affinity and the primary role of IGFBPs is
to regulate the availability of IGFs for interactions with IGF-1R
(10). From these, IGFBP-3 is the
most abundant IGFBP in the circulation under normal circumstances,
and has been focused on in numerous studies.
The association of the gene expression of the IGF
system in tumors with the prognosis or clinicopathological
characteristics of patients with CRC remains to be elucidated. In
the present study, mRNA expression levels of the IGF-1,
IGF-2, IGF-1R and IGFBP-3 genes were measured
in cancer tissue and adjacent normal mucosa obtained from 202
patients with CRC. The focus of the current study was to evaluate
the mRNA expression levels of the IGF-1, IGF-2,
IGF-1R and IGFBP-3 genes and to determine whether
expression levels are associated with clinicopathological
characteristics and the clinical outcomes of patients with CRC.
Materials and methods
Patients and surgical specimens
A total of 202 patients with untreated CRC were
enrolled into the present study. All patients underwent primary
tumor resection at Gastroenterological Center at Yokohama City
University Medical Center (Yokohama, Japan) or the Kanagawa Cancer
Center (Kanagawa, Japan) between December 2002 and June 2006.
Informed consent was obtained from each patient and the Ethical
Review Boards at Yokohama City University and Kanagawa Cancer
Center approved the present study. None of the patients had
received chemotherapy or radiotherapy prior to surgery or had any
other malignancies. Tumor staging was evaluated according to the
7th edition of the International Union Against Cancer
Tumor-Node-Metastasis classification of malignant tumors (11). The resected tumor and adjacent normal
mucosa were obtained from the resected colorectum, embedded in
Tissue Tek OCT medium (Sakura Finetek Europe B.V., Felmingweg,
Netherlands), frozen in liquid nitrogen and stored at −80°C until
used for RNA extraction. Sections of 5-µm thickness were stained
with hematoxylin and eosin, and histopathological features were
examined using a light microscope (CH30; Olympus Corporation,
Tokyo, Japan). Sections that consisted of >80% carcinoma cells
were defined as cancer tissue and used for total RNA extraction.
The clinicopathological characteristics of the patients with CRC
are presented in Table I.
 | Table I.Associations between the intratumoral
expression levels of IGF-1, IGF-2, IGF-1R and IGFBP-3 genes and the
clinicopathological characteristics of patients with colorectal
cancer. |
Table I.
Associations between the intratumoral
expression levels of IGF-1, IGF-2, IGF-1R and IGFBP-3 genes and the
clinicopathological characteristics of patients with colorectal
cancer.
|
| IGF-1
expression |
| IGF-2
expression |
| IGF-1R
expression |
| IGFBP-3
expression |
|
|---|
|
|
|
|
|
|
|
|
|
|---|
|
Characteristics | Low n=101 | High n=101 | P-value | Low n=101 | High n=101 | P-value | Low n=101 | High n=101 | P-value | Low n=101 | High n=101 | P-value |
|---|
| Age, years |
|
|
|
|
|
|
|
|
|
|
|
|
|
<60 | 28 | 28 | 1.000 | 27 | 29 | 0.753 | 30 | 26 | 0.530 | 28 | 28 | 1.000 |
|
≥60 | 73 | 73 |
| 74 | 72 |
| 71 | 75 |
| 73 | 73 |
|
| Gender |
|
|
|
|
|
|
|
|
|
|
|
|
|
Male | 52 | 58 | 0.397 | 51 | 59 | 0.258 | 50 | 60 | 0.158 | 56 | 54 | 0.778 |
|
Female | 49 | 43 |
| 50 | 42 |
| 51 | 41 |
| 45 | 47 |
|
| Tumor location |
|
|
|
|
|
|
|
|
|
|
|
|
|
Colon | 62 | 48 | 0.048 | 62 | 48 | 0.048 | 54 | 56 | 0.778 | 53 | 57 | 0.572 |
|
Rectum | 39 | 53 |
| 39 | 53 |
| 47 | 45 |
| 48 | 44 |
|
| Tumor diameter,
cm |
|
|
|
|
|
|
|
|
|
|
|
|
| ≤5 | 69 | 62 | 0.302 | 73 | 58 | 0.027 | 66 | 65 | 0.883 | 71 | 60 | 0.105 |
|
>5 | 32 | 39 |
| 28 | 43 |
| 35 | 36 |
| 30 | 41 |
|
| Histological
type |
|
|
|
|
|
|
|
|
|
|
|
|
| Well
differentiated | 30 | 29 | 0.987 | 30 | 29 | 0.668 | 27 | 32 | 0.605 | 29 | 30 | 0.668 |
|
Moderately differentiated | 57 | 58 |
| 55 | 60 |
| 58 | 57 |
| 60 | 55 |
|
| Poorly
differentiated | 14 | 14 |
| 16 | 12 |
| 16 | 12 |
| 12 | 16 |
|
| Depth of
invasion |
|
|
|
|
|
|
|
|
|
|
|
|
| T1 | 9 | 8 | 0.734 | 9 | 8 | 0.781 | 10 | 7 | 0.232 | 13 | 4 | 0.072 |
| T2 | 19 | 14 |
| 19 | 14 |
| 19 | 14 |
| 18 | 15 |
|
| T3 | 36 | 42 |
| 38 | 40 |
| 32 | 46 |
| 39 | 39 |
|
| T4 | 37 | 37 |
| 35 | 39 |
| 40 | 34 |
| 31 | 43 |
|
| Lymph node
metastasis |
|
|
|
|
|
|
|
|
|
|
|
|
|
Absent | 53 | 50 | 0.673 | 52 | 51 | 0.888 | 48 | 55 | 0.325 | 59 | 44 | 0.035 |
|
Present | 48 | 51 |
| 49 | 50 |
| 53 | 56 |
| 42 | 57 |
|
| Lymphatic
invasion |
|
|
|
|
|
|
|
|
|
|
|
|
|
Absent | 64 | 68 | 0.554 | 64 | 68 | 0.554 | 62 | 70 | 0.237 | 61 | 71 | 0.139 |
|
Present | 37 | 33 |
| 37 | 33 |
| 39 | 31 |
| 40 | 30 |
|
| Venous
invasion |
|
|
|
|
|
|
|
|
|
|
|
|
|
Absent | 41 | 34 | 0.308 | 44 | 31 | 0.058 | 31 | 44 | 0.058 | 43 | 62 | 0.109 |
|
Present | 60 | 67 |
| 57 | 70 |
| 70 | 57 |
| 58 | 69 |
|
| Liver
metastasis |
|
|
|
|
|
|
|
|
|
|
|
|
|
Absent | 83 | 77 | 0.298 | 82 | 78 | 0.488 | 80 | 80 | 1.000 | 84 | 76 | 0.165 |
|
Present | 18 | 24 |
| 19 | 23 |
| 21 | 21 |
| 17 | 25 |
|
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA in resected CRC and adjacent normal mucosa
was isolated with the use of TRIzol® Reagent (Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA). A total of 10
units of DNase I, RNase-free (Roche Applied Science, Penzberg,
Germany) was added and the samples were incubated for 20 min at
37°C. Complementary (c)DNA was synthesized from 0.2 µg of total RNA
using the iScript cDNA Synthesis kit (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). Reverse transcription was performed in a total
volume of 20 µl, which contained 4 µl iScript reaction mix, 1 µl
iScript reverse transcriptase, and 15 µl (13.3 ng/µl) total RNA.
The complete reaction mix was incubated for 5 min at 25°C, 30 min
at 42°C, 5 min at 85°C. Following synthesis, the cDNA was diluted
to 0.2 µg/µl with H2O and stored at −20°C until required
for experiments.
RT-qPCR was performed with an iQ SYBR-Green Supermix
kit (Bio-Rad Laboratories, Inc.). PCR reactions were performed in a
total volume of 15 µl, which contained cDNA derived from 75 ng of
RNA, 0.27 µM of each primer, 7.5 µl of iQ SYBR-Green Supermix
containing dATP, dCTP, dGTP, and dTTP (400 µM each) and iTag DNA
polymerase (50 units/ml). The PCR consisted of 10 min at 95°C,
followed by 40 cycles of denaturation of the cDNA for 10 sec at
95°C, annealing for 10 sec at an appropriate temperature (Table II) and a primer extension for 20 sec
at 72°C, followed by 10 min at 72°C. To distinguish specific from
nonspecific products and primer dimmers, melting curve analysis was
performed. RT-qPCR experiments were performed in triplicate, with
two wells for each gene in each experiment. To evaluate specific
mRNA expression in samples, a standard curve was produced for each
run, measuring three points of the human control cDNA (Clontech
Laboratories, Inc., Mountain View, CA, USA). The concentration of
each sample was calculated by relating its crossing point to the
standard curve (12).
 | Table II.Primers and conditions for the
polymerase chain reaction. |
Table II.
Primers and conditions for the
polymerase chain reaction.
| Gene/internal
control | Primers | Probes (5′-3′) | Annealing
temperature (°C) | Product size
(bp) |
|---|
| IGF-1 | Forward |
GTGGATGAGTGCTGCTTC | 58.0 | 134 |
|
| Reverse |
ACTTCCTTCTGGGTCTTGG |
|
|
| IGF-2 | Forward |
TACCGCCATCTCCCTTCTC | 60.0 | 122 |
|
| Reverse |
TCCCTCTGACTGCTCTGTG |
|
|
| IGF-1R | Forward |
TGCCTTGGTCTCCTTGTC | 58.0 | 154 |
|
| Reverse |
TTTCCCTGCTTTGATGGTC |
|
|
| IGFBP-3 | Forward |
TTTCATCTCTCATCTTTTGTCCTC | 60.0 | 77 |
|
| Reverse |
GCCATTCCTCCTTCCTGTTC |
|
|
| β-actin | Forward |
AGTTGCGTTACACCCTTTCTTGAC | 60.0 | 171 |
|
| Reverse |
GCTCGCTCCAACCGACTGC |
|
|
Statistical analysis
All statistical analyses were performed using IBM
SPSS Statistics 20 (IBM SPSS, Armonk, NY, USA). The gene expression
levels in cancer tissue were compared with those in adjacent normal
mucosa using the Wilcoxon signed-rank test. The associations
between gene expression and potential explanatory variables
(including age, gender, tumor location, tumor size, histological
type, depth of invasion, lymph node metastasis, lymphatic invasion,
venous invasion and liver metastasis) were evaluated using the χ2
test. The gene expression levels in the tumors were compared in the
presence or absence of lymph node metastasis. Kaplan-Meier curves
for the postoperative survival of patients with CRC were plotted
and differences in survival rate between groups were analyzed
according to the log-rank test. A Cox proportional-hazards model
was used to estimate the hazard ratios of variables for
postoperative survival. Univariate and multivariate analyses were
conducted using a Cox proportional-hazards model to identify
independent prognostic factors for postoperative survival.
Variables that had a P-value of <0.05 for at least one endpoint
on univariate analysis were subsequently included in multivariate
analysis. P<0.05 was considered to indicate a statistically
significant difference.
Results
Expression levels of the IGF-1, IGF-2,
IGF-1R and IGFBP-3 genes in cancer tissue and adjacent normal
mucosa
IGF-1 gene expression levels were
significantly reduced in cancer tissue compared with adjacent
normal mucosa (P<0.001; Fig. 1A).
IGF-2 gene expression levels did not differ significantly
between cancer tissue and adjacent normal mucosa (P=0.453; Fig. 1B). IGF-1R gene expression
levels were significantly higher in cancer tissue compared with
adjacent normal mucosa (P<0.001; Fig.
1C). IGFBP-3 gene expression levels did not differ
significantly between cancer tissue and adjacent normal mucosa
(P=0.126; Fig. 1D).
Association of IGF-1, IGF-2, IGF-1R
and IGFBP-3 mRNA expression levels to clinicopathological
characteristics
Expression levels of the IGF-1, IGF-2, IGF-1R
and IGFBP-3 genes were categorized as low or high according
to the median values (0.129, 0.362, 0.306 and 292.5, respectively).
The associations between gene expression and clinicopathological
characteristics were evaluated. Expression levels of the
IGFBP-3 gene were significantly associated with lymph node
metastasis (P=0.035; Table I). The
IGF-1 and IGF-2 gene expression levels were significantly
associated with tumor location (P=0.048, P=0.048,
respectively).
Association of IGF-1, IGF-2, IGF-1R
and IGFBP-3 mRNA expression levels and lymph node metastasis
No significant associations were identified between
IGF-1, IGF-2 and IGF-1R mRNA expression levels
and lymph node metastasis (Fig.
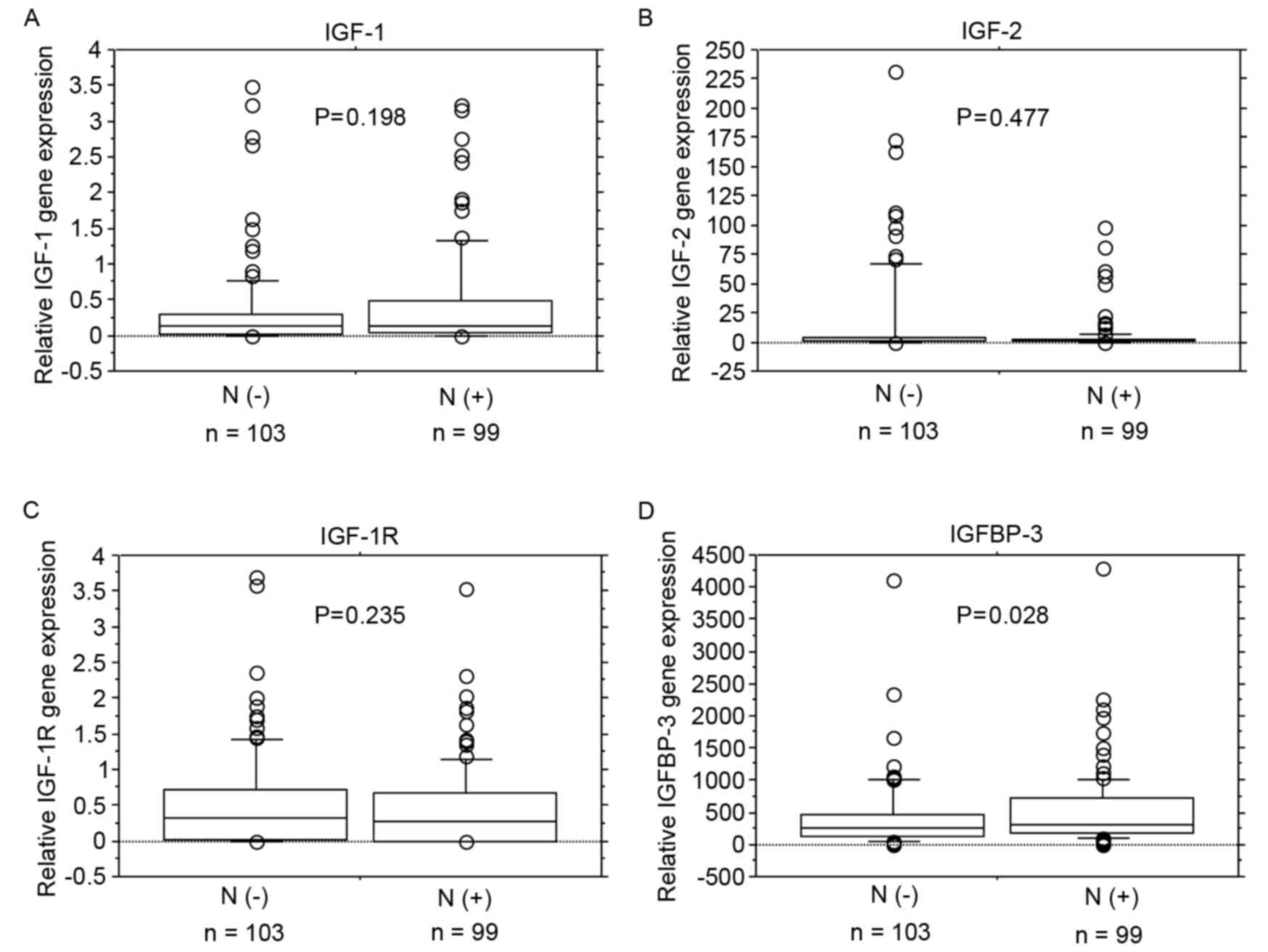
2A-C). IGFBP-3 mRNA expression levels were significantly
increased in patients with lymph node metastasis (P=0.028; Fig. 2D).
Association between IGF-1, IGF-2,
IGF-1R and IGFBP-3 mRNA expression levels and postoperative
survival rate
The expression levels of IGF-1, IGF-2,
IGF-1R and IGFBP-3 mRNA were categorized as low or
high according to the respective median values. Post-operative
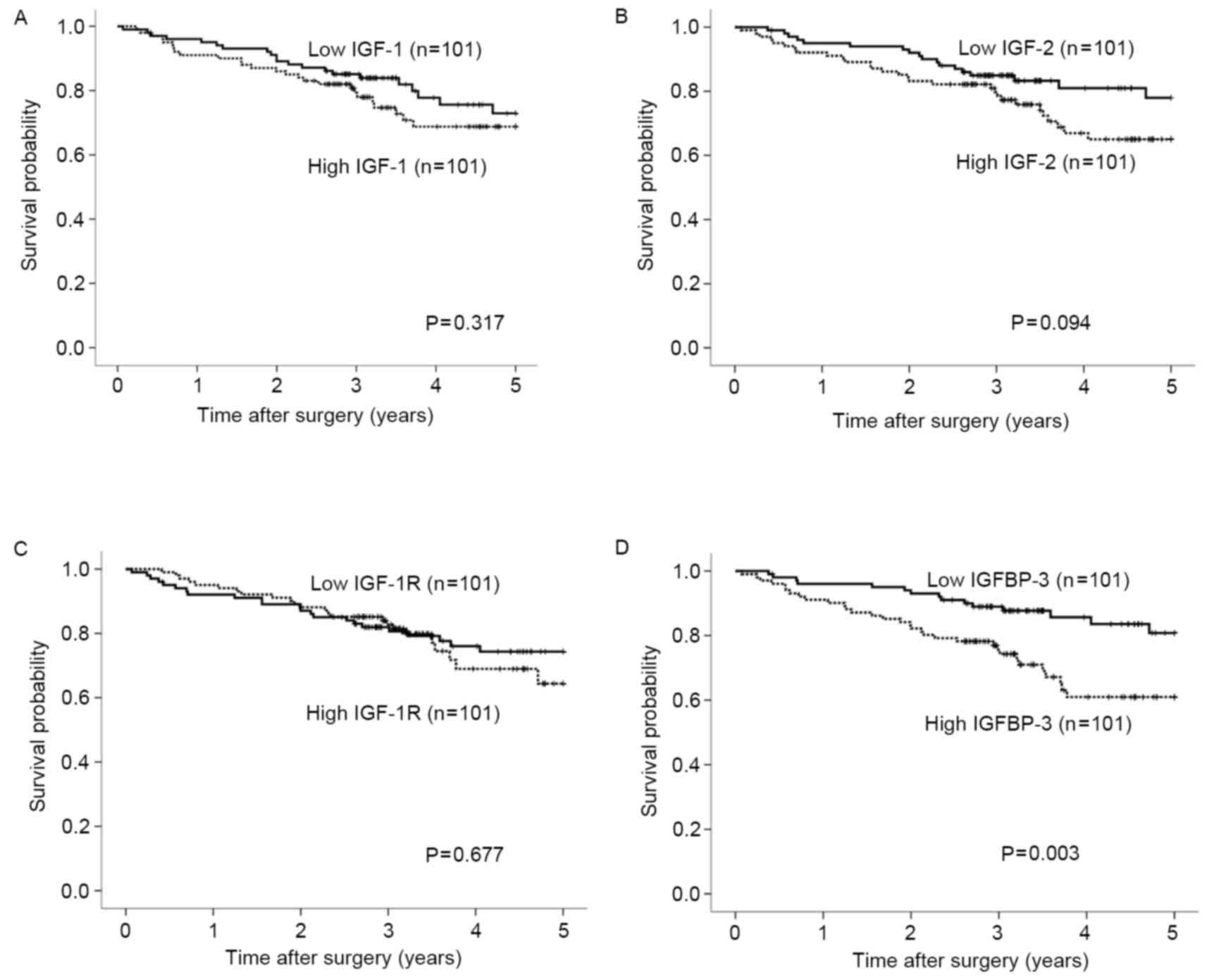
survival did not differ significantly according to expression
levels of the IGF-1, IGF-2 or IGF-1R genes
(Fig. 3A-C). By contrast,
postoperative survival was significantly poorer in patients with
high expression levels of the IGFBP-3 gene compared with
those with low expression levels (Fig.
3D; P=0.003). Univariate analysis revealed that the depth of
invasion, lymph node metastasis, lymphatic invasion, liver
metastasis, tumor diameter and IGFBP-3 expression were
associated with clinical outcomes (P<0.01). On multivariate Cox
proportional-hazards regression analysis, lymph node metastasis,
liver metastasis and IGFBP-3 gene expression were
independently inversely correlated with patient outcomes (P=0.011,
P<0.001, P=0.026, respectively; Table III).
 | Table III.Univariate and multivariate analyses
using the Cox proportional hazard model of variables associated
with the postoperative survival of patients with colorectal
cancer. |
Table III.
Univariate and multivariate analyses
using the Cox proportional hazard model of variables associated
with the postoperative survival of patients with colorectal
cancer.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Variables | Hazard ratio | 95% CI | P-value | Hazard ratio | 95% CI | P-value |
|---|
| Gender |
| Male
vs. female | 1.330 | 0.742–2.742 | 0.338 | – | – | – |
| Age, years |
|
|
|
| ≥60 vs.
<60 | 1.367 | 0.695–2.695 | 0.365 | – | – | – |
| Depth of
invasion |
|
|
|
|
|
|
| T3/4
vs. T1/2 | 17.687 | 2.439–128.439 | 0.004 | 5.650 | 0.746–42.746 | 0.094 |
| Lymph node
metastasis |
|
|
|
|
|
|
| Present
vs. absent | 6.383 | 2.979–13.979 | <0.001 | 3.038 | 1.292–7.292 | 0.011 |
| Tumor location |
|
|
|
| Rectum
vs. colon | 1.513 | 0.851–2.851 | 0.158 | – | – | – |
| Lymphatic
invasion |
|
|
|
|
|
|
| Present
vs. absent | 3.307 | 1.849–5.849 | <0.001 | 1.604 | 0.834–3.834 | 0.157 |
| Venous
invasion |
|
|
|
| Present
vs. absent | 1.601 | 0.827–3.827 | 0.163 | – | – | – |
| Liver
metastasis |
|
|
|
|
|
|
| Present
vs. absent | 7.258 | 4.033–13.033 | <0.001 | 4.695 | 2.511–8.511 | <0.001 |
| Histological
type |
|
|
|
|
Moderate and poor vs.
well | 2.045 | 0.955–4.955 | 0.066 | – | – | – |
| Tumor diameter,
cm |
|
|
|
|
|
|
| ≤5 vs.
>5 | 2.191 | 1.236–3.236 | 0.007 | 1.225 | 0.677–2.677 | 0.502 |
| IGF-1 expression
level |
|
|
|
| High
vs. low | 1.340 | 0.754–2.754 | 0.319 | – | – | – |
| IGF-2 expression
level |
|
|
|
| High
vs. low | 1.645 | 0.914–2.914 | 0.097 | – | – | – |
| IGF-1R expression
level |
|
|
|
| High
vs. low | 1.130 | 0.635–2.635 | 0.677 | – | – | – |
| IGFBP-3 expression
level |
|
|
|
|
|
|
| High
vs. low | 2.439 | 1.320–4.320 | 0.004 | 2.033 | 1.087–3.087 | 0.026 |
Discussion
The IGF system serves an important role in the
pathogenesis of dysplasia and neoplasia (8,13). The
present study investigated tissue expression levels of the
IGF-1, IGF-2, IGF-1R and IGFBP-3 genes,
the clinicopathological characteristics of 202 patients with
untreated CRC, and the associations of these expression levels with
postoperative survival.
A number of previous studies have compared the mRNA
expression levels of the IGF-1, IGF-2, IGF-1R
and IGFBP-3 genes in CRC tissue and adjacent normal mucosa.
Freier et al (14) reported
that there was no identifiable IGF-1 mRNA in healthy or malignant
human colonic tissue. Nosho et al (15) reported significantly increased mRNA
expression levels of the IGF-2 gene in colorectal tumor
tissue compared with those in adjacent normal mucosa. The mRNA
expression levels of the IGF-1R gene were higher in
adenocarcinoma tissue of the colon compared with adjacent normal
mucosa (14). Another previous study
demonstrated that mRNA expression of the IGF-IR gene was
detected in ~40% of CRC tissue specimens but was undetectable in
adjacent normal mucosa (15). Keku
et al (16) reported that
IGFBP-3 gene expression was significantly lower in
colorectal adenoma tissue compared with normal mucosa. In the
current study, the mRNA expression level of the IGF-1R gene
was higher in CRC tissue compared with adjacent normal mucosa.
However, the mRNA expression levels of the IGF-1 gene were
reduced in cancer tissue compared with adjacent normal mucosa. The
mRNA expression levels of the IGF-2 and IGFBP-3 genes
did not differ significantly between cancer tissue and adjacent
normal mucosa.
The association between IGF-1, IGF-2,
IGF-1R and IGFBP-3 mRNA expression levels and
clinicopathological characteristics were examined in patients with
CRC. Peters et al (17)
reported that IGF-1 gene expression was not associated with
any clinicopathological characteristic in CRC, whilst Shiratsuchi
et al (18) reported that
IGF-1 gene expression in CRC was associated with tumor size, depth
of tumor invasion, lymphatic invasion and venous invasion in CRC.
In another previous study, IGF-2 gene expression was
correlated with age and tumor size, whilst IGF-1R gene
expression did not associate with any clinicopathological
characteristic in patients with early CRC (15). Although IGF-1R expression
correlated with tumor size and depth of invasion in CRC (18), it did not associate any of the
clinicopathological characteristics in patients with prostate
cancer (19). Increased postoperative
tumor growth and the presence of liver metastasis were associated
with significantly elevated IGF-1R gene expression in
gastrinoma (20). In the present
study, IGF-1 gene expression was significantly associated
with tumor location. IGF-2 gene expression was significantly
associated with tumor location and tumor size; whilst IGF-1R
gene expression was not associated with any clinicopathological
characteristic in patients with CRC. In a previous study,
IGFBP-3 gene expression was significantly associated with
age and positively correlated with tumor stage in CRC (21). Higher mRNA levels of the
IGFBP-3 gene were associated with reduced levels of
apoptosis (16). Positive
associations of IGFBP-3 expression with tumor size and lymph node
metastasis have been identified in oral squamous cancer (22). An increased expression level of
IGFBP-3 has been associated with lymph node metastasis in
pancreatic endocrine neoplasms (23).
The IGFBP-3 gene was overexpressed in advanced pancreatic
cancer and the intratumoral levels of the IGFBP-3 gene was
associated with tumor size (24). The
results of the current study are in accordance with the literature
that IGFBP-3 gene overexpression is associated with lymph
node metastasis.
Finally, the association of IGF-1,
IGF-2, IGF-1R and IGFBP-3 gene expression
levels and the outcomes of patients with CRC were assessed. In
previous studies, IGF-1 expression was not observed to be
significantly associated with overall survival rate (17,25). IGF-2
expression has been associated with poorer clinical outcomes in
patients with CRC (7,17,26).
IGF-1R expression in primary CRC may promote an increased risk of
recurrence (27), but was not
associated with patients' 5-year survival rate (28). Increased tissue expression levels of
the IGFBP-3 gene have been associated with the rapid growth
of breast cancer and poor patient outcomes (29,30).
Previous immunohistochemical studies of breast cancer demonstrated
that IGFBP-3 expression was associated with shorter overall
survival (31). High expression
levels of the IGFBP-3 gene were associated with unfavorable
prognostic characteristics in breast cancer (32). Santosh et al (33) reported that IGFBP-3 overexpression in
tumor tissues was an independent predictor of reduced survival rate
in patients with newly diagnosed glioblastoma. By contrast, Aishima
et al (34) identified that
high expression levels of IGFBP-3 were independently associated
with an improved survival rate in patients with hepatocellular
carcinoma. In patients with squamous cell carcinoma of the tongue,
IGFBP-3 expression was associated with favorable outcomes (35). In the present study, the postoperative
survival rate was significantly poorer in patients with high
expression levels of the IGFBP-3 gene compared with those
with low expression levels of the IGFBP-3 gene.
The molecular mechanisms underlying the association
between IGFBP-3 expression and poor outcomes in cancer remain to be
fully elucidated. Schmid et al (36) reported that IGFBP-3 was overexpressed
in the endothelial cells of mouse breast tumor vessels. Granata
et al (37) reported that
IGFBP-3 dose-dependently promoted neovessels in subcutaneous
implants in vivo and suggested that IGFBP-3 promotes
angiogenesis and positively regulates the expression of
proangiogenic molecules, including vascular endothelial growth
factor (VEGF) (37). Yu et al
(38) reported a positive correlation
between the high expression of epidermal growth factor receptor
(EGFR) and the high expression of IGFBP-3 in breast cancer tissue,
and Butt et al (10)
identified that a blockade of EGFR kinase activity restored the
inhibitory effects of IGFBP-3 in vitro. Additionally, Martin
et al (39) demonstrated that
IGFBP-3 enhanced EGF signaling and its proliferative effects via
increased EGFR phosphorylation and the activation of MAP kinase
signaling pathways in breast cancer cells in vitro. An
associated previous study also reported that IGFBP-3 promotes the
proliferation of breast cancer cells through increasing EGFR
signaling mediated by SphK1 (40).
Therefore, IGFBP-3 has been suggested to promote angiogenesis by
inducing VEGF, thereby inducing EGFR signaling mediated by SphK1
and activation of MAP kinase signaling pathways. These effects are
considered to promote proliferation of cancer cells, potentially
leading to poor survival outcomes. Molecular investigations are
required to additionally investigate the role of IGFBP-3 as a
prognostic factor and to elucidate the pleiotropic functions of
this protein.
In conclusion, high expression of the IGFBP-3
gene was significantly associated with lymph node metastasis and
poor outcomes. The results of the present study suggest that
overexpression of the IGFBP-3 gene is an important
independent predictor of outcomes following surgery in patients
with CRC.
References
|
1
|
Ma J, Pollak MN, Giovannucci E, Chan JM,
Tao Y, Hennekens CH and Stampfer MJ: Prospective study of
colorectal cancer risk in men and plasma levels of insulin-like
growth factor (IGF)-I and IGF-binding protein-3. J Natl Cancer
Inst. 91:620–625. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shikata K, Ninomiya T and Kiyohara Y:
Diabetes mellitus and cancer risk: Review of the epidemiological
evidence. Cancer Sci. 104:9–14. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Clayton PE, Banerjee I, Murray PG and
Renehan AG: Growth hormone, the insulin-like growth factor axis,
insulin and cancer risk. Nat Rev Endocrinol. 7:11–24. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Renehan AG, Zwahlen M, Minder C, O'Dwyer
ST, Shalet SM and Egger M: Insulin-like growth factor (IGF)-I, IGF
binding protein-3, and cancer risk: Systematic review and
meta-regression analysis. Lancet. 363:1346–1353. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Durai R, Yang W, Gupta S, Seifalian AM and
Winslet MC: The role of the insulin-like growth factor system in
colorectal cancer: Review of current knowledge. Int J Colorectal
Dis. 20:203–220. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rinaldi S, Cleveland R, Norat T, Biessy C,
Rohrmann S, Linseisen J, Boeing H, Pischon T, Panico S, Agnoli C,
et al: Serum levels of IGF-I, IGFBP-3 and colorectal cancer risk:
Results from the EPIC cohort, plus a meta-analysis of prospective
studies. Int J Cancer. 126:1702–1715. 2010.PubMed/NCBI
|
|
7
|
Kawamoto K, Onodera H, Kondo S, Kan S,
Ikeuchi D, Maetani S and Imamura M: Expression of insulin-like
growth factor-2 can predict the prognosis of human colorectal
cancer patients: Correlation with tumor progression, proliferative
activity and survival. Oncology. 55:242–248. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Foulstone E, Prince S, Zaccheo O, Burns
JL, Harper J, Jacobs C, Church D and Hassan AB: Insulin-like growth
factor ligands, receptors, and binding proteins in cancer. J
Pathol. 205:145–153. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Firth SM and Baxter RC: Cellular actions
of the insulin-like growth factor binding proteins. Endocr Rev.
23:824–854. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Butt AJ, Martin JL, Dickson KA, McDougall
F, Firth SM and Baxter RC: Insulin-like growth factor binding
protein-3 expression is associated with growth stimulation of T47D
human breast cancer cells: The role of altered epidermal growth
factor signaling. J Clin Endocrinol Metab. 89:1950–1956. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sobin LH and Wittekind C: TNM:
Classification of Malignant Tumours. 6th. John Wiley & Sons;
Hoboken, NJ: 2002
|
|
12
|
Larionov A, Krause A and Miller W: A
standard curve based method for relative real time PCR data
processing. BMC Bioinformatics. 6:622005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pollak M: Insulin-like growth factor
physiology and cancer risk. Eur J Cancer. 36:1224–1228. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Freier S, Weiss O, Eran M, Flyvbjerg A,
Dahan R, Nephesh I, Safra T, Shiloni E and Raz I: Expression of the
insulin-like growth factors and their receptors in adenocarcinoma
of the colon. Gut. 44:704–708. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nosho K, Yamamoto H, Taniguchi H, Adachi
Y, Yoshida Y, Arimura Y, Endo T, Hinoda Y and Imai K: Interplay of
insulin-like growth factor-II, insulin-like growth factor-I,
insulin-like growth factor-I receptor, COX-2, and matrix
metalloproteinase-7, play key roles in the early stage of
colorectal carcinogenesis. Clin Cancer Res. 10:7950–7957. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Keku TO, Sandler RS, Simmons JG, Galanko
J, Woosley JT, Proffitt M, Omofoye O, McDoom M and Lund PK: Local
IGFBP-3 mRNA expression, apoptosis and risk of colorectal adenomas.
BMC Cancer. 8:1432008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Peters G, Gongoll S, Langner C, Mengel M,
Piso P, Klempnauer J, Rüschoff J, Kreipe H and von Wasielewski R:
IGF-1R, IGF-1 and IGF-2 expression as potential prognostic and
predictive markers in colorectal-cancer. Virchows Arch.
443:139–145. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shiratsuchi I, Akagi Y, Kawahara A,
Kinugasa T, Romeo K, Yoshida T, Ryu Y, Gotanda Y, Kage M and
Shirouzu K: Expression of IGF-1 and IGF-1R and their relation to
clinicopathological factors in colorectal cancer. Anticancer Res.
31:2541–2545. 2011.PubMed/NCBI
|
|
19
|
Mita K, Nakahara M and Usui T: Expression
of the insulin-like growth factor system and cancer progression in
hormone-treated prostate cancer patients. Int J Urol. 7:321–329.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Furukawa M, Raffeld M, Mateo C, Sakamoto
A, Moody TW, Ito T, Venzon DJ, Serrano J and Jensen RT: Increased
expression of insulin-like growth factor I and/or its receptor in
gastrinomas is associated with low curability, increased growth,
and development of metastases. Clin Cancer Res. 11:3233–3242. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Georges RB, Adwan H, Hamdi H, Hielscher T,
Linnemann U and Berger MR: The insulin-like growth factor binding
proteins 3 and 7 are associated with colorectal cancer and liver
metastasis. Cancer Biol Ther. 12:69–79. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhong LP, Yang X, Zhang L, Wei KJ, Pan HY,
Zhou XJ, Li J, Chen WT and Zhang ZY: Overexpression of insulin-like
growth factor binding protein 3 in oral squamous cell carcinoma.
Oncol Rep. 20:1441–1447. 2008.PubMed/NCBI
|
|
23
|
Hansel DE, Rahman A, House M, Ashfaq R,
Berg K, Yeo CJ and Maitra A: Met proto-oncogene and insulin-like
growth factor binding protein 3 overexpression correlates with
metastatic ability in well-differentiated pancreatic endocrine
neoplasms. Clin Cancer Res. 10:6152–6158. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xue A, Scarlett CJ, Jackson CJ, Allen BJ
and Smith RC: Prognostic significance of growth factors and the
urokinase-type plasminogen activator system in pancreatic ductal
adenocarcinoma. Pancreas. 36:160–167. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sheen-Chen SM, Chou FF, Hsu W, Huang CC,
Eng HL and Tang RP: Lack of prognostic value of insulin-like growth
factor-1 in patients with breast cancer: Analysis with tissue
microarray. Anticancer Res. 27:3541–3544. 2007.PubMed/NCBI
|
|
26
|
Xu Z, Liu F, Qi X and Li J: Relationship
between insulin-like growth factor II and prognosis of colorectal
cancer. Zhonghua Wai Ke Za Zhi. 37:718–720, 743. 1999.(In Chinese).
PubMed/NCBI
|
|
27
|
Nakamura M, Miyamoto S, Maeda H, Zhang SC,
Sangai T, Ishii G, Hasebe T, Endoh Y, Saito N, Asaka M and Ochiai
A: Low levels of insulin-like growth factor type 1 receptor
expression at cancer cell membrane predict liver metastasis in
Dukes' C human colorectal cancers. Clin Cancer Res. 10:8434–8441.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shiono S, Ishii G, Nagai K, Murata Y,
Tsuta K, Nitadori J, Kodama T and Ochiai A: Immunohistochemical
prognostic factors in resected colorectal lung metastases using
tissue microarray analysis. Eur J Surg Oncol. 32:308–309. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Vestey SB, Perks CM, Sen C, Calder CJ,
Holly JM and Winters ZE: Immunohistochemical expression of
insulin-like growth factor binding protein-3 in invasive breast
cancers and ductal carcinoma in situ: Implications for
clinicopathology and patient outcome. Breast Cancer Res.
7:R119–R129. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sheen-Chen SM, Zhang H, Huang CC and Tang
RP: Insulin-like growth factor-binding protein-3 in breast cancer:
Analysis with tissue microarray. Anticancer Res. 29:1131–1135.
2009.PubMed/NCBI
|
|
31
|
Kim JH, Cho YH, Park YL, Sohn JH and Kim
HS: Prognostic significance of insulin growth factor-I receptor and
insulin growth factor binding protein-3 expression in primary
breast cancer. Oncol Rep. 23:989–995. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yu H, Levesque MA, Khosravi MJ,
Papanastasiou-Diamandi A, Clark GM and Diamandis EP: Insulin-like
growth factor-binding protein-3 and breast cancer survival. Int J
Cancer. 79:624–628. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Santosh V, Arivazhagan A, Sreekanthreddy
P, Srinivasan H, Thota B, Srividya MR, Vrinda M, Sridevi S,
Shailaja BC, Samuel C, et al: Grade-specific expression of
insulin-like growth factor-binding proteins-2, −3, and −5 in
astrocytomas: IGFBP-3 emerges as a strong predictor of survival in
patients with newly diagnosed glioblastoma. Cancer Epidemiol
Biomarkers Prev. 19:1399–1408. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Aishima S, Basaki Y, Oda Y, Kuroda Y,
Nishihara Y, Taguchi K, Taketomi A, Maehara Y, Hosoi F, Maruyama Y,
et al: High expression of insulin-like growth factor binding
protein-3 is correlated with lower portal invasion and better
prognosis in human hepatocellular carcinoma. Cancer Sci.
97:1182–1190. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Papadimitrakopoulou VA, Brown EN, Liu DD,
El-Naggar AK, Lee Jack J, Hong WK and Lee HY: The prognostic role
of loss of insulin-like growth factor-binding protein-3 expression
in head and neck carcinogenesis. Cancer Lett. 239:136–143. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Schmid MC, Bisoffi M, Wetterwald A,
Gautschi E, Thalmann GN, Mitola S, Bussolino F and Cecchini MG:
Insulin-like growth factor binding protein-3 is overexpressed in
endothelial cells of mouse breast tumor vessels. Int J Cancer.
103:577–586. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Granata R, Trovato L, Lupia E, Sala G,
Settanni F, Camussi G, Ghidoni R and Ghigo E: Insulin-like growth
factor binding protein-3 induces angiogenesis through IGF-I- and
SphK1-dependent mechanisms. J Thromb Haemost. 5:835–845. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yu H, Levesque MA, Khosravi MJ,
Papanastasiou-Diamandi A, Clark GM and Diamandis EP: Associations
between insulin-like growth factors and their binding proteins and
other prognostic indicators in breast cancer. Br J Cancer.
74:1242–1247. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Martin JL, Weenink SM and Baxter RC:
Insulin-like growth factor-binding protein-3 potentiates epidermal
growth factor action in MCF-10A mammary epithelial cells.
Involvement of p44/42 and p38 mitogen-activated protein kinases. J
Biol Chem. 278:2969–2976. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Martin JL, de Silva HC, Lin MZ, Scott CD
and Baxter RC: Inhibition of insulin-like growth factor-binding
protein-3 signaling through sphingosine kinase-1 sensitizes
triple-negative breast cancer cells to EGF receptor blockade. Mol
Cancer Ther. 13:316–328. 2014. View Article : Google Scholar : PubMed/NCBI
|