Introduction
Endoscopic ultrasound (EUS)-guided fine-needle
aspiration (EUS-FNA) has been reported to be a sensitive method for
tissue sampling of suspicious lesions of the gastrointestinal lumen
and adjacent structures, including pancreaticobiliary and
esophageal lesions, gastric malignancies and mediastinal and
intra-abdominal lymphadenopathies (1–6). The
diagnostic accuracy of EUS-FNA ranges between 60 and 90%, according
to the site being evaluated (1,7–11). Cytological study of the material
obtained by FNA allows for the evaluation of cellular findings that
are indicative of malignancy. However, EUS-FNA has a number of
limitations. Certain neoplasms, including lymphomas, stromal tumors
and well-differentiated neoplasias are difficult to diagnose
without histological samples, since tissue architecture and cell
morphology are essential for accurate pathological assessments,
which include immunohistochemical analyses in such cases (12–14). In
addition, the accuracy of EUS-FNA depends on the presence of an
on-site cytopathologist or cytotechnician to assess the specimen
adequacy (15), and to determine
whether additional samples are required to perform ancillary
studies (16,17).
In an attempt to overcome these diagnostic
limitations and optimize the accuracy, efficiency and quality of
EUS-FNA specimens, various investigators have attempted to obtain
tissue fragments with high negative pressure or with needles of
varying diameters (18–21). The use of suction during FNA varies
widely. No standard suction technique has been established. A
randomized trial involving 52 patients compared suction and no
suction during EUS-FNA of the pancreas (22). No significant differences in
diagnostic yield were observed. In a previous study, Kudo et
al (23) utilized high negative
pressure mechanical suction (35 ml of a 60 ml syringe) using a
22-gauge (G) needle, and this process yielded tissue cores that
were adequate for histological evaluation in 96% of the solid
masses, however, the approach was not advantageous compared with
cytology alone. In addition, it may be assumed that suctioning
dilutes the specimen with blood, and the stylet injures malignant
cells. These assumptions raise the possibility of atypical
results.
Therefore, a retrospective study was performed to
investigate the feasibilities and yields of EUS-FNA combined with
10 ml suction (negative pressure applied with 10 ml syringes), 5 ml
suction (negative pressure applied with a 5 ml syringe) and
slow-pull (no stylet) techniques, and to compare characteristics of
the samples obtained with each of the three techniques in terms of
contamination with blood.
Materials and methods
Study design and patients
The present study was a retrospective, case-control
study. A total of 149 patients who were referred for EUS-guided FNA
tissue acquisition for the evaluation of intra-intestinal or
extra-intestinal mass lesions and/or peri-intestinal lymph nodes
between February 2013 and July 2014 were retrospectively identified
from a prospectively collected endoscopy database at Tongji
Hospital Endoscope Center (Wuhan, China). Patient characteristics
are presented in Table I. Patients
were classified into EUS/slow-pull, EUS/5 ml suction or EUS/10 ml
suction groups (the patients who underwent EUS-FNA with the 22-G
needle system with no stylet, with 5 ml negative pressure and with
10 ml negative pressure). Only patients with surgical pathology or
with ≥6 months of clinical follow-up subsequent to EUS were
included in the present study. The present authors reviewed the
computerized patient record system to obtain patient demographics,
lesion sites, EUS characteristics of the lesion and clinical
follow-up information.
 | Table I.Patient demographic and mediastinal
and intra-abdominal lesion characteristics. |
Table I.
Patient demographic and mediastinal
and intra-abdominal lesion characteristics.
| Characteristic | Total | 0 ml | 5 ml | 10 ml | P-value |
|---|
| n |
| 34 | 37 | 78 |
|
| Age,
median | 54 | 56 | 56 | 53 | 0.89a |
| Gender, n
(%) |
|
|
|
| 0.21b |
| Male | 95 | 26 (76.5) | 22 (59.5) | 47 (60.3) |
|
|
Female | 54 | 8
(23.5) | 15 (40.5) | 31 (39.7) |
|
| Lesion location, n
(%) |
|
|
|
| 0.67c |
|
Pancreatic mass | 69 (46.3) | 15 (10.1) | 19 (12.8) | 36 (24.2) |
|
|
Mediastinal nodes | 33 (22.1) | 9 (6.0) | 7 (4.7) | 17 (11.4) |
|
|
Retroperitoneal lesion | 32 (21.5) | 8 (5.4) | 9 (6.0) | 14 (9.4) |
|
| Othersd | 15 (10.1) | 2 (1.3) | 2 (1.3) | 11 (7.4) |
|
| Needle passes
(SD) | 3.4 (0.7) | 3.5 (0.7) | 3.4 (0.8) | 3.4 (0.6) | 0.50a |
| Final diagnosis, n
(%) |
|
|
|
| 0.078b |
|
Malignancy | 102 (68.5) | 26 (17.4) | 29 (19.5) | 47 (31.5) |
|
| Benign
processes | 47 (31.5) | 8 (5.4) | 8 (5.4) | 31 (20.8) |
|
The EUS-FNA cytology and histology results were
compared with those of the gold standard technique of surgical
histopathology or long-term clinical follow-up. Intra-procedural
and immediate post-procedural complications were monitored and
recorded for all patients as part of a standard hospital protocol.
The study protocol conformed to the guidelines of the 1975
Declaration of Helsinki (6th revision, 2008) and was approved by
the Ethical Committee of Tongji Hospital, Tongji Medical College,
Huazhong University of Science and Technology (Wuhan, China).
Written informed consent was obtained from all patients prior to
undergoing EUS-FNA. Patients described in the present study
provided written informed consent to publish their case
details.
Procedural technique
The patients underwent EUS-FNAs with 22-G needles
(EchoTip Ultra needle; Wilson-Cook, Winston-Salem, NC, USA)
(24). These EUS-FNAs were performed
by an experienced endosonographer (>150 EUS procedures/year;
>10 years of experience). All procedures were performed with a
standard technique, which utilized a linear array echoendoscope
(Olympus GF-UCT 240; Olympus Corporation, Tokyo, Japan) and an
Alpha 5 Aloka processor (Hitachi-Aloka Medical, Ltd., Tokyo,
Japan). During the individual EUS-FNA passes, the stylet was
reproducibly removed with a slow-pull technique, and a 10 ml
syringe with 5 or 10 ml suction technique (25–27) was
attached to the proximal end of the needle. The needle was then
moved back and forth 12–16 times while applying suction. EUS-FNA
was performed using fanning techniques. The lock of the syringe was
finally closed prior to the withdrawal of the needle from the
lesion. Needle aspirate was placed on glass slides. Ethanol-fixed
smears (95% ethanol) were prepared, stained with Papanicolaou stain
for 6 h at room temperature and evaluated the next working day by a
cytopathologist to perform the preliminary diagnosis. Any visible
core specimens and residual aspirate were collected into a liquid
preservative (formalin) for subsequent preparation of histological
analysis. Immunocytochemistry was performed within 24 h. No
cytopathologist was present in the endoscopy room for the on-site
sample evaluation.
Pathological assessments of the
samples obtained
The pathologist evaluated the quantity and quality
of each specimen and determined a histological diagnosis while
blinded to the clinical information, cytology and final diagnoses.
The quantities of the samples were assessed with the scoring system
described by Gerke et al (28). Malignancies and borderline lesions
were defined as positive for malignancy. Atypical cells and benign
cells were defined as negative for malignancy.
An accurate diagnosis was defined as follows:
Positive for malignancy with a final diagnosis of a malignant
disease, including carcinoma, neuroendocrine tumor or solid
pseudopapillary neoplasm (true positive); and negative for
malignancy with the condition ultimately being diagnosed as a
nonmalignant disease, including pancreatitis and non-neoplastic
pancreatic tissue (true negative). Diagnostic accuracy was defined
as the sum of the true positive and true negative values divided by
the total number of samples. The adequacy rate was calculated with
the following formula: Number of adequate samples/total number of
samples.
Clinical diagnostic methodology used
for the ultimate diagnosis
Malignant disease was ultimately identified in the
patients according to the following criteria: Diagnosis at autopsy
following pancreatic cancer-associated mortality; diagnosis based
on histopathological analysis of surgically resected specimens;
radiological or clinical data indicating evidence of disease
progression; and diagnosis based on histopathological analysis of
nodules in other organs that demonstrated metastatic progression.
In the present study, benign disease was defined by a decrease or
lack of change in mass and no change in the obtained clinical data
for at least 6.5 months (23).
Outcome measurements
The primary objectives of the present study were to
determine the adequacy of tissue acquisition via the EUS-FNA/high
negative pressure (HNP) combined technique and to determine the
accuracies of the histological diagnoses that were achievable using
EUS-FNA combined with slow-pull, 5 ml suction and 10 ml suction.
The secondary objectives of the present study were to assess the
qualities and quantities of the obtained tissues and the potential
for adverse events resulting from the application of this
procedure.
Statistical analysis
Statistical analyses were performed with the SPSS
(version 18.0; SPSS, Inc., Chicago, IL, USA) and MedCalc software
packages (version 12.7.7; MedCalc Software bvba, Ostend, Belgium).
The baseline characteristics of the patient population, mass
lesions and technical details were calculated. Continuous variables
were presented as medians and ranges of values. Categorical
variables were reported as proportions with 95% confidence
intervals where appropriate. Categorized variables were compared
using the Fisher's exact or χ2 two-tailed tests, as appropriate.
Quantitative variables were analyzed by the two-sample Student's
t-test/one-way analysis of variance (for normal distributions) or
the Mann-Whitney U-test (for skewed distributions). P<0.05 was
considered to indicate a statistically significant difference.
Normally distributed data (n=149) are presented as the mean ±
standard deviation.
Results
Patients and lesions
characteristics
During the study period, 95 males and 54 females
(149 patients) were enrolled. The median age of the patients was 54
years. All lesions were visible via EUS. There were 69 lesions in
the pancreas, 33 in the mediastinum, 32 in the retroperitoneal
area, 8 in the thickened esophagogastric wall, 4 in the abdominal
cavity, 2 in the liver and 1 in the left adrenal gland (Table I). No significant differences were
observed between the slow-pull, 5 ml suction and 10 ml suction
techniques in terms of patient demographics or lesion locations.
Surgical histopathological findings were available for
corroboration in 49 (33%) of the cases, flow cytometry data
collected following EUS-FNA were available for 6 (4%) patients, and
the remaining cases (63%) were corroborated based on long-term
clinical follow-up data. The mean clinical follow-up period
following EUS was 6.5 months. The final histological diagnoses and
diagnostic yields are shown in Tables
II and III, respectively. All
EUS-FNA procedures were performed with on-site cytopathology
evaluations.
 | Table II.Final diagnosis, independent of
tissue biopsies (EUS-FNA). |
Table II.
Final diagnosis, independent of
tissue biopsies (EUS-FNA).
| Diseases | Final diagnosis,
n | Adequate histology
sample, % | Correct diagnosis,
% |
|---|
| Malignant | 82 |
80.39 |
67.7 |
|
Secondary metastatic
tumors | 33 |
81.8 |
69.7 |
|
Pancreatic carcinoma | 22 |
72.7 |
81.8 |
|
Lymphoma | 9 | 100 |
77.8 |
|
Gallbladder and biliary
cancer | 7 |
57.1 |
57.1 |
| Lung
carcinoma | 5 | 80 | 80 |
|
Gastroesophageal
carcinoma | 5 | 80 | 60 |
| Adrenal
carcinoma | 1 |
0 | 100 |
| Borderline
lesions | 21 |
90.5 |
90.5 |
|
Neuroendocrine tumor | 12 |
77.8 |
77.8 |
|
Gastrointestinal stromal
tumor | 9 | 100 | 100 |
| Benign | 46 |
82.97 |
80.85 |
|
Pancreatitis | 12 |
91.7 |
83.3 |
|
Tuberculosis | 11 |
72.8 |
90.9 |
| No
evidence of malignancy | 16 |
88.9 |
66.7 |
| Solid
pseudopapillary neoplasm | 2 | 100 | 100 |
| Othersa | 4 | 60 | 60 |
 | Table III.Diagnostic yield and accuracy of
normal, moderate and high negative pressure suction techniques in
endoscopic ultrasound-guided fine-needle aspiration. |
Table III.
Diagnostic yield and accuracy of
normal, moderate and high negative pressure suction techniques in
endoscopic ultrasound-guided fine-needle aspiration.
|
| Type of negative
pressure |
|
|---|
|
|
|
|
|---|
| Lesion
location | 0 ml, % (n=34) | 5 ml, % (n=37) | 10 ml, %
(n=78) | P-value
(χ2 test) |
|---|
| Pancreatic lesion
(n=69) |
|
|
| 0.0005a |
|
Sensitivity | 90 |
86.7 | 64 |
|
|
Specificity | 75 | 100 |
88.2 |
|
|
PPV | 90 | 100 |
81.8 |
|
|
NPV | 75 | 60 | 75 |
|
|
Accuracy |
85.7 |
88.9 |
77.4 |
|
| Non-pancreatic
lesionb (n=80) |
|
|
| 0.0086a |
|
Sensitivity |
85.7 |
84.6 |
64.7 |
|
|
Specificity | 50 | 80 |
92.9 |
|
|
PPV |
92.3 |
91.7 |
94.4 |
|
|
NPV |
33.3 |
66.7 |
54.3 |
|
|
Accuracy |
81.2 |
83.3 |
71.9 |
|
| Total (n=149) |
|
|
|
<0.0001a |
|
Sensitivity |
87.5 |
85.7 |
61.9 |
|
|
Specificity |
66.7 |
87.5 |
90.3 |
|
|
PPV |
91.3 | 96 |
89.7 |
|
|
NPV |
57.1 |
63.6 |
63.6 |
|
|
Accuracy |
83.3 |
86.1 |
69.9 |
|
Accuracy
The final clinical diagnoses, the percentages of
adequate histology samples and the numbers of correct diagnoses are
listed in Table II. Of the 149
patients, the final diagnoses were: Malignancy in 82 patients;
borderline lesions in 21 patients; and benign lesions in 46
patients. Of the patients with malignancies, 33 patients ultimately
received a diagnosis of metastatic tumor, 22 exhibited pancreatic
carcinomas, 9 exhibited lymphomas, 7 exhibited gallbladder and
biliary cancer, 5 exhibited lung carcinomas, 5 exhibited
gastroesophageal carcinomas and 1 exhibited an adrenal carcinoma.
Of the patients with borderline lesions, 12 received a diagnosis of
neuroendocrine tumors and 9 exhibited gastrointestinal stromal
tumors. Among the benign patients, 12 patients received a diagnosis
of pancreatitis, 11 exhibited tuberculosis, 16 exhibited benign
lesions with histological types that could not be classified
(without evidence of malignancy), 2 exhibited solid
pseudo-papillaryneoplasma, and the remaining 4 cases exhibited 3
atypical hyperplasias and 1 reactive lymph node, and 1 patient
exhibited Castleman disease. Representative cases of lymphoma
(Fig. 1), tuberculosis (Fig. 2), pancreatic carcinoma and
pancreatitis (Fig. 3) are presented
in Figs. 1–3, respectively. Independent of the tissue
biopsies, the final diagnoses were categorized as malignant or
benign lesions.
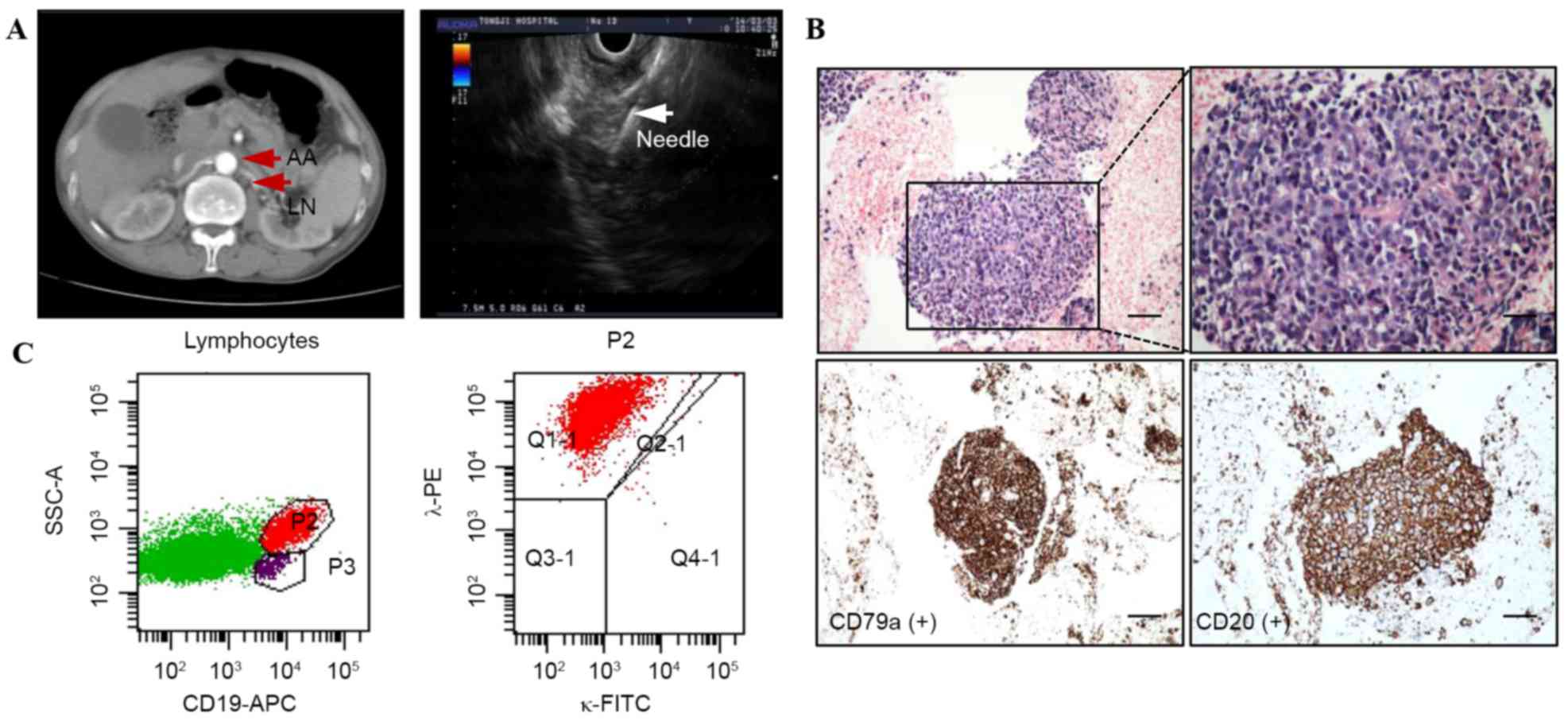 | Figure 1.A representative case of lower
para-aortic lymphoma. (A) Computed tomography revealed that there
is a lymphadenopathy located in the lower para-aortic. Endoscopic
ultrasound-guided fine-needle aspiration was performed on the
lesion. The white arrow indicated the needle with no interposing
vessels. (B) Histological findings demonstrated lymphoid follicles
(H&E; magnification, ×20; scale, 10 µm) and a monotonous
population of small lymphoid cells (H&E; original
magnification, ×400; scale, 5 µm). These cells exhibited positive
expression of CD79a and CD20 on immunohistological staining
(original magnification; magnification, ×400; scale, 5 µm). (C) The
P2 subpopulation of lymphocytes with CD19-positive expression was
isolated by flow cytometry, and λ monoclonal expression was
analyzed. The patients received a final diagnosis of diffuse large
B cell lymphoma. AA, abdominal aorta; LN, lymph nodes; H&E,
hematoxylin and eosin; FITC, fluorescein isothiocyanate; CD,
cluster of differentiation; APC, antigen-presenting cell; Q,
quadrant. |
 | Figure 3.Patients with pancreatic carcinoma or
autoimmune pancreatitis. (A-C) A representative case of pancreatic
carcinoma. (A) A linear EUS image showed the FNA needle inside a
round, undefined lesion (3.5 ×3.0 cm), which was detected at the
level of the pancreatic head and neck. The white arrow indicated
the needle with no interposing vessels. (B) Histological specimen
composed of sheets of neoplastic cells with hyperchromatic, molded
nuclei and scant cytoplasm. The tissue architecture was
recognizable (H&E; original magnification, ×200; scale, 10 µm;
original magnification, ×400; scale, 5 µm). (C) Cytological
diagnosis of adenocarcinoma cells (H&E; original magnification,
×400; scale, 5 µm). (D-F) A representative case of autoimmune
pancreatitis. (D) Computed tomography indicted the lesion in the
pancreas head (red arrows). An EUS image showed the FNA needle
(white arrow) following penetration into the target tissue. (E) The
pancreas returned back to a normal size (green arrows) and normal
echoes following immunotherapy. (F) A photomicrograph showed
neoplastic cells with strong positive staining (brown areas) for
immunoglobulin 4 (original magnification, ×200; scale, 10 µm).
EUS-FNA, endoscopic ultrasound-guided fine-needle aspiration;
H&E, hematoxylin and eosin. |
Based on the locations, lesions were classified into
69 pancreatic lesions and 80 non-pancreatic lesions (Table III). The non-pancreatic lesion group
consisted of 33 patients with mediastinal nodes, 32 patients with
retroperitoneal lesions, 8 patients with thickened esophagogastric
walls, 4 cases with abdominal masses, 2 cases with liver masses and
1 case with a left adrenal mass. Among the 69 pancreatic lesions
that were detected with the normal, moderate and HNP suction
techniques, the sensitivities of slow-pull (90%) and 5 ml suction
(86.7%) were increased compared with that of 10 ml suction (64%),
but the specificity of slow-pull (75%) was worse than those of 5 ml
(100%) and 10 ml suction (88.2%). Consequently, the accuracy of 5
ml suction (88.9%) was superior to those of slow-pull (85.7%) and
10 ml suction (77.4%). Similarly, among the non-pancreatic lesion
cases, the sensitivities of slow-pull, 5 ml suction and 10 ml
suction were 85.7, 84.6 and 64.7%, respectively, and the
specificities were 50, 80 and 92.9%, respectively. The accuracy of
5 ml suction (83.3%) was superior to those of slow-pull (81.25%)
and 10 ml suction (71.9%). Overall, the total accuracy of 5 ml
suction (86.1%) was greater than those of slow-pull (83.3%) and 10
ml suction (69.9%). Collectively, these results indicated that the
lesions were diagnosed more accurately with the EUS-FNA with 5 ml
suction technique regardless of the lesion location.
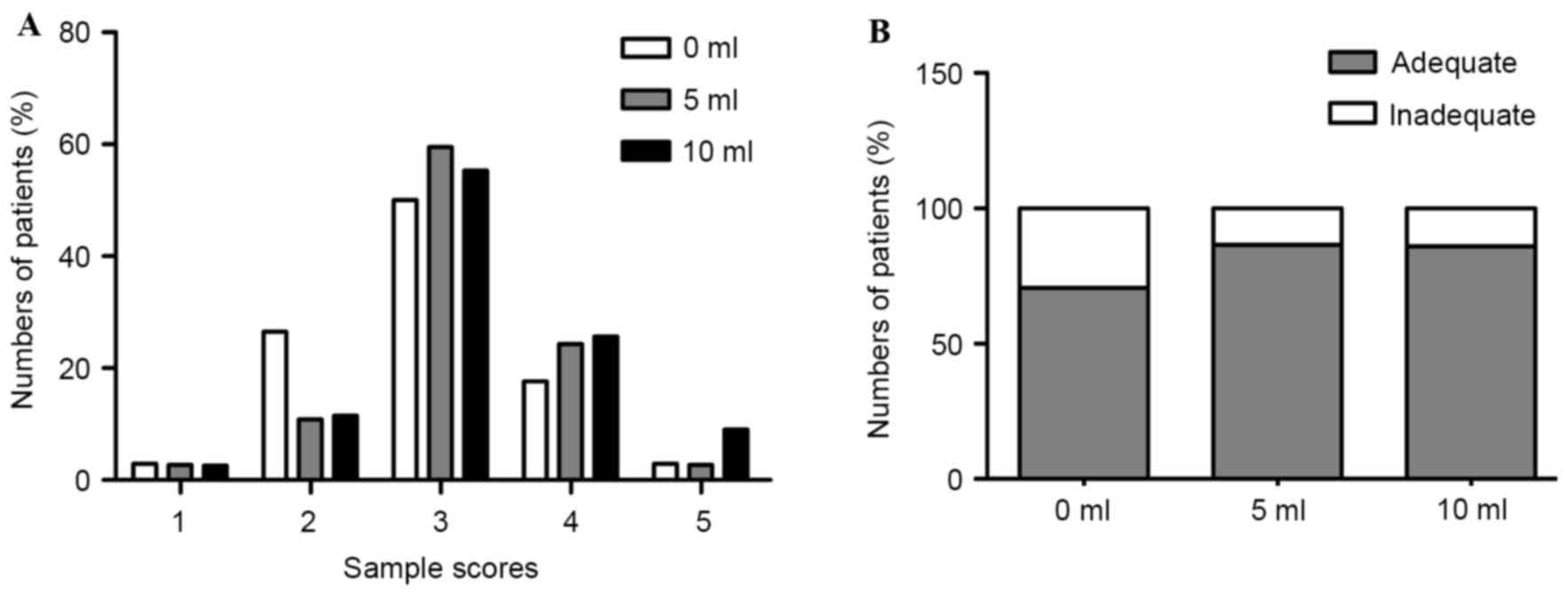
Adequacy scores and tissue quality of
specimens
The adequacy scores for histological diagnosis of
the obtained tissues are shown in Fig.
4A. The numbers of adequate and inadequate samples in the
slow-pull, 5 ml suction and 10 ml suction groups are provided in
Fig. 4B. Among the samples obtained
from the slow-pull group, 70.6% (24/34) were determined to be
adequate for histological diagnosis. By comparison, 86.5 (32/37)
and 85.9% (67/78) of the samples obtained from the 5 ml suction and
10 ml suction groups were found to be adequate for histological
diagnosis. Therefore, the samples that were obtained for
histopathological diagnosis using the 5 ml suction and 10 ml
suction techniques were superior to those obtained using normal
negative pressure (NNP), although no significant difference was
observed (P=0.1118; χ2 test). By contrast, the samples obtained
using 10 ml suction contained more blood compared with those
obtained using slow-pull or 5 ml suction techniques (P=0.0056; χ2
test; Table IV).
 | Table IV.Degree of the amount of blood in the
specimens. |
Table IV.
Degree of the amount of blood in the
specimens.
| Amount of
blood | 0 ml, n (%) | 5 ml, n (%) | 10 ml, n (%) | P-value
(χ2 test) |
|---|
| Minimal | 12 (35.3) | 13 (35.1) | 10 (12.8) | 0.0056a |
| Moderate | 15 (44.1) | 15 (40.5) | 31 (39.7) |
|
| Significant | 7
(20.5) | 9
(24.3) | 37 (47.4) |
|
Complications
Among the 149 enrolled patients with solid lesions,
no complications developed following the EUS-FNA procedures.
Discussion
In the present retrospective comparative analysis,
the use of the slow-pull and 5 ml suction techniques during EUS-FNA
for pancreatic or non-pancreatic solid lesions with regular FNA
needles (22-G) was associated with superior specificities and
accuracies compared with the use of the 10 ml suction technique.
Although the sensitivities of the cytological examinations
conducted with 5 ml suction and 10 ml suction were worse than that
of slow-pull, the increase in diagnostic yield based on
histological examination resulted in improved overall diagnostic
yields for the 5 ml suction and 10 ml suction techniques.
Furthermore, the samples obtained using slow-pull and 5 ml suction
contained less contamination with blood compared with those
obtained with 10 ml suction. Collectively, these results indicated
that the lesions were diagnosed more accurately using EUS-FNA with
5 ml suction techniques regardless of the lesion location.
The requirement for suction during EUS-FNA has been
evaluated in previous reports but remains controversial (24,29,30). The
application of suction in EUS-FNA was first investigated during the
sampling of lymph nodes. In a previous study on the application of
EUS-FNA to malignant lymph nodes that were dissected at autopsy
(31), continuous and intermittent
suction with syringes of between 10 and 30 ml were compared, and
continuous low-level suction resulted in optimum cellularity.
Another study by Wallace et al (32) compared the application of EUS-FNA with
and without suction to lymph nodes, and the application of suction
increased the cellularity but decreased the specimen quality due to
blood contamination. In another previous randomized controlled
trial on the application of EUS-FNA with and without suction to
pancreatic solid masses (33), which
utilized 22 and 25-G needles, the application of suction resulted
in greater cellularity, bloodiness and sensitivity. Therefore, the
effect of suction during EUS-FNA for pancreatic masses has not yet
been fully elucidated. However, the use of suction during EUS-FNA
is generally considered to increase the cellularity and blood
contamination, which may hinder cytological interpretation.
The European Society of Gastrointestinal Endoscopy
(Munich, Germany) technical guidelines advocate the use of suction
for the EUS-FNA of solid masses/cystic lesions (34). However, regarding the application of
EUS-FNA to lymph nodes (22), the
types of negative pressure that should be used with pancreatic and
non-pancreatic solid lesions remain vague. Therefore, three
different types of negative pressure suction techniques were
utilized: A normal condition without negative pressure or a stylet;
a moderate negative pressure condition using a 5 ml syringe; and a
HNP condition using a 10 ml syringe. The results of the present
study revealed that EUS-FNA with the 5 ml suction technique enabled
superior specificity and accuracy compared with the 10 ml suction
technique, and greater sensitivity of cytological examination
compared with the slow-pull technique.
A total of two previous studies have indicated that
EUS-FNA approaches that employ HNP suction for the aspiration of
tissue enable the acquisition of adequate tissue samples (24,28). In
addition, a technique has been proposed that reportedly enables the
acquisition of tissue cores for histological assessment with
standard 22 or 25-G EUS-FNA needles (29,30). The
needle is connected to a balloon inflation gun (Alliance II
inflation system; Microvasive Endoscopy, Boston Scientific
Corporation, Marlborough, MA, USA), which is turned into suction
mode to apply HNP (35–60 ml). In a previous study, Larghi et
al (24) applied this technique
prospectively in 27 patients with solid masses. These authors
reported that tissue samples for histological examination were
obtained in 96% of the cases. Kudo et al (23) also used this system and confirmed that
biopsy procedures involving the combination of EUS-FNA and HNP
techniques of between 35 and 60 ml, are superior to EUS-FNA
combined with 10 ml negative pressure procedures in terms of tissue
acquisition. One identified problem with the use of EUS-FNA with
HNP is that the obtained specimens contain more blood. However,
there were no differences between HNP and NNP in terms of
diagnostic accuracy. Consequently, as demonstrated in the present
study, the samples obtained using 5 ml suction contained less blood
contamination compared with those obtained using 10 ml suction. In
addition, the samples obtained for histopathological diagnosis via
5 ml suction remained superior to those obtained using slow-pull,
although this difference was not significant. Therefore, it appears
that the application of EUS-FNA with 5 ml suction is preferable for
the diagnosis of mediastinal and intra-abdominal lesions compared
with techniques that employ negative pressure applied with
slow-pull and 10 ml suction techniques.
There were a number of limitations in the protocol
of the present study. One limitation was the relatively low number
of cases. The low number of randomized lesions also led to uneven
randomization of the different target lesions and diagnostic
entities, which may have affected the results. The majority of the
patients presented with malignancies and only a few had benign
tumors. Specifically, only a few patients possessed hypervascular
tumors (n=9, neuroendocrine tumors). Additionally, this is an
observational and retrospective study. Although the majority of
baseline characteristics are balanced, selective bias and
heterogeneity could not be avoided. Although the evidence presented
here indicated that EUS-FNA with 5 ml suction is feasible, an
additional multicenter, prospective, double-blind, randomized,
controlled crossover trial study will be performed to resolve these
issues.
Acknowledgements
The present study was supported by the National
Science Foundation of China (grant nos. 81172063 and 81372352), and
the Innovation Foundation of Huazhong University of Science and
Technology. Dr Ronghua Wang was supported by the China Scholarship
Association. The authors thank Dr Patrick Varley and Dr Hai Huang
(both from Department of Surgery, University of Pittsburgh,
Pittsburgh, PA, USA), and Dr Wensheng Zhang (Department of
Med-Plastic Surgery, University of Pittsburgh, Pittsburgh, PA, USA)
for their review of the present manuscript.
References
|
1
|
Abdalla EK and Pisters PW: Staging and
preoperative evaluation of upper gastrointestinal malignancies.
Semin Oncol. 31:513–529. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pei Q, Wang L, Pan J, Ling T, Lv Y and Zou
X: Endoscopic ultrasonography for staging depth of invasion in
early gastric cancer: A meta-analysis. J Gastroenterol Hepatol.
30:1566–1573. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Guo T, Yao F, Yang AM, Li XY, Zhong DR, Wu
DS, Wu X and Lu XH: Endoscopic ultrasound in restaging and
predicting pathological response for advanced gastric cancer
patients after neoadjuvant chemotherapy. Asia Pac J Clin Oncol.
10:e28–e32. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chandran S, Efthymiou M, Kaffes A, Chen
JW, Kwan V, Murray M, Williams D, Nguyen NQ, Tam W, Welch C, et al:
Management of pancreatic collections with a novel endoscopically
placed fully covered self-expandable metal stent: A national
experience (with videos). Gastrointest Endosc. 81:127–135. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Iglesias-Garcia J, Lariño-Noia J,
Vallejo-Senra N, de-la-Iglesia-Garcia D, Abdulkader-Nallib I and
Dominguez-Muñoz JE: Feasibility of endoscopic ultrasound (EUS)
guided fine needle aspiration (FNA) and biopsy (FNB) with a new
slim linear echoendoscope. Rev Esp Enferm Dig. 107:359–365.
2015.PubMed/NCBI
|
|
6
|
Singh R, Jayanna M, Wong J, Lim LG, Zhang
J, Lv J, Liu D, Lee YC, Han ML, Tseng PH, et al: Narrow-band
imaging and white-light endoscopy with optical magnification in the
diagnosis of dysplasia in Barrett's esophagus: Results of the
Asia-Pacific barrett's consortium. Endosc Int Open. 3:E14–E18.
2015.PubMed/NCBI
|
|
7
|
Barawi M and Gress F: EUS-guided
fine-needle aspiration in the mediastinum. Gastrointest Endosc.
52:(6 Suppl). S12–S17. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Horwhat JD and Gress FG: Defining the
diagnostic algorithm in pancreatic cancer. JOP. 5:289–303.
2004.PubMed/NCBI
|
|
9
|
Fritscher-Ravens A: Endoscopic ultrasound
evaluation in the diagnosis and staging of lung cancer. Lung
Cancer. 41:259–267. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shami VM, Parmar KS and Waxman I: Clinical
impact of endoscopic ultrasound and endoscopic ultrasound-guided
fine-needle aspiration in the management of rectal carcinoma. Dis
Colon Rectum. 47:59–65. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lee YT, Chan FK, Leung WK, Chan HL, Wu JC,
Yung MY, Ng EK, Lau JY and Sung JJ: Comparison of EUS and ERCP in
the investigation with suspected biliary obstruction caused by
choledocholithiasis: A randomized study. Gastrointest Endosc.
67:660–668. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ribeiro A, Vazquez-Sequeiros E, Wiersema
LM, Wang KK, Clain JE and Wiersema MJ: EUS-guided fine-needle
aspiration combined with flow cytometry and immunocytochemistry in
the diagnosis of lymphoma. Gastrointest Endosc. 53:485–491. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Erickson RA, Sayage-Rabie L and Beissner
RS: Factors predicting the number of EUS-guided fine-needle passes
for diagnosis of pancreatic malignancies. Gastrointest Endosc.
51:184–190. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mesa H, Stelow EB, Stanley MW, Mallery S,
Lai R and Bardales RH: Diagnosis of nonprimary pancreatic neoplasms
by endoscopic ultrasound-guided fine-needle aspiration. Diagn
Cytopathol. 31:313–318. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Klapman JB, Logrono R, Dye CE and Waxman
I: Clinical impact of on-site cytopathology interpretation on
endoscopic ultrasound-guided fine needle aspiration. Am J
Gastroenterol. 98:1289–1294. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Logroño R and Waxman I: Interactive role
of the cytopathologist in EUS-guided fine needle aspiration: An
efficient approach. Gastrointest Endosc. 54:485–490. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jhala NC, Jhala DN, Chhieng DC, Eloubeidi
MA and Eltoum IA: Endoscopic ultrasound-guided fine-needle
aspiration. A cytopathologist's perspective. Am J Clin Pathol.
120:351–367. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wani S, Gupta N, Gaddam S, Singh V,
Ulusarac O, Romanas M, Bansal A, Sharma P, Olyaee MS and Rastogi A:
A comparative study of endoscopic ultrasound guided fine needle
aspiration with and without a stylet. Dig Dis Sci. 56:2409–2414.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rastogi A, Wani S, Gupta N, Singh V,
Gaddam S, Reddymasu S, Ulusarac O, Fan F, Romanas M, Dennis KL, et
al: A prospective, single-blind, randomized, controlled trial of
EUS-guided FNA with and without a stylet. Gastrointest Endosc.
74:58–64. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mertz H and Gautam S: The learning curve
for EUS-guided FNA of pancreatic cancer. Gastrointest Endosc.
59:33–37. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Siddiqui UD, Rossi F, Rosenthal LS, Padda
MS, Murali-Dharan V and Aslanian HR: EUS-guided FNA of solid
pancreatic masses: A prospective, randomized trial comparing
22-gauge and 25-gauge needles. Gastrointest Endosc. 70:1093–1097.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Puri R, Vilmann P, Săftoiu A, Skov BG,
Linnemann D, Hassan H, Garcia ES and Gorunescu F: Randomized
controlled trial of endoscopic ultrasound-guided fine-needle
sampling with or without suction for better cytological diagnosis.
Scand J Gastroenterol. 44:499–504. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kudo T, Kawakami H, Hayashi T, Yasuda I,
Mukai T, Inoue H, Katanuma A, Kawakubo K, Ishiwatari H, Doi S, et
al: High and low negative pressure suction techniques in EUS-guided
fine-needle tissue acquisition by using 25-gauge needles: A
multicenter, prospective, randomized, controlled trial.
Gastrointest Endosc. 80:1030–1037.e1. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Larghi A, Noffsinger A, Dye CE, Hart J and
Waxman I: EUS-guided fine needle tissue acquisition by using high
negative pressure suction for the evaluation of solid masses: A
pilot study. Gastrointest Endosc. 62:768–774. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Harris K, Maroun R, Attwood K and Chalhoub
M: Comparison of cytologic accuracy of endobronchial ultrasound
transbronchial needle aspiration using needle suction versus no
suction. Endosc Ultrasound. 4:115–119. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Alizadeh Mohammad AH, Hadizadeh M, Padashi
M, Shahbaazi S, Molaee M and Shariatpanahi ZV: Comparison of two
techniques for endoscopic ultrasonography fine-needle aspiration in
solid pancreatic mass. Endosc Ultrasound. 3:174–178. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Aadam AA, Oh YS, Shidham VB, Khan A, Hunt
B, Rao N, Zhang Y, Tarima S and Dua KS: Eliminating the residual
negative pressure in the endoscopic ultrasound aspirating needle
enhances cytology yield of pancreas masses. Dig Dis Sci.
61:890–899. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gerke H, Rizk MK, Vanderheyden AD and
Jensen CS: Randomized study comparing endoscopic ultrasound-guided
Trucut biopsy and fine needle aspiration with high suction.
Cytopathology. 21:44–51. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang KX, Ben QW, Jin ZD, Du YQ, Zou DW,
Liao Z and Li ZS: Assessment of morbidity and mortality associated
with EUS-guided FNA: A systematic review. Gastrointest Endosc.
73:283–290. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Haba S, Yamao K, Bhatia V, Mizuno N, Hara
K, Hijioka S, Imaoka H, Niwa Y, Tajika M, Kondo S, et al:
Diagnostic ability and factors affecting accuracy of endoscopic
ultrasound-guided fine needle aspiration for pancreatic solid
lesions: Japanese large single center experience. J Gastroenterol.
48:973–981. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bhutani MS, Suryaprasad S, Moezzi J and
Seabrook D: Improved technique for performing endoscopic ultrasound
guided fine needle aspiration of lymph nodes. Endoscopy.
31:550–553. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wallace MB, Kennedy T, Durkalski V,
Eloubeidi MA, Etamad R, Matsuda K, Lewin D, Van Velse A, Hennesey
W, Hawes RH and Hoffman BJ: Randomized controlled trial of
EUS-guided fine needle aspiration techniques for the detection of
malignant lymphadenopathy. Gastrointest Endosc. 54:441–447. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lee JK, Choi JH, Lee KH, Kim KM, Shin JU,
Lee JK, Lee KT and Jang KT: A prospective, comparative trial to
optimize sampling techniques in EUS-guided FNA of solid pancreatic
masses. Gastrointest Endosc. 77:745–751. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Polkowski M, Larghi A, Weynand B,
Boustière C, Giovannini M, Pujol B and Dumonceau JM: European
Society of Gastrointestinal Endoscopy (ESGE): Learning, techniques,
and complications of endoscopic ultrasound (EUS)-guided sampling in
gastroenterology: European Society of Gastrointestinal Endoscopy
(ESGE) technical guideline. Endoscopy. 44:190–206. 2012. View Article : Google Scholar : PubMed/NCBI
|