Introduction
The incidence of cholangiocarcinoma (CC), which is a
major cause of cancer-associated mortality in Asia, is rising
worldwide (1). Despite recent
advances in cancer therapy, it is difficult to completely cure CC
as radical surgical resection is the only effective curative
treatment (2). Additionally,
chemotherapy regimens for patients with CC with recurrent and
metastatic lesions have been insufficient. However, small
populations of patients with CC have obtained significant benefits
from conventional chemotherapy (3,4).
Therefore, to improve patient prognoses and risk assessments,
additional studies are required to identify novel markers and
therapeutic targets.
F-box and WD repeat domain-containing 7 (FBXW7),
which is an F-box protein consisting of 1 of the 4 subunits of the
Skp Cullin F-box containing ubiquitin ligase complex, induces the
degradation of oncoproteins including c-Myc, CyclinE, mammalian
target of rapamycin (mTOR), Notch and myeloid cell leukemia 1
(MCL1) via ubiquitin-mediated degradation pathways (5,6). The
degradation of c-Myc by FBXW7 leads to cell cycle dormancy or the
G0 state, therefore, FBXW7 alteration serves an important role in,
and is a major cause of, carcinogenesis (7). Takeishi et al (8) revealed that ablation of the FBXW7
gene in mouse leukemia-initiating cells of chronic myeloid leukemia
induced cell cycle re-entry via accumulation of c-Myc, and
subsequently increased the sensitivity of the cells to
chemotherapy. Concomitantly, it is known that loss of FBXW7
function is associated with resistance to anti-tubulin agents via
the accumulation of the anti-apoptotic protein MCL1, a member of
the B-cell lymphoma 2 family (9).
Therefore, the role of FBXW7 in chemosensitivity is
controversial.
In a previous study of clinical samples, low FBXW7
expression levels were associated with poor prognosis and cancer
progression in a number of human malignancies including breast
cancer (10), hepatocellular
(11) and esophageal carcinoma
(12,13). In intrahepatic and perihilar CC, it
was demonstrated that low FBXW7 expression levels are associated
with cancer progression and the increase of migration and invasion
rates due to the accumulation of mTOR, an FBXW7 degradation target,
as demonstrated in vivo and in vitro (14). Furthermore, Enkhbold et al
(15) revealed that low FBXW7
expression levels in clinical samples of intrahepatic CC were
associated with significantly poor prognosis, although their number
of cases was small. However, few studies have addressed the role of
FBXW7 in the prognosis and chemosensitivity of patients with CC,
whether intrahepatic or extrahepatic disease.
The purpose of the present study was to determine
the clinical significance of FBXW7 expression in CC. The expression
levels of FBXW7 were investigated in CC tissue samples using
immunohistochemistry to evaluate whether this protein qualifies as
a marker of poor prognosis and chemosensitivity for patients with
this disease.
Materials and methods
Patients and samples
A total of 100 patients with CC who underwent
surgical resection at the Gunma University Hospital (Maebashi,
Japan) and the Gunma Prefecture Saiseikai-Maebashi Hospital
(Maebashi, Japan) between January 1996 and December 2011 were
included in the present study. There were 69 males and 31 females.
The mean age of patients was 68.1 years, range, 36–94 years. Intra-
and extrahepatic CC were diagnosed in 14 and 86 patients,
respectively. Operative procedures were as follows: Among patients
diagnosed with intrahepatic CC, 11 underwent right or left
hepatectomy and 3 underwent sectionectomy, and among those
diagnosed with extrahepatic CC, 14 underwent hepatectomy with bile
duct resection, 63 underwent pancreatoduodenectomy, 3 underwent
hepato-pancreatoduodenectomy and 6 underwent bile duct resection
alone. For all cases, lymph node dissection was performed according
to the location of the tumor. The median follow-up period for
survivors was 30.4 months, range, 2.2–115.2 months. The
pathological stage of CC was determined according to the 7th
edition of tumor-node-metastasis (TNM) classification of Union for
International Cancer Center (16). A
total of 80 patients were staged as R0, no local residual tumor,
and 20 patients were staged as R1, microscopic residual tumor.
Recurrence occurred in 56 patients, while 64 patients received
chemotherapy. Chemotherapy was recommended for all patients,
however, those with dysfunction of the vital organs and those who
chose not to receive chemotherapy were excluded. The details of
chemotherapy are as follows: 30 patients received
tegafur/gimeracil/oteracil (S-1), 24 received gemcitabine, 17
received tegafur-uracil, 7 received gemcitabine + S-1, and 2
received gemcitabine + cisplatin, including overlap. None of the
patients had received neoadjuvant chemotherapy and irradiation
prior to surgical resection. Patient backgrounds are summarized in
Table I. Written informed consent was
obtained from all patients included in the present study, which was
approved by the institutional review board at Gunma University
Hospital.
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Factors | Number |
|---|
| Age (mean ±
standard deviation) | 68.1±9.0 |
| Sex |
|
|
Male | 69 |
|
Female | 31 |
| Location |
|
|
Intrahepatic | 14 |
|
Extrahepatic | 86 |
| Histology |
|
Well | 21 |
|
Moderate | 49 |
|
Poor | 30 |
| Tumor size
(mm) |
|
|
≤60 | 84 |
|
>60 | 9 |
|
N/A | 7 |
| Tumor stage |
|
| T1,
T2 | 47 |
| T3,
T4 | 53 |
| Lymph node
metastasis |
|
|
Absent | 58 |
|
Present | 42 |
| Stage (UICC) |
|
| 0, I,
II | 77 |
| III,
IV | 23 |
| Recurrence |
|
|
Absent | 44 |
|
Present | 56 |
| Chemotherapy |
|
|
Absent | 36 |
|
Present | 64 |
Immunohistochemical staining
A paraffin-embedded block of CC specimens was cut
into 2-µm thick sections and mounted on glass slides. Each section
was deparaffinized by xylene and dehydrated in alcohol. Endogenous
peroxidase was inhibited using 0.3%
H2O2/methanol for 30 min at room temperature.
The sections were soaked in heated water with 0.5% Immunosaver
(Nisshin EM Co. Ltd., Tokyo, Japan) at 98°C for 45 min.
Non-specific antigens were blocked by Protein Block, Serum-Free
(Dako; Agilent Technologies, Inc., Santa Clara, CA, USA) at room
temperature for 30 min. The sections were then incubated with
rabbit polyclonal primary antibody against FBXW7 (cat. no.,
ab109617; dilution, 1:300; Abcam, Cambridge, MA, USA) for 24 h at
4°C. Subsequent to washing in PBS, the Histofine Simple Stain
MAX-PO (MULTI) kit (Nichirei Biosciences, Inc., Tokyo, Japan) was
applied for visualizing primary antibody and incubated at room
temperature for 45 min. The chromogen 3,3′-diaminobenzidine
tetrahydrochloride (DAB) was applied as a 0.02% solution containing
0.005% H2O2 in 50 mM ammonium acetate-citrate
acid buffer (pH 6.0). Finally, counterstaining of the nucleus was
performed using Mayer's hematoxylin solution. A negative control
was included by replacing the primary antibody with PBS in 0.1%
bovine serum albumin, and confirmed no detectable staining in this
case.
Additionally, 3 sequential sections of each sample
were treated with primary antibodies against c-Myc (cat. no.,
ab32072; dilution, 1:50; Abcam) and the cell proliferation marker
Ki-67 (cat. no., M7240; dilution, 1:150; Dako; Agilent
Technologies, Inc.), according to the aforementioned protocol, to
evaluate the correlations between FBXW7, c-Myc and cell
proliferation.
Assessment of FBXW7 and c-Myc
expression
Nuclear staining of FBXW7 in cancerous tissue was
evaluated in 5 randomly selected fields, and the intensity scored.
The intensity score ranged from 1 to 3, with 1, weak; 2, moderate;
and 3, strong, and was evaluated in each sample.
The expression level of c-Myc was evaluated in the
nucleus of the cancerous tissue. The proportion of positively
staining tumor cells was evaluated, regardless of the staining
intensity. A percentage of positive tumor cells ≥10% was defined as
‘positive’ expression and <10% was defined as ‘negative’
expression (17).
Statistical analysis
Data for the continuous variables were expressed as
the mean ± standard deviation, or median and range. The association
between FBXW7 expression and clinicopathological characteristics
was analyzed using the χ2 test and the Wilcoxon signed-rank test.
Survival curves were described using the Kaplan-Meier method, and
differences in survival between groups were compared using the
log-rank test. Univariate and multivariate analysis by the Cox
proportional hazards model was used to identify prognostic factors.
P<0.05 was considered to indicate a statistically significant
difference. All statistical analyses were performed using the JMP
software package (version 10.0.0; SAS Institute Inc., Cary, NC,
USA).
Results
Analysis of FBXW7 expression in CC
tissues by immunohistochemistry
FBXW7 expressed in the nucleus was measured by
immunohistochemistry. Therefore, nuclear FBXW7 expression was
evaluated in 100 CC samples. FBXW7 expression was observed in
normal bile duct epithelium (Fig.
1A); however, the expression was lower in cancerous areas
(Fig. 1B and C). Similarly, FBXW7
expression was reduced at the site of lymph node metastases
(Fig. 1E and F). These CC samples
were divided into 2 groups according to the intensity of nuclear
FBXW7 staining in the cancerous areas. A score of 1 in cancerous
tissue samples was considered a low expression level of FBXW7
(Fig. 1B and C); scores of 2 or 3
were considered as high expression (Fig.
1D). A total of 54 samples exhibited high FBXW7 expression,
whilst 46 exhibited low FBXW7 expression. Additionally, the
correlation among FBXW7, c-Myc and marker of proliferation Ki67
(Ki-67) expression was investigated using immunohistochemistry in 3
sequential sections (Fig. 2). Low
FBXW7 expression samples demonstrated enhanced expression of c-Myc
and Ki-67 (Fig. 2A), while high FBXW7
expression samples exhibited lower expression of c-Myc and Ki-67
(Fig. 2B). Low FBXW7 expression was
significantly associated with nuclear c-Myc accumulation
(P<0.001).
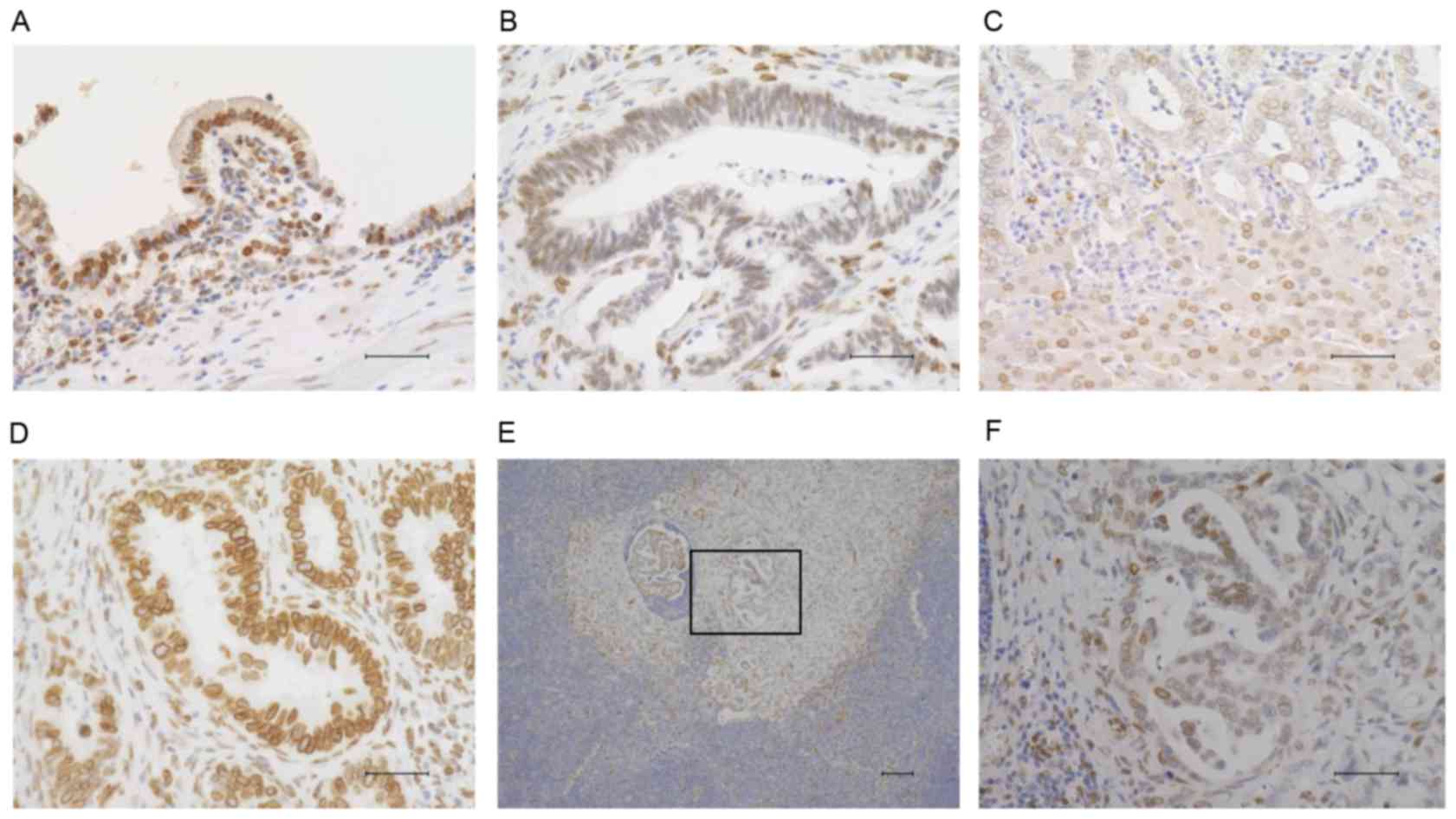 | Figure 1.Immunohistochemical staining of
FBXW7. (A) FBXW7 expression in normal bile duct epithelium
(magnification, ×400; scale bar, 50 µm). (B) Representative
extrahepatic CC sample demonstrating weak expression of FBXW7
(magnification, ×400; scale bar, 50 µm). (C) Representative
intrahepatic CC sample that demonstrates weak expression of FBXW7
(magnification, ×200; scale bar, 100 µm). (D) Representative
extrahepatic CC sample demonstrating strong expression of FBXW7
(magnification, ×400; scale bar, 50 µm). (E) FBXW7 expression in a
lymph node metastasis of CC on a low power field (magnification,
×100; scale bar 100 µm). (F) High power field of Fig. 1E (square); the lymph node metastasis
site of CC demonstrated low expression of FBXW7 (magnification,
×400; scale bar, 50 µm). Immunohistochemical staining was performed
using 3,3′-diaminobenzidine and counterstaining of the nucleus was
performed using Mayer's hematoxylin solution. All bright images
were obtained using a fluorescence microscope (BZ-X700, KEYENCE,
Osaka, Japan) FBXW7, F-box and WD repeat domain-containing 7; CC,
cholangiocarcinoma. |
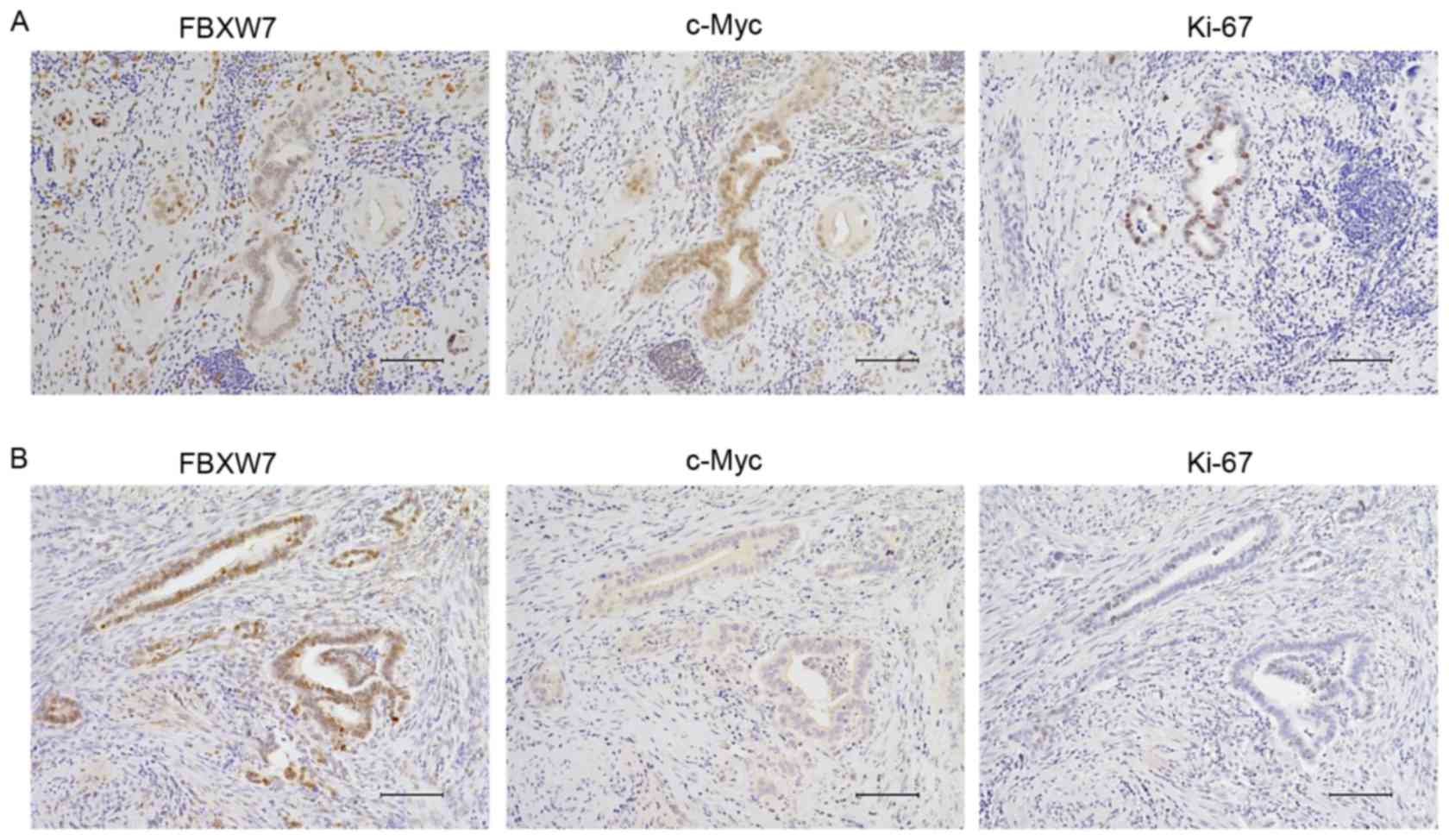 | Figure 2.Correlation between FBXW7, c-Myc, and
Ki-67 expression in representative cholangiocarcinoma tissue
samples. Proteins were detected by immunohistochemistry. (A) Low
levels of FBXW7 expression (left) in tumors exhibited enhanced
c-Myc (middle) and Ki-67 (right) expression. (B) High FBXW7
expression (left) in tumors exhibited decreased c-Myc (middle) and
Ki-67 (right) expression. Magnification, ×200; scale bar, 100 µm.
Immunohistochemical staining was performed using
3,3′-diaminobenzidine and counterstaining of the nucleus was
performed using Mayer's hematoxylin solution. All bright images
were obtained using a fluorescence microscope (BZ-X700, KEYENCE,
Osaka, Japan) Ki-67, marker of proliferation Ki67; FBXW7, F-box and
WD repeat domain-containing 7. |
Association between FBXW7 expression
and clinicopathological characteristics of CC
The clinicopathological characteristics of patients
listed according to FBXW7 expression are summarized in Table II. Low FBXW7 expression levels were
significantly associated with progressive tumor size (P=0.049).
Furthermore, the median recurrence-free survival time of the low
FBXW7 expression group was significantly shorter compared with that
of the high FBXW7 expression group (P=0.044). However, there were
no significant differences with respect to age, sex, histology,
tumor stage, lymph node metastasis, recurrence and
chemotherapy.
 | Table II.Clinicopathological characteristics
according to FBXW7 expression in 100 patients with
cholangiocarcinoma. |
Table II.
Clinicopathological characteristics
according to FBXW7 expression in 100 patients with
cholangiocarcinoma.
|
| FBXW7 expression
(%) |
|
|---|
|
|
|
|
|---|
| Factors | High (n=54) | Low (n=46) | P-value |
|---|
| Age |
|
| 0.684 |
|
≤65 | 35 (64.8) | 28 (60.9) |
|
|
>65 | 19 (35.2) | 18 (39.1) |
|
| Sex |
|
| 0.450 |
|
Male | 39 (72.2) | 30 (65.2) |
|
|
Female | 15 (27.8) | 16 (34.8) |
|
| Histology |
|
| 0.364 |
|
Well | 14 (25.9) | 7 (15.2) |
|
|
Moderate | 26 (48.2) | 23 (50.0) |
|
|
Poor | 14 (25.9) | 16 (34.8) |
|
| Location |
|
| 0.116 |
|
Distal | 42 (77.8) | 31 (67.4) |
|
|
Perihilar | 8 (14.8) | 5 (10.9) |
|
|
Intrahepatic | 4 (7.4) | 10 (21.7) |
|
| Tumor size
(mm) |
|
| 0.049a |
|
≤60 | 47 (95.9) | 37 (84.1) |
|
|
>60 | 2 (4.1) | 7 (15.9) |
|
| Tumor stage |
|
| 0.515 |
| T1,
T2 | 27 (50.0) | 20 (43.5) |
|
| T3,
T4 | 27 (50.0) | 26 (56.5) |
|
| Lymph node
metastasis |
|
| 0.591 |
|
Absent | 30 (55.6) | 28 (60.9) |
|
|
Present | 24 (44.4) | 18 (39.1) |
|
| Stage
(UICC) |
|
| 0.249 |
| 0, I,
II | 44 (81.5) | 33 (71.7) |
|
| III,
IV | 10 (18.5) | 13 (28.3) |
|
| Recurrence |
|
| 0.923 |
|
Absent | 24 (44.4) | 20 (43.5) |
|
|
Present | 30 (55.6) | 26 (56.5) |
|
| Chemotherapy |
|
| 0.547 |
|
Absent | 18 (33.3) | 18 (39.1) |
|
|
Present | 36 (66.7) | 28 (60.9) |
|
| Nuclear c-Myc
accumulation |
|
|
<0.001a |
|
Negative | 42 (77.8) | 17 (37.0) |
|
|
Positive | 12 (22.2) | 29 (63.0) |
|
| Recurrence free
survival (Months) |
|
| 0.044a |
| Median
(range) | 12 (0.5–123.5) | 7.6 (0.3–83.3) |
|
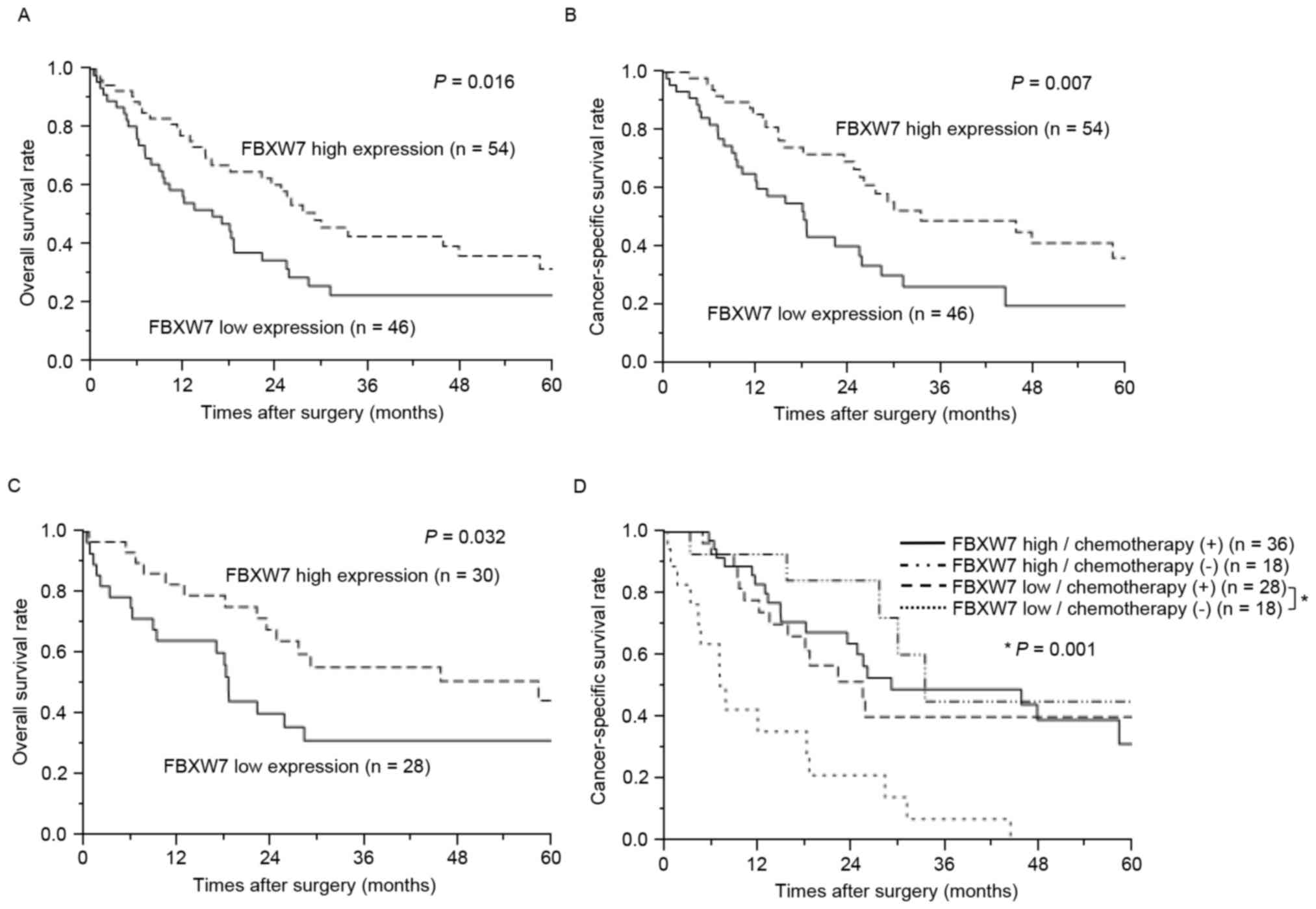
Prognostic value of FBXW7 expression
in CC
Patients with low FBXW7 expression exhibited
significantly poorer prognoses compared with those with high FBXW7
expression in terms of overall and cancer-specific survival
(P=0.016 and P=0.007, respectively; Fig.
3A and B). Notably, even in cases of negative lymph node
metastasis, the low FBXW7 expression group also exhibited
significantly poorer prognosis compared with the high FBXW7
expression group for overall survival (P=0.032; Fig. 3C). The association between FBXW7
expression and survival subsequent to chemotherapy was also
examined. In the high FBXW7 expression group, cancer-specific
survival times were not significantly different among patients with
or without chemotherapy (Fig. 3D). In
the low FBXW7 expression group, however, cancer-specific survival
times in patients who received chemotherapy were significantly
longer compared with that of patients who did not (P=0.001;
Fig. 3D). Due to various chemotherapy
regimens in the cohort of the present study, a sub-analysis was
performed according to chemotherapy regimen, such as S-1,
gemcitabine, and tegafur-uracil. However, each regimen demonstrated
similar results, and there were no significant differences between
the regimens (data not shown). Additionally, the present study
considered whether differences in patient characteristics,
including exhibiting a more aggressive-stage tumor, may explain the
difference in survival outcomes between patients with low
FBXW7-expression who underwent chemotherapy compared with those who
did not. However, no such differences were observed (Table III). These data therefore suggest
that patients with low FBXW7 expression are sensitive to
chemotherapy compared with patients with high FBXW7 expression.
 | Table III.Association between
clinicopathological characteristics and chemotherapy in patients
with low FBXW7 expression. |
Table III.
Association between
clinicopathological characteristics and chemotherapy in patients
with low FBXW7 expression.
|
| Chemotherapy
(%) |
|
|---|
|
|
|
|
|---|
| Factors | Absent (n=18) | Present (n=28) | P-value |
|---|
| Age |
|
| 0.554 |
|
≤65 | 8 (44.4) | 10 (35.7) |
|
|
>65 | 10 (55.6) | 18 (64.3) |
|
| Sex |
|
| 0.639 |
|
Male | 11 (61.1) | 19 (67.9) |
|
|
Female | 7 (38.9) | 9 (32.1) |
|
| Histology |
|
| 0.833 |
|
Well | 3 (16.7) | 4 (14.3) |
|
|
Moderate | 8 (44.4) | 15 (53.6) |
|
|
Poor | 7 (38.9) | 9 (32.1) |
|
| Tumor size
(mm) |
|
| 0.697 |
|
≤60 | 13 (81.3) | 24 (85.7) |
|
|
>60 | 3 (18.7) | 4 (14.3) |
|
| Tumor stage |
|
| 0.474 |
| T1,
T2 | 9 (50.0) | 11 (39.3) |
|
| T3,
T4 | 9 (50.0) | 17 (60.7) |
|
| Lymph node
metastasis |
|
| 0.554 |
|
Absent | 10 (55.6) | 18 (64.3) |
|
|
Present | 8 (44.4) | 10 (35.7) |
|
| Stage (UICC) |
|
| 0.954 |
| 0, I,
II | 13 (72.2) | 20 (71.4) |
|
| III,
IV | 5 (27.8) | 8 (28.6) |
|
Univariate and multivariate analyses of overall
survival are summarized in Table IV.
Low FBXW7 expression was a prognostic factor on univariate
analysis, including with histology, tumor stage, lymph node
metastasis and recurrence. However, on multivariate analysis, only
low FBXW7 expression in CC was an independent poor prognostic
factor, thus outweighing the existing clinicopathological factors
for overall survival in the cohort of the present study
(P=0.043).
 | Table IV.Univariate and Multivariate analysis
for overall survival using the Cox proportional hazards model. |
Table IV.
Univariate and Multivariate analysis
for overall survival using the Cox proportional hazards model.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Variable | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Age (≤65 vs.
>65) | 1.23 | 0.74–2.74 | 0.404 | – | – | – |
| Sex (Male vs. |
| Female) | 1.25 | 0.75–2.75 | 0.379 | – | – | – |
| Histology (Well
vs. |
| Moderately vs.
Poor) | 2.52 | 1.33–5.33 | 0.004a | 1.83 | 0.93–3.93 | 0.082 |
| Tumor stage (T1-2
vs. T3-4) | 2.07 | 1.26–3.26 | 0.004a | 1.57 | 0.91–2.91 | 0.101 |
| Lymph node |
| metastasis (− vs.
+) | 1.86 | 1.14–3.14 | 0.014a | 1.4 | 0.80–2.80 | 0.229 |
| Recurrence (− vs.
+) | 2.24 | 1.32–3.32 | 0.002a | 1.56 | 0.90–2.90 | 0.598 |
| FBXW7 (High vs.
Low) | 1.81 | 1.10–2.10 | 0.018a | 1.67 | 1.01–2.01 | 0.043a |
Discussion
In the present study, it was demonstrated that low
FBXW7 expression levels in the nucleus are associated with tumor
size and poor prognosis in patients with CC, and is associated with
enhanced c-Myc and Ki-67 expression. Furthermore, the efficacy of
chemotherapy differed according to FBXW7 expression levels, as the
low FBXW7 expression group was more sensitive to chemotherapy
compared with the high FBXW7 expression group.
c-Myc is a target protein of FBXW7, and is regulated
and degraded via ubiquitin-proteasome pathways. Therefore, the loss
of FBXW7 function results in the accumulation of c-Myc (18,19).
Additionally, as c-Myc serves an important role in cell cycle
regulation, particularly in G0-G1 phase transition (20), the overexpression of c-Myc in tumors
is associated with aggressive proliferation (21,22). It
has been demonstrated that hematopoietic stem cells from
conditional FBXW7 gene knockout mice exhibited c-Myc
accumulation, and were therefore difficult to maintain in a
quiescent state, leading to excessive acceleration of their cell
cycle, exhaustion and even leukemogenesis (23). Additionally, the suppression of FBXW7
by small interference RNA in human colorectal cancer cell lines
resulted in enhanced c-Myc expression and increased proliferation
(24). In a previous study of
clinical samples, low FBXW7 expression correlated with greater
tumor size in gastric cancer and intrahepatic CC (14,25). The
present study was consistent with previous findings, as cancer
tissue samples with low FBXW7 expression exhibited enhanced nuclear
c-Myc and Ki-67 staining. Thus, the data of the present study
suggest that the greater tumor sizes observed in the low FBXW7
expression group were caused by an acceleration of the cell cycle,
and subsequently of proliferation, due to c-Myc accumulation.
Low levels of FBXW7 protein and/or messenger RNA
were revealed to be associated with poor prognoses in breast
(10,26), gastric (25), non-small cell lung (27) and esophageal cancer (12,13). The
present study demonstrated that low FBXW7 expression in CC was an
independent poor prognostic factor that outweighed existing
clinicopathological factors, including TNM stage. Thus, the
evaluation of FBXW7 expression is a potentially a useful marker for
predicting prognosis in patients with CC.
Previously, molecular pathways that regulate FBXW7
stability and cellular functions have been investigated
extensively. Gene mutation, copy number variations and
transcriptional- and post-transcriptional controls are hypothesized
to suppress FBXW7 function in cancer (28). FBXW7 gene alterations are often
detected in human malignancies, with the rate identified to be ~6%
(29). As for mechanisms for the
modification of FBXW7 activity other than gene alteration, previous
studies have revealed that FBXW7 is regulated by p53 (30,31),
microRNA (miR-223 and miR-27) (32,33) and
self-ubiquitination in a phosphorylation-dependent manner (34,35). The
mutation rate of FBXW7 in CC is ~15–35%, which is as high as in
patients with T-cell acute lymphoblastic leukemia (36,37). These
data suggest that FBXW7 mutations serve an important role in
CC development, and are one of the important suppression mechanisms
of FBXW7 in CC. However, the association between FBXW7
mutations and FBXW7 protein expression, as evaluated by
immunohistochemistry in the present study, remains unclear and
requires additional investigation.
Previous studies have examined the association
between FBXW7 and chemosensitivity. It was demonstrated that low
FBXW7 expression produced resistance to anti-tubulin agents such as
paclitaxel due to MCL1 accumulation (9,27).
Additionally, another report revealed that low FBXW7 expression is
associated with resistance to doxorubicin in hepatocellular
carcinoma (38). However,
FBXW7 depletion in leukemia-initiating cells of chronic
myeloid leukemia promotes cell cycle re-entry through c-Myc
accumulation, and leads to the high sensitivity to imatinib in
leukemia-initiating cells and thus enhanced eradication (8). Thus, FBXW7 has a dichotomous role with
respect to chemosensitivity (39). In
the present study, the cancer-specific survival in patients with CC
with low FBXW7 expression levels who underwent chemotherapy
exhibited improved prognosis compared with patients who did not,
despite there being no difference in outcomes according to
chemotherapy administration in patients with CC with high FBXW7
levels (Fig. 3D). c-Myc accumulation
and increased Ki-67 positive cells were also confirmed in the low
FBXW7 expression group. Notably, it was demonstrated that the
overexpression of c-Myc sensitized pancreatic cancer cell lines to
cisplatin (40), and that pediatric
medulloblastoma cells are sensitive to chemo- and radiation therapy
because of their high c-Myc expression (41). Based on these observations, it appears
that c-Myc accumulation as regulated by FBXW7 induces
chemosensitivity in patients with clinical CC. The evaluation of
FBXW7 expression in CC clinical samples may contribute to
predicting the efficacy of chemotherapy and selecting those
patients who are expected to benefit from it.
The present study had several limitations, including
the retrospective design, single-center cohort and disease
heterogeneity, such as intra- and extrahepatic disease, and
heterogeneous treatment groups. In particular, as aforementioned, a
sample bias of patients according to receipt of chemotherapy was an
important issue in the present study, as patients who did not wish
to undergo chemotherapy were excluded from the adaptation criteria
of chemotherapy. Accordingly, additional studies in larger series,
preferably prospective multi-center cohorts, are required to
confirm these data.
In conclusion, low expression levels of the cell
cycle regulator and tumor suppressor FBXW7 contributed to shorter
survival and greater tumor size in patients with CC. The evaluation
of FBXW7 expression levels may therefore be a useful predictor of
poor prognosis and cancer progression in CC, particularly
extrahepatic CC, considering the cohort of the present study.
Conversely, low FBXW7 expression was associated with prolonged
survival times in patients with CC treated by chemotherapy; this
suggests that FBXW7 may be a surrogate marker for predicting the
efficacy of chemotherapy in CC.
Acknowledgements
The present study was supported by Grants-in-Aid for
Scientific Research from the Japan Society for the Promotion of
Science (JSPS; grant numbers 26461969, 15K10129 and 15K10085). The
present study was also supported in part by Uehara Zaidan, the
Medical Research Encouragement Prize of The Japan Medical
Association, the Promotion Plan for the Platform of Human Resource
Development for Cancer and New Paradigms - Establishing Centers for
Fostering Medical Researchers of the Future programs by the
Ministry of Education, Culture, Sports, Science, and Technology of
Japan, and Gunma University Initiative for Advanced Research
(GIAR).
Glossary
Abbreviations
Abbreviations:
|
CC
|
cholangiocarcinoma
|
|
FBXW7
|
F-box and WD repeat domain-containing
7
|
|
TNM
|
tumor-node-metastasis
|
References
|
1
|
Rizvi S and Gores GJ: Pathogenesis,
diagnosis and management of cholangiocarcinoma. Gastroenterology.
145:1215–1229. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Blechacz B and Gores GJ:
Cholangiocarcinoma: Advances in pathogenesis, diagnosis and
treatment. Hepatology. 48:308–321. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Valle JW: Advances in the treatment of
metastatic or unresectable biliary tract cancer. Ann Oncol 21
Suppl. 7:vii345–vii348. 2010.
|
|
4
|
Valle JW, Furuse J, Jitlal M, Beare S,
Mizuno N, Wasan H, Bridgewater J and Okusaka T: Cisplatin and
gemcitabine for advanced biliary tract cancer: A meta-analysis of
two randomised trials. Ann Oncol. 25:391–398. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Davis RJ, Welcker M and Clurman BE: Tumor
suppression by the Fbw7 ubiquitin ligase: Mechanisms and
opportunities. Cancer Cell. 26:455–464. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tan Y, Sangfelt O and Spruck C: The
Fbxw7/hCdc4 tumor suppressor in human cancer. Cancer Lett.
271:1–12. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Onoyama I and Nakayama KI: Fbxw7 in cell
cycle exit and stem cell maintenance: Insight from gene-targeted
mice. Cell Cycle. 7:3307–3313. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Takeishi S, Matsumoto A, Onoyama I, Naka
K, Hirao A and Nakayama KI: Ablation of Fbxw7 eliminates
leukemia-initiating cells by preventing quiescence. Cancer Cell.
23:347–361. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wertz IE, Kusam S, Lam C, Okamoto T,
Sandoval W, Anderson DJ, Helgason E, Ernst JA, Eby M, Liu J, et al:
Sensitivity to antitubulin chemotherapeutics is regulated by MCL1
and FBW7. Nature. 471:110–114. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ibusuki M, Yamamoto Y, Shinriki S, Ando Y
and Iwase H: Reduced expression of ubiquitin ligase FBXW7 mRNA is
associated with poor prognosis in breast cancer patients. Cancer
Sci. 102:439–445. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Imura S, Tovuu LO, Utsunomiya T, Morine Y,
Ikemoto T, Arakawa Y, Kanamoto M, Iwahashi S, Saito Y, Takasu C, et
al: The role of Fbxw7 expression in hepatocellular carcinoma and
adjacent non-tumor liver tissue. J Gastroenterol Hepatol.
29:1822–1829. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yokobori T, Mimori K, Iwatsuki M, Ishii H,
Tanaka F, Sato T, Toh H, Sudo T, Iwaya T, Tanaka Y, et al: Copy
number loss of FBXW7 is related to gene expression and poor
prognosis in esophageal squamous cell carcinoma. Int J Oncol.
41:253–259. 2012.PubMed/NCBI
|
|
13
|
Kurashige J, Watanabe M, Iwatsuki M,
Kinoshita K, Saito S, Hiyoshi Y, Kamohara H, Baba Y, Mimori K and
Baba H: Overexpression of microRNA-223 regulates the ubiquitin
ligase FBXW7 in oesophageal squamous cell carcinoma. Br J Cancer.
106:182–188. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yang H, Lu X, Liu Z, Chen L, Xu Y, Wang Y,
Wei G and Chen Y: FBXW7 suppresses epithelial-mesenchymal
transition, stemness and metastatic potential of cholangiocarcinoma
cells. Oncotarget. 6:6310–6325. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Enkhbold C, Utsunomiya T, Morine Y, Imura
S, Ikemoto T, Arakawa Y, Kanamoto M, Iwahashi S, Saito Y, Ishikawa
D and Shimada M: Loss of FBXW7 expression is associated with poor
prognosis in intrahepatic cholangiocarcinoma. Hepatol Res.
44:E346–E352. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
TNM classification of malignant tumorsInt
Union Again Cancer (UICC). 7th. New York: Wiley-Liss; 2009
|
|
17
|
Huang W, Guo L, Liu H, Zheng B, Ying J and
Lv N: C-MYC overexpression predicts aggressive transformation and a
poor outcome in mucosa-associated lymphoid tissue lymphomas. Int J
Clin Exp Pathol. 7:5634–5644. 2014.PubMed/NCBI
|
|
18
|
Welcker M, Orian A, Jin J, Grim JE, Harper
JW, Eisenman RN and Clurman BE: The Fbw7 tumor suppressor regulates
glycogen synthase kinase 3 phosphorylation-dependent c-Myc protein
degradation. Proc Natl Acad Sci USA. 101:9085–9090. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yada M, Hatakeyama S, Kamura T, Nishiyama
M, Tsunematsu R, Imaki H, Ishida N, Okumura F, Nakayama K and
Nakayama KI: Phosphorylation-dependent degradation of c-Myc is
mediated by the F-box protein Fbw7. EMBO J. 23:2116–2125. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Singh AM and Dalton S: The cell cycle and
Myc intersect with mechanisms that regulate pluripotency and
reprogramming. Cell Stem Cell. 5:141–149. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Spencer CA and Groudine M: Control of
c-myc regulation in normal and neoplastic cells. Adv Cancer Res.
56:1–48. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Grandori C, Cowley SM, James LP and
Eisenman RN: The Myc/Max/Mad network and the transcriptional
control of cell behavior. Annu Rev Cell Dev Biol. 16:653–699. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Matsuoka S, Oike Y, Onoyama I, Iwama A,
Arai F, Takubo K, Mashimo Y, Oguro H, Nitta E, Ito K, et al: Fbxw7
acts as a critical fail-safe against premature loss of
hematopoietic stem cells and development of T-ALL. Genes Dev.
22:986–991. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Iwatsuki M, Mimori K, Ishii H, Yokobori T,
Takatsuno Y, Sato T, Toh H, Onoyama I, Nakayama KI, Baba H and Mori
M: Loss of FBXW7, a cell cycle regulating gene, in colorectal
cancer: Clinical significance. Int J Cancer. 126:1828–1837.
2010.PubMed/NCBI
|
|
25
|
Yokobori T, Mimori K, Iwatsuki M, Ishii H,
Onoyama I, Fukagawa T, Kuwano H, Nakayama KI and Mori M:
P53-Altered FBXW7 expression determines poor prognosis in gastric
cancer cases. Cancer Res. 69:3788–3794. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mao JH, Kim IJ, Wu D, Climent J, Kang HC,
DelRosario R and Balmain A: FBXW7 targets mTOR for degradation and
cooperates with PTEN in tumor suppression. Science. 321:1499–1502.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yokobori T, Yokoyama Y, Mogi A, Endoh H,
Altan B, Kosaka T, Yamaki E, Yajima T, Tomizawa K, Azuma Y, et al:
FBXW7 mediates chemotherapeutic sensitivity and prognosis in
NSCLCs. Mol Cancer Res. 12:32–37. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang L, Ye X, Liu Y, Wei W and Wang Z:
Aberrant regulation of FBW7 in cancer. Oncotarget. 5:2000–2015.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Akhoondi S, Sun D, von der Lehr N,
Apostolidou S, Klotz K, Maljukova A, Cepeda D, Fiegl H, Dafou D,
Marth C, et al: FBXW7/hCDC4 is a general tumor suppressor in human
cancer. Cancer Res. 67:9006–9012. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kimura T, Gotoh M, Nakamura Y and Arakawa
H: HCDC4b, a regulator of cyclin E, as a direct transcriptional
target of p53. Cancer Sci. 94:431–436. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mao JH, Perez-Losada J, Wu D, Delrosario
R, Tsunematsu R, Nakayama KI, Brown K, Bryson S and Balmain A:
Fbxw7/Cdc4 is a p53-dependent, haploinsufficient tumour suppressor
gene. Nature. 432:775–779. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Xu Y, Sengupta T, Kukreja L and Minella
AC: MicroRNA-223 regulates cyclin E activity by modulating
expression of F-box and WD-40 domain protein 7. J Biol Chem.
285:34439–34446. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lerner M, Lundgren J, Akhoondi S, Jahn A,
Ng HF, Moqadam Akbari F, Oude Vrielink JA, Agami R, Den Boer ML,
Grandér D and Sangfelt O: MiRNA-27a controls FBW7/hCDC4-dependent
cyclin E degradation and cell cycle progression. Cell Cycle.
10:2172–2183. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Min SH, Lau AW, Lee TH, Inuzuka H, Wei S,
Huang P, Shaik S, Lee DY, Finn G, Balastik M, et al: Negative
regulation of the stability and tumor suppressor function of Fbw7
by the Pin1 prolyl isomerase. Mol Cell. 46:771–783. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ji S, Qin Y, Shi S, Liu X, Hu H, Zhou H,
Gao J, Zhang B, Xu W, Liu J, et al: ERK kinase phosphorylates and
destabilizes the tumor suppressor FBW7 in pancreatic cancer. Cell
Res. 25:561–573. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Cheng Y and Li G: Role of the ubiquitin
ligase Fbw7 in cancer progression. Cancer Metastasis Rev. 31:75–87.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Churi CR, Shroff R, Wang Y, Rashid A, Kang
HC, Weatherly J, Zuo M, Zinner R, Hong D, Meric-Bernstam F, et al:
Mutation profiling in cholangiocarcinoma: Prognostic and
therapeutic implications. PLoS One. 9:e1153832014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yu J, Zhang W, Gao F, Liu YX, Chen ZY,
Cheng LY, Xie SF and Zheng SS: FBW7 increases chemosensitivity in
hepatocellular carcinoma cells through suppression of
epithelial-mesenchymal transition. Hepatobiliary Pancreat Dis Int.
13:184–191. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wang Z, Fukushima H, Gao D, Inuzuka H, Wan
L, Lau AW, Liu P and Wei W: The two faces of FBW7 in cancer drug
resistance. BioEssays. 33:851–859. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Biliran H Jr, Banerjee S, Thakur A, Sarkar
FH, Bollig A, Ahmed F, Wu J, Sun Y and Liao JD: C-Myc-induced
chemosensitization is mediated by suppression of cyclin D1
expression and nuclear factor-kappa B activity in pancreatic cancer
cells. Clin Cancer Res. 13:2811–2821. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
von Bueren AO, Oehler C, Shalaby T, von
Hoff K, Pruschy M, Seifert B, Gerber NU, Warmuth-Metz M, Stearns D,
Eberhart CG, et al: C-MYC expression sensitizes medulloblastoma
cells to radio- and chemotherapy and has no impact on response in
medulloblastoma patients. BMC Cancer. 11:742011. View Article : Google Scholar : PubMed/NCBI
|