Introduction
Particulate matter (PM), a key type of air
pollutant, is regarded as a group 1 human carcinogen by the
International Agency for Research on Cancer (1,2). A number
of epidemiological studies have demonstrated an association between
high concentrations of PM, particularly that with an aerodynamic
diameter of <2.5 µm (PM2.5), and an increased risk of
cancer development (3). In Northern
China, coal combustion is used widely and extensively in rural
areas for cooking and heating (4).
The high concentration of PM2.5 caused by this type of
energy production, and chemical and metallurgical industries, in
the cities of China may cause serious health problems in the
population (5).
Previous studies have demonstrated an association
between PM2.5 and lung cancer cell metastasis (6,7). The
results revealed that PM2.5 enhanced lung cancer cell
migration and invasion, and promoted reactive oxygen species (ROS)
levels -mediated extracellular matrix (ECM) degradation. However,
the molecular mechanisms underlying PM2.5-induced
carcinogenesis are not yet well understood.
Hepatocellular carcinoma (HCC) is one of the
predominant causes of cancer-associated mortality worldwide
(8). HCC cell metastasis is the
primary cause of HCC development. HCC metastasis occurs through
complex processes, including the migration and invasion of tumor
cells (9,10). PM2.5 induced the metastatic
capabilities of lung cancer, including migration and invasion
(11). The patient observational
reports indicated that PM2.5 exposure was associated
with HCC via chronic liver inflammation (12). The incidence of HCC may also be
associated with PM2.5, therefore the effects of
PM2.5 on HCC cells require further study.
Aberrant ROS expression may lead to a number of
physiological and pathological changes, such as cell cycle
progression (13) and apoptosis
(14,15). Notably, ROS can stimulate the
expression of numerous metastatic factors, which leads to HCC cell
migration and invasion (13,16). In addition, ROS production is an
important etiological mechanism in PM2.5-induced tissue
injury (17–19). However, whether PM2.5
affects HCC through the production of ROS is not yet known, to the
best of our knowledge.
HCC metastasis occurs through a complex mechanism,
during which matrix metalloproteinases (MMPs) are responsible for
ECM degradation (20). MMP13 is
overexpressed in numerous types of invasive tumors (21–23),
suggesting that MMP13 may be associated with the cell migration and
invasion induced by PM2.5.
The phosphoinositide 3-kinase (PI3K)-RAC-alpha
serine/threonine-protein kinase (AKT) signaling pathway is
important for the development of HCC (24,25).
Activated AKT is necessary for the metastasis, proliferation and
evasion of apoptosis of tumor cells, therefore the PI3K/AKT
signaling pathway may be activated during PM2.5-mediated
cancer cell migration and invasion.
The results from the present study demonstrate that
PM2.5 induces HCC invasion and migration, and revealed
that the underlying molecular mechanism involves the PI3K/AKT
signaling pathway, which in turn promotes ROS and MMP13
expression.
Materials and methods
Preparation of ambient
PM2.5 water-soluble extracts
A total of 50 mg of particulate matter 2.5
(PM2.5; SRM® 1650b; NIST, Boulder, CO, USA)
was suspended in 5 ml PBS for 24 h at 37°C and sonicated at 40 W
for 20 min. The PM2.5 suspension was centrifuged at
13,000 × g for 10 min at 4°C, and filtered using a 0.22-µm syringe
filter.
Cell culture and exposure
The human HCC cell lines HepG2 and HuH-7, and human
normal human HL7702 hepatocytes were purchased from the China
Center for Type Culture Collection and were cultured in Dulbecco's
modified Eagle's medium (DMEM) with 10% fetal bovine serum (FBS),
100 U/ml penicillin and 100 µg/ml streptomycin (all Invitrogen;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) in a humidified
incubator with 5% CO2 at 37°C. LY294002 was purchased
from Sigma Aldrich (#L9908; Merck KGaA, Darmstadt, Germany).
Cell invasion assay
HepG2 and HuH-7 cells (cultured at 37°C) invasion
were measured using a 24-well Matrigel®-coated
Transwell® assay, as previously described (26). Briefly, the upper surface of the
filter was coated with Matrigel (1 mg/ml) at room temperature.
Prior to the assay, HCC cells (5×105 cells/ml) were seeded into
plates, 10 µg/ml PM2.5 was added and the plates were
cultured for 24 h. The PM2.5-treated HCC cells were
harvested, and 8×104 cells in DMEM were added to the upper chamber
of the Transwell plate. DMEM medium with 10% FBS was added to the
lower chamber. Cells were allowed to migrate through the Matrigel
for 24 h. Migrated cells were fixed with 4% paraformaldehyde and
stained with crystal violet.
Transwell migration assay
Cell migration was assessed using a Transwell assay.
HCC cells (5×104) were incubated with PM2.5 at various
doses (0–10 µg/ml) for 24 h prior to seeding into the upper
chambers. DMEM containing 10% FBS was placed into the bottom
chambers. Following 8 h of incubation, cells in the upper chamber
that had not migrated were removed. The migrated cells were fixed
with 4% paraformaldehyde and stained with crystal violet. Images
were captured using an Olympus light microscope. A total of three
independent experiments were performed. The migration index was
defined as follows: (the migrated cells number in the experimental
group/the migrated cells number in the control group) ×100.
ROS assay
A total of 5×105 cells/well of HepG2 or HuH-7 cells
were seeded into 35 mm Petri dishes with DMEM containing 10% FBS.
The cells were treated with PM2.5 at various doses (0–10
µg/ml) for 6 h. The cells were harvested, resuspended in DMEM and
incubated with 2′,7′-dichlorofluoresceindiacetate (DCFH-DA; 10 µM)
at 37°C for 30 min. The intracellular ROS levels were monitored at
488 nm (excitation) and 519 nm (emission) using a confocal
fluorescence microscope and analyzed using flow cytometry. The data
were processed using the FlowJo Vx 10.0 software (Tree Star Inc.,
Ashland, OR, USA).
ELISA
MMP13 levels of HepG2 and HuH-7 cells were
determined using the Human MMP13 Quantitation ELISA kit (DM1300;
R&D Systems, Inc., Minneapolis, MN, USA) according to the
manufacturer's protocol. The optical density of the plates was read
at 450 nm (excitation) and 540 nm (emission) using a microplate
reader. The amount of MMP13 (µg/ml) was evaluated from a standard
curve and expressed as µg/ml.
Western blotting
Total protein from HepG2 and HuH-7 cells was
extracted using radioimmunoprecipitation assay lysis buffer
containing 1% protease inhibitor cocktail (#5500; R&D Systems,
Inc.). The proteins (40 µg) were separated by SDS-PAGE and
transferred onto a polyvinylidene fluoride membrane (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). The membranes were
incubated for 1 h at room temperature with 5% nonfat milk to block
nonspecific binding and then incubated with the primary antibodies,
including GAPDH (dilution, 1:100; #2118; Cell Signaling Technology,
Inc., Dancers, MA, USA) and p-AKT (dilution, 1:100; #4058; Cell
Signaling Technology, Inc.) overnight at 4°C. Following washing
with Tris-buffered saline with 0.1% Tween-20, the membranes were
incubated with the anti-rabbit (#W4011) or anti-mouse (#W4021)
immunoglobulin conjugated to horseradish peroxidase secondary
antibody (dilution, 1:500; Promega Corporation, Madison, WI, USA)
for 1 h at room temperature. The blots were visualized using
enhanced chemiluminescence kit (#32106, Thermo Fisher Scientific,
Inc.).
Cytotoxicity assay
HL7702 cell viability was measured using the Cell
Counting Kit-8 (CCK-8; Dojindo Molecular Technologies, Inc.,
Kumamoto, Japan) according to the manufacturer's protocol. HL7702
cells (5×103 cells/well) were seeded into 96-well plates overnight
and then treated with serial concentrations of PM2.5
(0–400 µg/ml) for 24 h. A total of 10 µl of CCK-8 solution was
added to each well for 1 h, and the absorbance at 450 nm was
measured using a microplate reader and analyzed using Image-Pro
Plus 6.0 software (Media Cybernetics, Inc., Shanghai, China).
Apoptosis assay
Cell apoptosis was detected using the Annexin
V-fluorescein isothiocyanate (FITC)/propidium iodide (PI) assay.
HL7702 cells (2×105 cells/well) were seeded into 6-well plates and
treated with PM2.5 (0–400 µg/ml) for 24 h. Apoptotic
cells were then identified using an Annexin V-FITC apoptosis
detection kit (BD Biosciences), according to the manufacturer's
protocol. Flow cytometry data were performed using the CellQuest
software (BD Biosciences, San Jose, CA, USA).
Statistical analysis
The data are presented as the mean ± standard
deviation of three independent experiments. All analyses were
performed using analysis of variance tests followed by a Fisher's
least significant difference test. P<0.05 was considered to
indicate a statistically significant difference.
Results
PM2.5 induces HCC cell
invasion
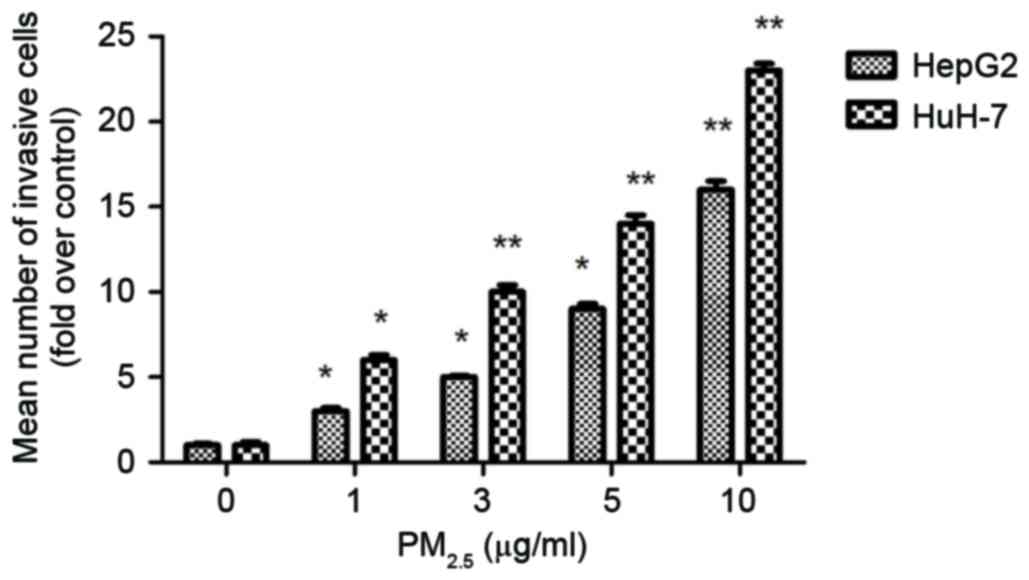
Matrigel chamber assays were used to investigate the
role of PM2.5 in cell invasion. Following
PM2.5 exposure, the number of HCC cells that migrated
from the upper chamber to the lower chamber compared with the
control (untreated cells) was significantly increased (Fig. 1), which suggested that
PM2.5 promotes HCC invasion.
PM2.5 induces HCC cell
migration
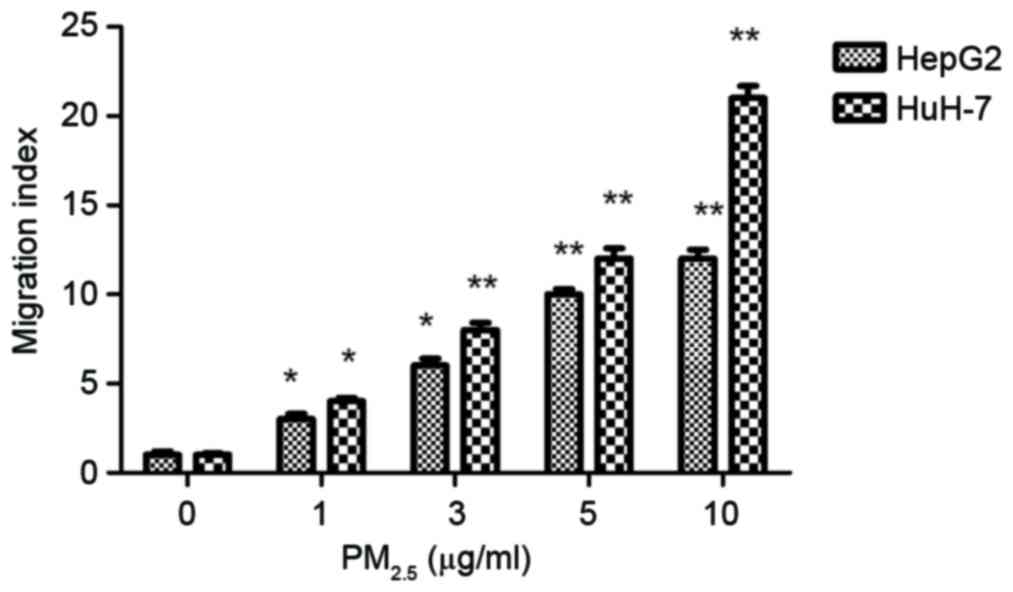
To evaluate the effect of PM2.5 on cell
motility, cell migration assays were carried out using a Transwell
assay. Following exposure to PM2.5 (1–10 µg/ml) for 24
h, the number of cells that migrated significantly increased
compared with the control (Fig. 2).
PM2.5 stimulated HCC cell migration in a dose-dependent
manner.
PM2.5 induces ROS
production in HCC cells
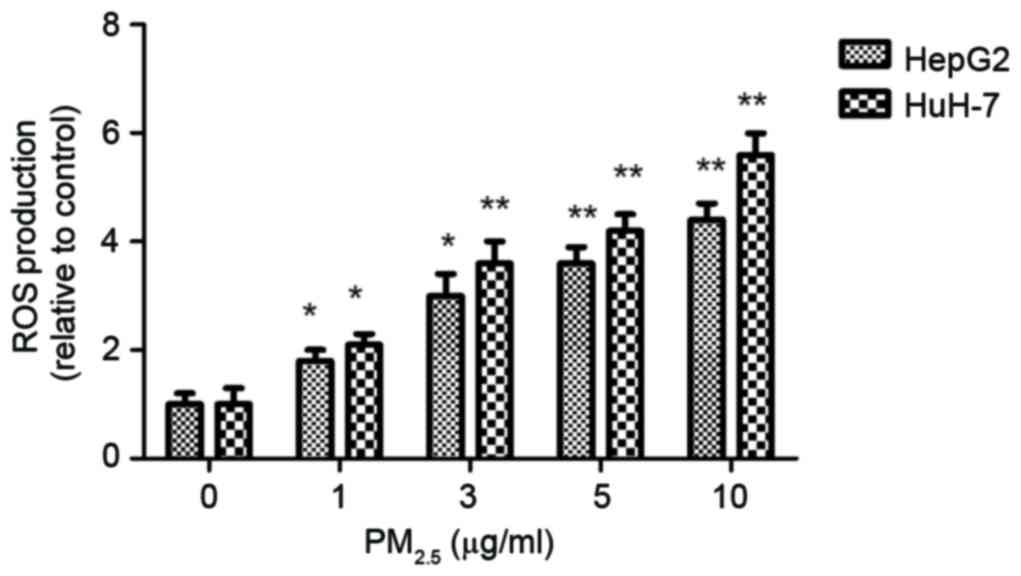
To investigate the involvement of ROS in
PM2.5-induced HCC metastasis, ROS levels were measured
following exposure to PM2.5 for 6 h. The DCFH-DA
staining data demonstrated that PM2.5 significantly
induces ROS overproduction compared with the control (Fig. 3).
Underlying molecular mechanisms of
PM2.5-induced HCC cell migration and invasion
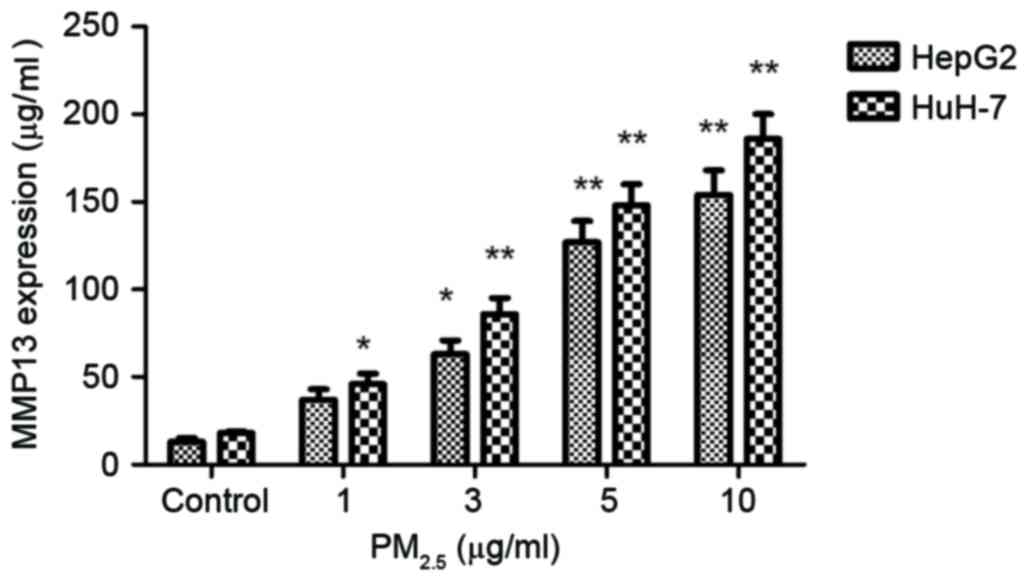
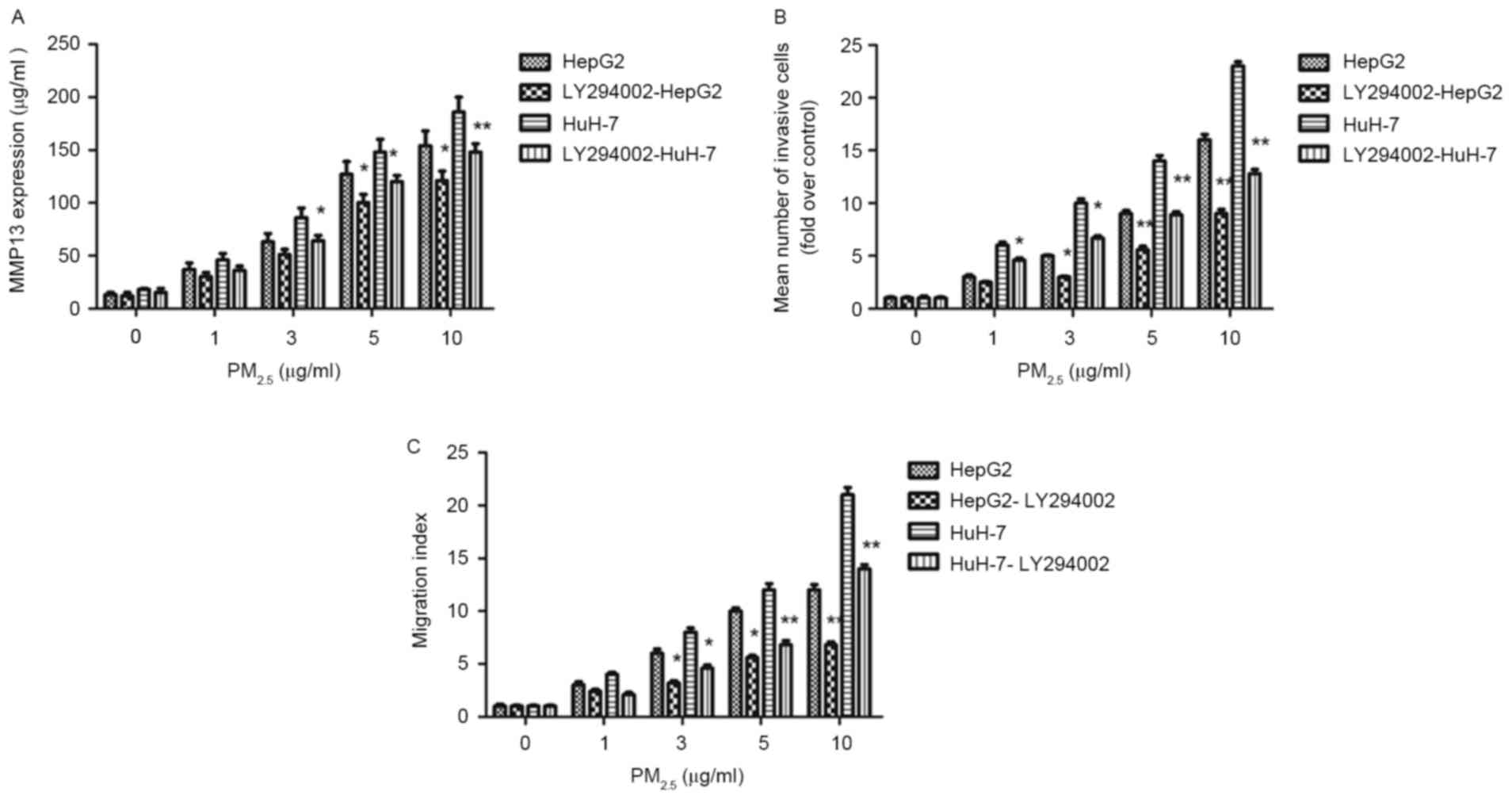
The expression of MMP13 was measured following
PM2.5 stimulation. The expression of MMP13 was
positively associated with the PM2.5 dose (1–10 µg/ml)
in HepG2 cells (Fig. 4). In addition,
MMP13 expression in HCC cells increased significantly following
PM2.5 treatment compared with the control.
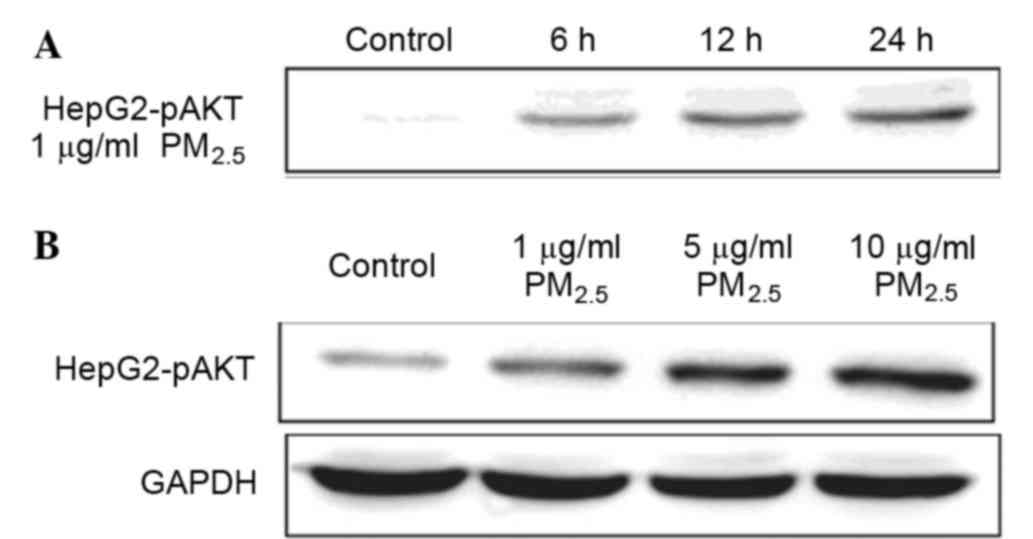
To test whether the PI3K/AKT signaling pathway was
involved in the response to PM2.5 exposure in HCC cells,
HepG2 cells were treated with PM2.5 at different doses
and time points. AKT phosphorylation increased 6 h following
PM2.5 exposure (Fig. 5).
The data revealed that PM2.5 increased levels of
phosphorylated AKT in a dose-dependent manner.
LY294002 significantly suppressed the MMP13 protein
expression induced by PM2.5 (Fig. 6A), in addition to the increased
invasion (Fig. 6B) and migration
(Fig. 6C) induced by
PM2.5. These data indicate that the inhibition of the
AKT signaling pathway reduces MMP13 expression, and may suppress
PM2.5-induced HCC migration and invasion.
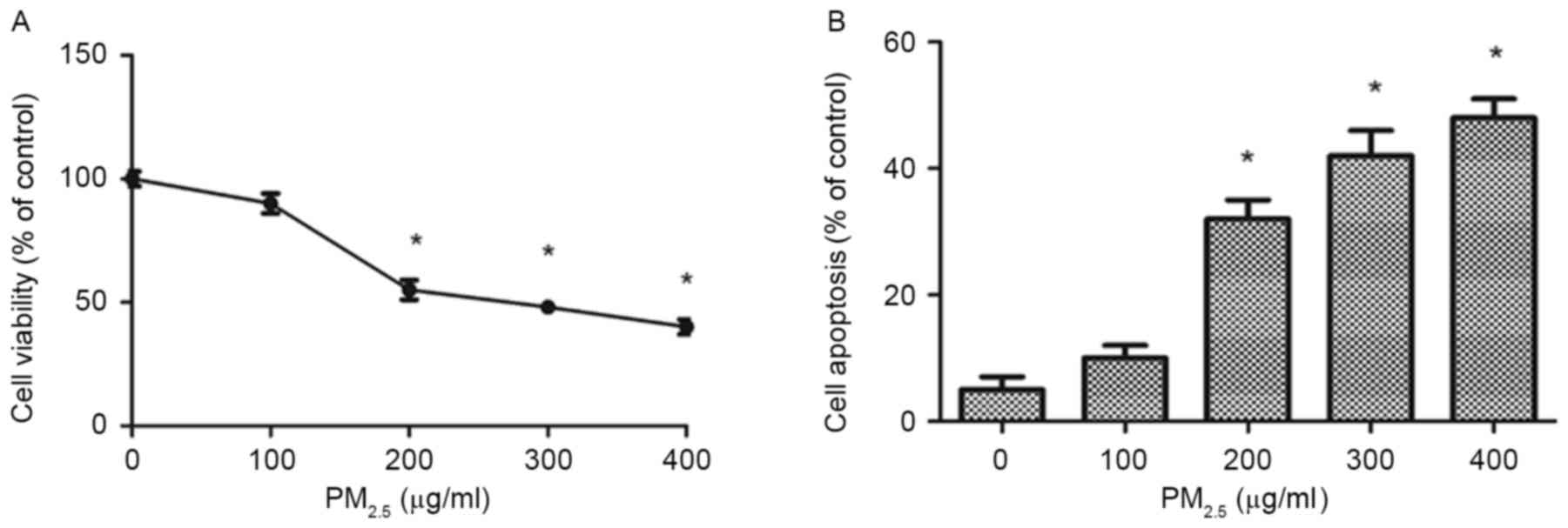
High concentrations of
PM2.5 decreases HL7702 proliferation in a dose-dependent
manner
The CCK-8 assay results revealed that 200, 300 and
400 µg/ml PM2.5 significantly reduced HL7702 viability
following exposure for 24 h compared with the control group. The
half-maximal inhibitory concentration (IC50) value of
PM2.5 was 200 µg/ml (Fig.
7A).
High concentrations of
PM2.5 induces HL7702 apoptosis in a dose-dependent
manner
The Annexin V-FITC/PI double staining assays
demonstrated that PM2.5 induced apoptosis in HL7702
cells in a dose-dependent manner (Fig.
7B). In addition, 200–400 µg/ml PM2.5 significantly
increased the rate of apoptosis in HL7702 cells following exposure
for 24 h compared with the control group.
Discussion
The aim of the present study was to explore the
effect of PM2.5 on the invasion and migration of HCC
cells, and to identify the underlying mechanisms of this effect.
The results from the present study demonstrated that
PM2.5 could induce the migration and invasion of HCC
cells. Additionally, PM2.5 increased ROS and MMP13
production in a dose-dependent manner. Western blotting results
indicated that the activation of the AKT signaling pathway may be
involved in these effects of PM2.5. The results from the
present study suggest that PM2.5 promotes the
development of HCC via inducing cell invasion and migration.
Invasion and metastasis are typical characteristics
of HCC and a contributing factor to the poor prognosis of patients
with HCC. PM2.5 exposure was associated with the risk of
developing HCC and PM2.5 exposure induced inflammation
cytokine levels that may contributed to HCC risk. Considering the
frequent occurrence of metastasis in patients with HCC, the
association of PM2.5 exposure with HCC cell invasion and
migration requires further study. HCC cell invasion is the first
step for distant metastasis, therefore increased HCC cell invasion
may have a significant effect on tumor development. The data from
the present study demonstrated that PM2.5 exposure
significantly promoted HCC migration and invasion in a
dose-dependent manner.
ROS production has been revealed to serve an
important role in mediating the cytotoxic effects of
PM2.5. Exposure to PM2.5 is regarded as a
cardiovascular risk factor via ROS overproduction (27), but whether it promotes HCC via
inducing ROS production remains unclear. In the present study,
PM2.5 significantly increased HCC cell production of ROS
in a dose-dependent manner.
MMP13 serves a crucial role in HCC invasion and
metastasis, and has demonstrated to serve a role in the chronic
inflammatory response (28). MMP13
mediates the release of inflammatory cytokines (21). Tumor necrosis factor (TNF)-α is a
proinflammatory cytokine that serves a role in the pathogenesis of
numerous diseases, including HCC (29). Therefore, PM2.5 is likely
to promote HCC development by affecting MMP13 expression, which
could promote cancer invasion and migration, in addition to
promoting the expression of inflammatory cytokines. This hypothesis
is supported by a previous study, which revealed that
PM2.5 is associated with inflammatory cytokines as it
induces TNF-α expression (30).
Previous studies have demonstrated that the PI3K/AKT
signaling pathway is associated with MMP13 expression (31). Additionally, the data from the present
study revealed that the PI3K/AKT signaling pathway was activated in
PM2.5-treated HCC cells. The AKT inhibitor LY294002
significantly decreased PM2.5-induced MMP13
overexpression in HCC cells. These findings suggest that
PM2.5-induced MMP13 upregulation is dependent on the
PI3K/AKT signaling pathway. The data also revealed that
PM2.5 effectively inhibited proliferation of HL7702
cells in vitro with an IC50 value of 200
µg/ml.
In conclusion, the present study demonstrated that
exposure to PM2.5 promotes the migration and invasion of
HCC cells. The present study also highlighted the role of the
PI3K/AKT signaling pathway and MMP13 expression in regulating
PM2.5-induced HCC cell migration and invasion. The
results demonstrating that PM2.5 exposure promotes the
invasion and migration ability of HCC cells provides an insight
into the association between a higher incidence of HCC and
PM2.5 exposure. Further studies are required to address
the chronic exposure to higher PM2.5 levels on the
effect of public health.
Acknowledgements
The present study was supported by the Provisions of
Shanghai Municipality on Science and Technology Awards from the
Shanghai Municipal Science and Technology Commission (grant no.
2013sy036).
References
|
1
|
Hou X, Strickland MJ and Liao KJ:
Contributions of regional air pollutant emissions to ozone and fine
particulate matter-related mortalities in eastern U.S. urban areas.
Environ Res. 137:475–484. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Eze IC, Schaffner E, Fischer E, Schikowski
T, Adam M, Imboden M, Tsai M, Carballo D, von Eckardstein A, Künzli
N, et al: Long-term air pollution exposure and diabetes in a
population-based Swiss cohort. Environ Int. 70:95–105. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chow JC: Health effects of fine
particulate air pollution: Lines that connect. J Air Waste Manag
Assoc. 56:707–708. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Loomis D, Grosse Y, Lauby-Secretan B, El
Ghissassi F, Bouvard V, Benbrahim-Tallaa L, Guha N, Baan R, Mattock
H and Straif K: International Agency for Research on Cancer
Monograph Working Group IARC: The carcinogenicity of outdoor air
pollution. Lancet Oncol. 14:1262–1263. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Loomis D, Huang W and Chen G: The
International Agency for Research on Cancer (IARC) evaluation of
the carcinogenicity of outdoor air pollution: Focus on China. Chin
J Cancer. 33:189–196. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Boström CE, Gerde P, Hanberg A, Jernström
B, Johansson C, Kyrklund T, Rannug A, Törnqvist M, Victorin K and
Westerholm R: Cancer risk assessment, indicators, and guidelines
for polycyclic aromatic hydrocarbons in the ambient air. Environ
Health Perspect. 110:(Suppl 3). S451–S488. 2002. View Article : Google Scholar
|
|
7
|
Xia Z, Duan X, Tao S, Qiu W, Liu D, Wang
Y, Wei S, Wang B, Jiang Q, Lu B, et al: Pollution level, inhalation
exposure and lung cancer risk of ambient atmospheric polycyclic
aromatic hydrocarbons (PAHs) in Taiyuan, China. Environ Pollut.
173:150–156. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tang D, Sun B, Yu H, Yang Z and Zhu L:
Tumor-suppressing effect of miR-4458 on human hepatocellular
carcinoma. Cell Physiol Biochem. 35:1797–1807. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gores GJ: Decade in review-hepatocellular
carcinoma: HCC-subtypes, stratification and sorafenib. Nat Rev
Gastroenterol Hepatol. 11:645–647. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang X, Ng HL, Lu A, Lin C, Zhou L, Lin
G, Zhang Y, Yang Z and Zhang H: Drug delivery system targeting
advanced hepatocellular carcinoma: Current and future.
Nanomedicine. 12:853–869. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shu Y, Zhu L, Yuan F, Kong X, Huang T and
Cai YD: Analysis of the relationship between PM2.5 and lung cancer
based on protein-protein interactions. Comb Chem High Throughput
Screen. 19:100–108. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pan WC, Wu CD, Chen MJ, Huang YT, Chen CJ,
Su HJ and Yang HI: Fine Particle Pollution, Alanine Transaminase,
and Liver Cancer: A Taiwanese Prospective Cohort Study
(REVEAL-HBV). J Natl Cancer Inst. 108:pii: djv341. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Deng G, Hu C, Zhu L, Huang F, Huang W, Xu
H and Nie W: Downregulation of ROS-FIG inhibits cell proliferation,
colony formation, cell cycle progression, migration and invasion,
while inducing apoptosis in intrahepatic cholangiocarcinoma cells.
Int J Mol Med. 34:661–668. 2014.PubMed/NCBI
|
|
14
|
Shao J, Xue J, Dai Y, Liu H, Chen N, Jia L
and Huang J: Inhibition of human hepatocellular carcinoma HepG2 by
phthalocyanine photosensitiser PHOTOCYANINE: ROS production,
apoptosis, cell cycle arrest. Eur J Cancer. 48:2086–2096. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hou YQ, Yao Y, Bao YL, Song ZB, Yang C,
Gao XL, Zhang WJ, Sun LG, Yu CL, Huang YX, et al: Juglanthraquinone
C Induces Intracellular ROS Increase and Apoptosis by Activating
the Akt/Foxo Signal Pathway in HCC Cells. Oxid Med Cell Longev.
2016:49416232016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Adhikary A, Mohanty S, Lahiry L, Hossain
DM, Chakraborty S and Das T: Theaflavins retard human breast cancer
cell migration by inhibiting NF-kappaB via p53-ROS cross-talk. FEBS
Lett. 584:7–14. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhou W, Tian D, He J, Wang Y, Zhang L, Cui
L, Jia L, Zhang L, Li L, Shu Y, et al: Repeated PM2.5 exposure
inhibits BEAS-2B cell P53 expression through ROS-Akt-DNMT3B
pathway-mediated promoter hypermethylation. Oncotarget.
7:20691–21703. 2016.PubMed/NCBI
|
|
18
|
Torres-Ramos YD, Montoya-Estrada A,
Guzman-Grenfell AM, Mancilla-Ramirez J, Cardenas-Gonzalez B,
Blanco-Jimenez S, Sepulveda-Sanchez JD, Ramirez-Venegas A and Hicks
JJ: Urban PM2.5 induces ROS generation and RBC damage in COPD
patients. Front Biosci (Elite Ed). 3:808–817. 2011.PubMed/NCBI
|
|
19
|
Verma V, Fang T, Xu L, Peltier RE, Russell
AG, Ng NL and Weber RJ: Organic aerosols associated with the
generation of reactive oxygen species (ROS) by water-soluble PM2.5.
Environ Sci Technol. 49:4646–4656. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang YH, Sui XM, Sui YN, Zhu QW, Yan K,
Wang LS, Wang F and Zhou JH: BRD4 induces cell migration and
invasion in HCC cells through MMP-2 and MMP-9 activation mediated
by the Sonic hedgehog signaling pathway. Oncol Lett. 10:2227–2232.
2015.PubMed/NCBI
|
|
21
|
Yang Z, Zhang Y and Wang L: A feedback
inhibition between miRNA-127 and TGFβ/c-Jun cascade in HCC cell
migration via MMP13. PLoS One. 8:e652562013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kotepui M, Thawornkuno C,
Chavalitshewinkoon-Petmitr P, Punyarit P and Petmitr S:
Quantitative real-time RT-PCR of ITGA7, SVEP1, TNS1, LPHN3, SEMA3G,
KLB and MMP13 mRNA expression in breast cancer. Asian Pac J Cancer
Prev. 13:5879–5882. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shah M, Huang D, Blick T, Connor A, Reiter
LA, Hardink JR, Lynch CC, Waltham M and Thompson EW: An
MMP13-selective inhibitor delays primary tumor growth and the onset
of tumor-associated osteolytic lesions in experimental models of
breast cancer. PLoS One. 7:e296152012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang Y, Huang X, Han J, Zheng W and Ma W:
Extract of Perilla frutescens inhibits tumor proliferation of HCC
via PI3K/AKT signal pathway. Afr J Tradit Complement Altern Med.
10:251–257. 2013.PubMed/NCBI
|
|
25
|
Sui Y, Zheng X and Zhao D: Rab31 promoted
hepatocellular carcinoma (HCC) progression via inhibition of cell
apoptosis induced by PI3K/AKT/Bcl-2/BAX pathway. Tumour Biol.
36:8661–8670. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li W, Jiang G, Zhou J, Wang H, Gong Z,
Zhang Z, Min K, Zhu H and Tan Y: Down-regulation of miR-140 induces
EMT and promotes invasion by targeting Slug in esophageal cancer.
Cell Physiol Biochem. 34:1466–1476. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cao J, Qin G, Shi R, Bai F, Yang G, Zhang
M and Lv J: Overproduction of reactive oxygen species and
activation of MAPKs are involved in apoptosis induced by PM2.5 in
rat cardiac H9c2 cells. J Appl Toxicol. 36:609–617. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jin D, Tao J, Li D, Wang Y, Li L, Hu Z,
Zhou Z, Chang X, Qu C and Zhang H: Golgi protein 73 activation of
MMP-13 promotes hepatocellular carcinoma cell invasion. Oncotarget.
6:33523–33533. 2015.PubMed/NCBI
|
|
29
|
Ji Y, Wang Z, Li Z, Zhang A, Jin Y, Chen H
and Le X: Angiotensin II Enhances Proliferation and Inflammation
through AT1/PKC/NF-κB Signaling Pathway in Hepatocellular Carcinoma
Cells. Cell Physiol Biochem. 39:13–32. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tong GQ, Zhang ZH, Zhao Y, Liu JJ and Han
JB: Traffic-related PM2.5 induces cytosolic [Ca2+]
increase regulated by Orai1, alters the CaN-NFAT signaling pathway,
and affects IL-2 and TNF-α cytoplasmic levels in Jurkat T-cells.
Arch Environ Contam Toxicol. 68:31–37. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Xu L, Sun K, Xia M, Li X and Lu Y: MMP13
regulates aggressiveness of pediatric multiple myeloma through
VEGF-C. Cell Physiol Biochem. 36:509–516. 2015. View Article : Google Scholar : PubMed/NCBI
|