Introduction
Breast cancer is the most prevalent cancer in
females worldwide, with an overall lifetime risk of >10%
(1). Paclitaxel, isolated from the
bark of Taxus brevifolia, is a potent chemotherapeutic agent
used in the treatment of breast cancer (2). Paclitaxel mechanistically interferes
with the dynamic instability of microtubules, leading to G2/M cell
cycle arrest and subsequent apoptosis (3). However, paclitaxel chemotherapy has had
limited success (4) due to the
activation of cytoprotective signaling pathways in tumors,
including the nuclear factor kappa B (NF-κB), AKT serine/threonine
kinase (Akt) and mitogen-activated protein kinase signaling
pathways, which induce drug resistance (3–6). In
addition, paclitaxel has been implicated in the regulation of
NF-κB-dependent genes that promote cell survival and inhibit
apoptosis, including apoptosis regulator Bcl-2 and Bcl-2 like 1
(Bcl-xL) (7). Therefore, agents that
inhibit paclitaxel-induced NF-κB activation may be effective
chemotherapeutic candidates for the treatment of breast cancer.
Sulforaphane (SFN), an isothiocyanate naturally
present in cruciferous vegetables, including broccoli, sprouts and
kale, has been reported to reduce the risk of developing a number
of common types of cancer, including breast cancer (8,9). Previous
studies have demonstrated that SFN induces G2/M cell cycle arrest
by elevating cyclin B1 expression, and induces apoptosis by
activating poly(ADP-ribose) polymerase 1 and caspase family
proteins in human breast cancer cell lines (8,10). In
addition, SFN was reported to attenuate histone deacetylase
activity in prostate epithelial cells (11) and inhibit tubulin polymerization in
breast cancer cells (12).
Furthermore, SFN has been demonstrated to inhibit NF-κB activation
in various diseases, including cancer, skin disorders and spinal
cord injury, by inhibiting NF-κB inhibitor alpha (IκBα)
phosphorylation, IκBα degradation and p65 nuclear translocation
(13). Therefore, SFN may be an
effective chemotherapeutic agent.
Building upon previous paclitaxel and SFN research,
the present study investigated the effect of combined
SFN-paclitaxel treatment on breast cancer cells. The underlying
molecular mechanisms of combined SFN and paclitaxel treatment were
investigated by evaluating the inhibition of the anti-apoptotic
signal transducers Akt and NF-κB. It was observed that treatment
with SFN inhibits paclitaxel-induced NF-κB activation, Bcl-2 gene
expression and proliferation in breast cancer cells.
Materials and methods
Cell culture
The human breast cancer cell lines MDA-MB-231 and
MCF-7 were purchased from the American Type Culture Collection
(Manassas, VA, USA). Cells were cultured in Dulbecco's modified
Eagle's medium supplemented with 10% fetal bovine serum (Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) and antibiotics
(100 µg/ml streptomycin and 100 U/ml penicillin; Thermo Fisher
Scientific, Inc.), and maintained at 37°C in a humidified
atmosphere containing 5% CO2. Overexpression of Bcl-2
was induced by transfecting a PC3.1-Bcl-2 plasmid into MDA-MB-231
cells with Lipofectamine® 2000 (Invitrogen; Thermo
Fisher Scientific, Inc.): At 1 day prior to transfection,
1×105 cells in 500 µl of growth medium without
antibiotics were seeded in each well of a 24-well plate. When
90–95% confluence was obtained, 0.8 µg of the PC3.1-Bcl-2 plasmid
in serum-free 50 µl Opti-MEM I (cat. no. 31985; Invitrogen; Thermo
Fisher Scientific, Inc.) was prepared, and 2 µl of
Lipofectamine® 2000 was diluted with 50 µl of Opti-MEM
I. After waiting 5 min, the diluted Lipofectamine® 2000
was combined with the diluted PC3.1-Bcl-2 plasmid for a total
volume of 100 µl, gently mixed, and left to stand for 20 min at
room temperature. The 100 µl volume was then added to each well.
Following transfection, a stable Bcl-2-overexpressing cell line was
generated using neomycin (1000 µg/µl) to select cells. Cells were
then maintained at 37°C, in a humidified atmosphere with 5%
CO2.
Reagents
Propidium iodide (PI), MTT, paclitaxel and SFN were
obtained from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). The
Caspase 3, 8 and 9 Fluorometric Assay kit was obtained from R&D
Systems, Inc. (Minneapolis, MN, USA). The rabbit anti-human primary
antibodies anti-Bcl-2 (cat no. MABC573), cytochrome c (cat no.
04–1043), caspase-3 (cat no. AB1899), caspase-8 (cat no. 06–775)
and caspase-9 (cat no. AB16969) were purchased from Calbiochem (La
Jolla, CA, USA). The rabbit anti-human primary antibodies directed
against Akt (cat no. 4691), phosphorylated (P)-Akt (cat no. 4060),
IκBα (cat no. 4812), GADPH (cat. no. 5174) and P-IκB kinase (P-IKK)
(cat no. 2697) were purchased from Cell Signaling Technology, Inc.
(Danvers, MA, USA). The primary antibodies were used at a dilution
of 1:1,000. All other chemicals not specifically cited here were
purchased from Sigma-Aldrich (Merck Millipore).
Cell viability assay
Cells were grown to 70% confluence and treated with
increasing concentrations (0–20 µM) of SFN, alone or in combination
with 10 nM paclitaxel. Control untreated cells were incubated, with
complete media containing 0.1% dimethyl sulfoxide, at 37°C with 5%
CO2 in a humidified atmosphere, for 24 h. Following
treatment, cell number and viability was determined using an MTT
assay. A volume of 200 µl DMEM containing 2×104 cells
was seeded in each well of 48-well plates. Following treatment with
SFN and paclitaxel, the plates were incubated for 2 days. For each
measurement, 50 µl MTT (5 mg/ml) was added into each well and
incubated at 37°C with 5% CO2 for 2 h. The wells were
then decanted, and formed formazan crystals were dissolved in 200
µl DMSO. The absorbance of the plate was measured at 595 nm in an
ELISA plate reader. A hemocytometer was additionally used for
viable cell counts. All assays were performed in triplicate.
Flow cytometric analysis
Cells were seeded in 6-well plates at a density of
2×105 cells/well and subsequently treated with
increasing concentrations (0–20 µM) SFN and paclitaxel alone, or
combined, at 37°C for 24 h. Treated and control cells were
harvested by trypsinization and stained with PI, according to the
manufacturer's protocol. Cell cycle progression was determined
using flow cytometry (FACSCalibur™; BD Biosciences, Franklin Lakes,
NJ, USA) and data analysis was performed using FlowJo 9.3 software
for Mac OS X (Tree Star, Inc., Ashland, OR, USA). To detect
apoptosis, cells were harvested and stained using the Annexin-V
Apoptosis Detection kit (R&D Systems, Inc.) In brief, the cells
were harvested and washed with cold PBS, centrifuged at ~300 × g
for 5 to 10 min at room temperature, resuspended in 100 µl of the
binding buffer from the kit containing 2.5 µl FITC conjugated
Annexin-V and incubated for 15 min at room temperature in the dark.
A total of >10,000 events were detected and analyzed by flow
cytometry.
Mitochondrial labelling within live
cells
SFN-treated cells were stained with 1 µg/ml
MitoTracker for 15 min and observed under a fluorescence
microscope. Samples were then further analyzed by FACScan (BD
Biosciences). Data analysis was performed using FlowJo 9.3 software
(Tree Star, Inc.).
Western blotting
Cells were washed with cold PBS and lysed in a
commercial lysis buffer PRO-PREP® (Intron Biotechnology
Inc., Seongnam, Korea). Protein concentration was determined using
a Bio-Rad Protein Assay kit II (cat. no. 5000002; Bio-Rad
Laboratories, Inc., Hercules, CA, USA) according to the
manufacturer's protocol. Total protein (30 µg) was separated using
12% SDS-PAGE and transferred to a nitrocellulose membrane
(Schleicher & Schuell BioScience, Inc., Keene, NH, USA). The
primary antibodies were incubated with the membrane at 4°C
overnight. Membranes were then washed and incubated with 5% skimmed
milk in tris-buffered saline with Tween 20 for 1 h. Anti-rabbit IgG
conjugated to horseradish peroxidase (cat no. 7074; Cell Signaling
Technology, Inc.) was used as the secondary antibody. The secondary
antibody (dilution, 1:2,000) was incubated at room temperature, for
1 h. Protein bands were visualized using an Amersham Imager 600
enhanced chemiluminescence detection system (GE Healthcare Life
Sciences, Chalfont, UK). GADPH was used as a protein loading
control.
Preparation of cytosolic extracts
Cells were harvested by gentle scraping and washed
in PBS. For preparation of cytosolic extracts, cells were
resuspended in ice-cold lysis buffer (250 mM sucrose, 80 mM KCl, 50
µg/ml digitonin, PMSF, and complete™ protease inhibitor in PBS).
Following 5 min of incubation on ice the cell suspensions were
centrifuged at 10,000 × g for 5 min at-20°C and the supernatants
were frozen at −70°C until analysis by western blotting.
Electrophoretic mobility shift assay
(EMSA)
EMSA was performed on nuclear extracts. Briefly, the
preparation of nuclear extracts was conducted using NE-PER nuclear
extraction reagents (Pierce; Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol. Briefly, MDA-MB231 cells
treated with SFN and paclitaxel, were harvested using trypsin-EDTA
and subsequently washed with PBS. Cytosolic proteins were first
extracted by disrupting the cell membranes, followed by
centrifugation at 16,000 × g for 5 min at −20°C. Intact nuclei were
washed with cold PBS and then lysed with high salt NE-PER buffer.
Nuclear fractions were stored at-80°C for use in subsequent
experiments. A synthetic complementary NF-κB-binding
oligonucleotide (5′-AGTTGAGGGGACTTTCCCAGGC-3′; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) was 3′-biotinylated with a
Pierce Biotin 3′ End DNA Labeling kit used according to the
manufacturer's protocol (Thermo Fisher Scientific, Inc.). Binding
reactions were conducted with 50 ng/µl poly (dI-dC), 0.05% Nonidet
P-40, 5 mM MgCl2, 10 mM EDTA and 2.5% glycerol in 1X
binding buffer (Pierce LightShift™ Chemiluminescent EMSA kit,
Thermo Fisher Scientific, Inc.), using 20 fmol of
biotin-end-labeled target DNA and 10 µg of nuclear extract, for 20
min at room temperature. Assays were loaded onto native 4%
polyacrylamide gels, pre-electrophoresed for 60 min in 0.5X Tris
borate in EDTA and electrophoresed at 100 V, followed by transfer
onto a positively charged nylon membrane (Hybond™-N+; GE
Healthcare Life Sciences) in 0.5x Tris borate/EDTA at 100 V for 30
min. Transferred DNAs were cross-linked to the membrane at 120
mJ/cm2 and detected using horseradish
peroxidase-conjugated streptavidin (from the LightShift™
Chemiluminescent EMSA kit) according to the manufacturer's
instructions.
Determination of caspase activity
Caspase activity was determined using the Caspase 3,
8 and 9 Fluorometric Assay kit (R&D Systems, Inc.) according to
the manufacturer's protocol. The kit utilized synthetic
tetrapeptides labeled with P-nitroaniline. Cells were lysed in the
supplied lysis buffer, and supernatants were collected by
centrifugation (10,000 × g, 20 min, 4°C), then incubated at 37°C
with the supplied reaction buffer. Caspase activity was determined
by measuring the change in absorbance at 405 nm with a microplate
reader.
Statistical analysis
Values are presented as the mean ± standard
deviation of triplicate data. The statistical significance of
differences was assessed using a two-way ANOVA followed by
Bonferroni's multiple comparison test. GraphPad Software 6 was used
for statistical analyses (GraphPad Software Inc., La Jolla, CA,
USA). P<0.05 was considered to indicate a statistically
significant difference; P<0.01 and P<0.001 were additionally
considered as further thresholds of significance.
Results
Combined treatment with SFN and
paclitaxel reduces human breast cancer cell viability
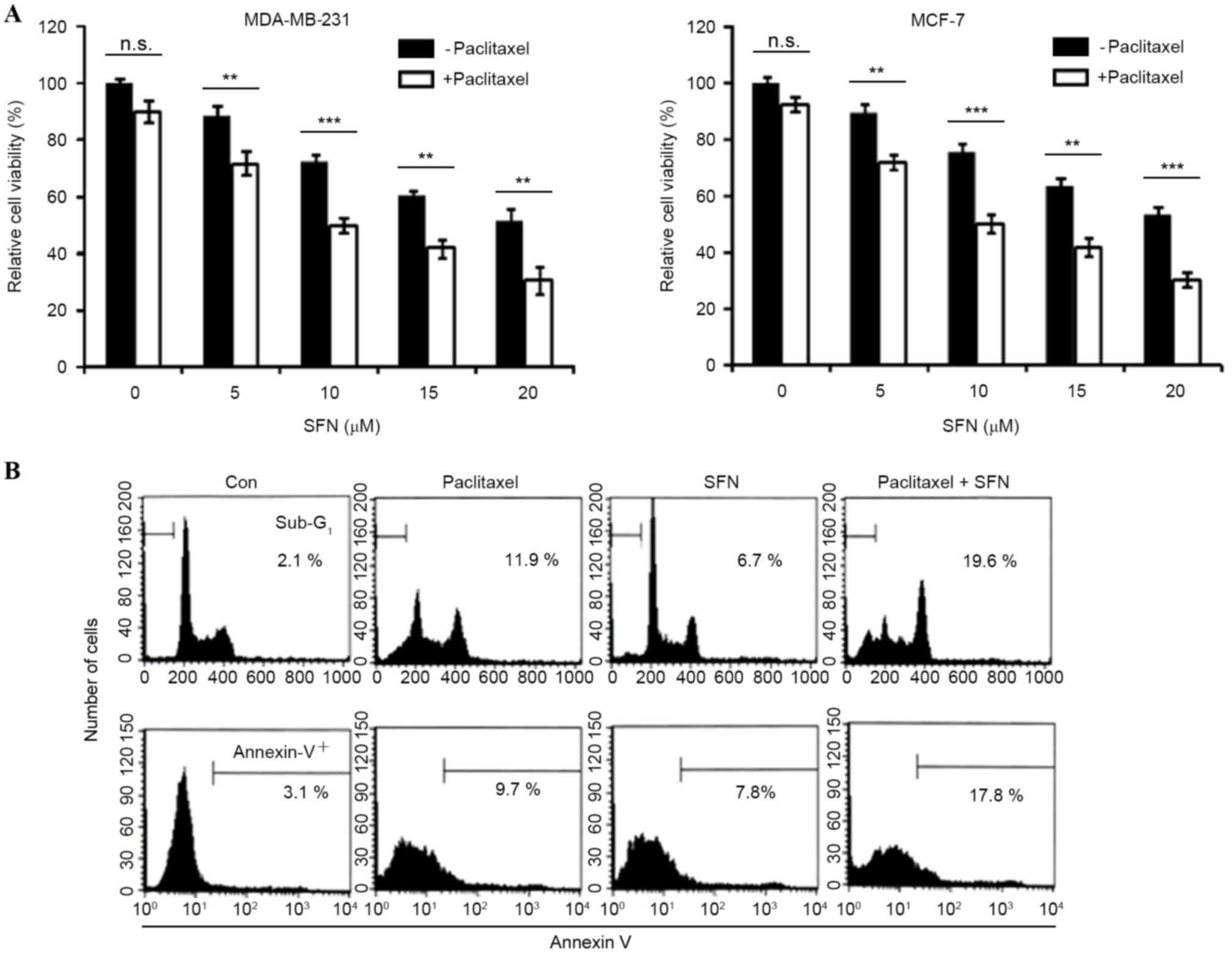
The effect of paclitaxel on cell viability was
analyzed in MDA-MB-231 and MCF-7 breast cancer cell lines.
Treatment with 10 nM paclitaxel induced limited inhibition of cell
viability (<10%) at 24 h, suggesting that these cells are
resistant to the apoptotic effects of paclitaxel. The effect of
combined treatment with SFN and paclitaxel on cell viability was
also examined. Cell viability was significantly decreased by
combined treatment with SFN and paclitaxel (MDA-MB231, P=0.001,
0.0001, 0.009, 0.001, respectively; MCF-7, P=0.005, 0.001, 0.004,
0.0002, respectively, for paclitaxel with SFN doses 5, 10, 15 and
20 µM, compared with SFN-only groups; Fig. 1A). The dependence of combined SFN and
paclitaxel treatment on apoptosis was subsequently investigated. As
shown in Fig. 1B, treatment of
MDA-MB2-31 cells with 5 µM SFN and 10 nM paclitaxel for 24 h
markedly increased the accumulation of sub-G1 phase cells and
Annexin V staining (Fig. 1B). These
results indicate that SFN enhances paclitaxel-induced apoptosis and
reduces viability in breast cancer cells.
Combined SFN-paclitaxel treatment
activates extrinsic and intrinsic apoptotic pathways
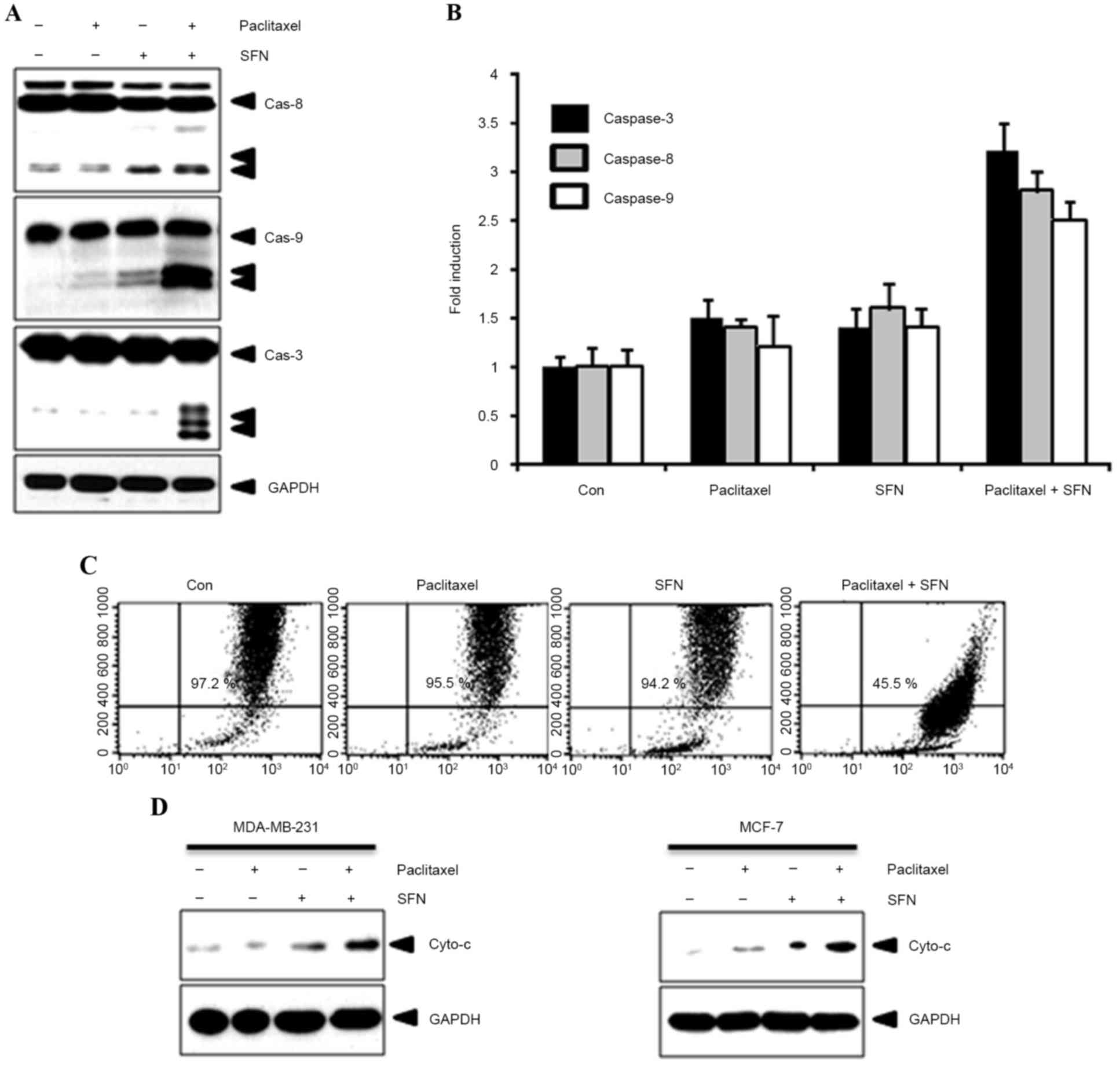
To confirm the effect of combined treatment with SFN
and paclitaxel on apoptosis, caspase-8, −9 and −3 expression and
activity was evaluated using western blotting and caspase activity
kits, respectively. As shown in Fig.
2A, cleavage of caspase-8, −9 and −3 was observed following
treatment with SFN and paclitaxel. By contrast, cleaved caspases
were not observed in cells treated with SFN or paclitaxel alone.
Furthermore, caspase activity was increased following treatment
with SFN and paclitaxel (Fig. 2B).
Apoptotic events in the mitochondria were detected by measuring the
mitochondrial membrane potential with 3,3′-dihexyloxacarbocyanine
iodide. A marked reduction in mitochondrial membrane potential was
observed in cells following treatment with SFN and paclitaxel
(Fig. 2C). In addition, this process
was accompanied by the release of cytochrome c from the
mitochondria into the cytosol (Fig.
2D). These results indicate that combined SFN-paclitaxel
treatment increases apoptosis in breast cancer cells.
SFN inhibits paclitaxel-induced NF-κB
activation and IκBα degradation
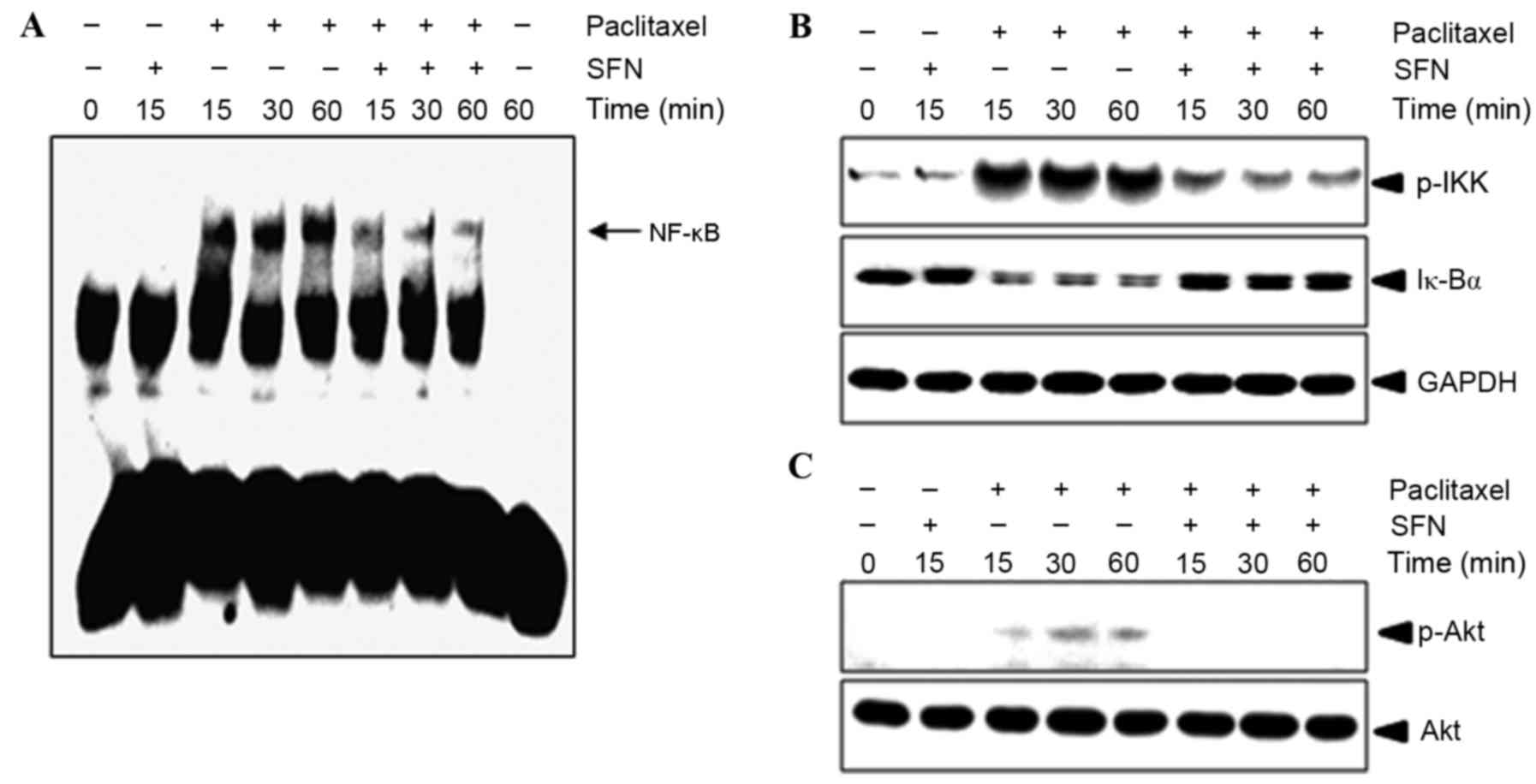
The effect of treatment with SFN on
paclitaxel-mediated NF-κB activation was evaluated using an EMSA.
As shown in Fig. 3A, 10 nM paclitaxel
induced an increase in NF-κB binding to complementary
oligonucleotides after 15 min, which was sustained for 1 h.
Pretreatment with SFN inhibited paclitaxel-induced NF-κB activation
in a time-dependent manner (Fig. 3A).
To confirm whether paclitaxel-induced NF-κB activation was caused
by IKK phosphorylation and IκBα degradation, western blotting of
P-IKK and IκBα was performed. As shown in Fig. 3B, IKK phosphorylation and IκBα
degradation began 15 min following treatment with paclitaxel, but
was markedly decreased following pretreatment with SFN. These
results suggest that paclitaxel induces NF-κB activation in
MDA-MB-231 cells through the degradation of IκBα and that SFN
inhibits IκBα degradation. Since Akt signaling was previously
demonstrated to regulate NF-κB activation (5,14), it was
evaluated in the present study whether SFN had an effect on the
phosphorylation of AKT. Pretreatment with SFN abolished
paclitaxel-induced Akt phosphorylation (Fig. 3C), indicating that Akt serves a role
in the mechanism of action of SFN.
Bcl-2 overexpression attenuates
paclitaxel- and SFN- induced apoptosis
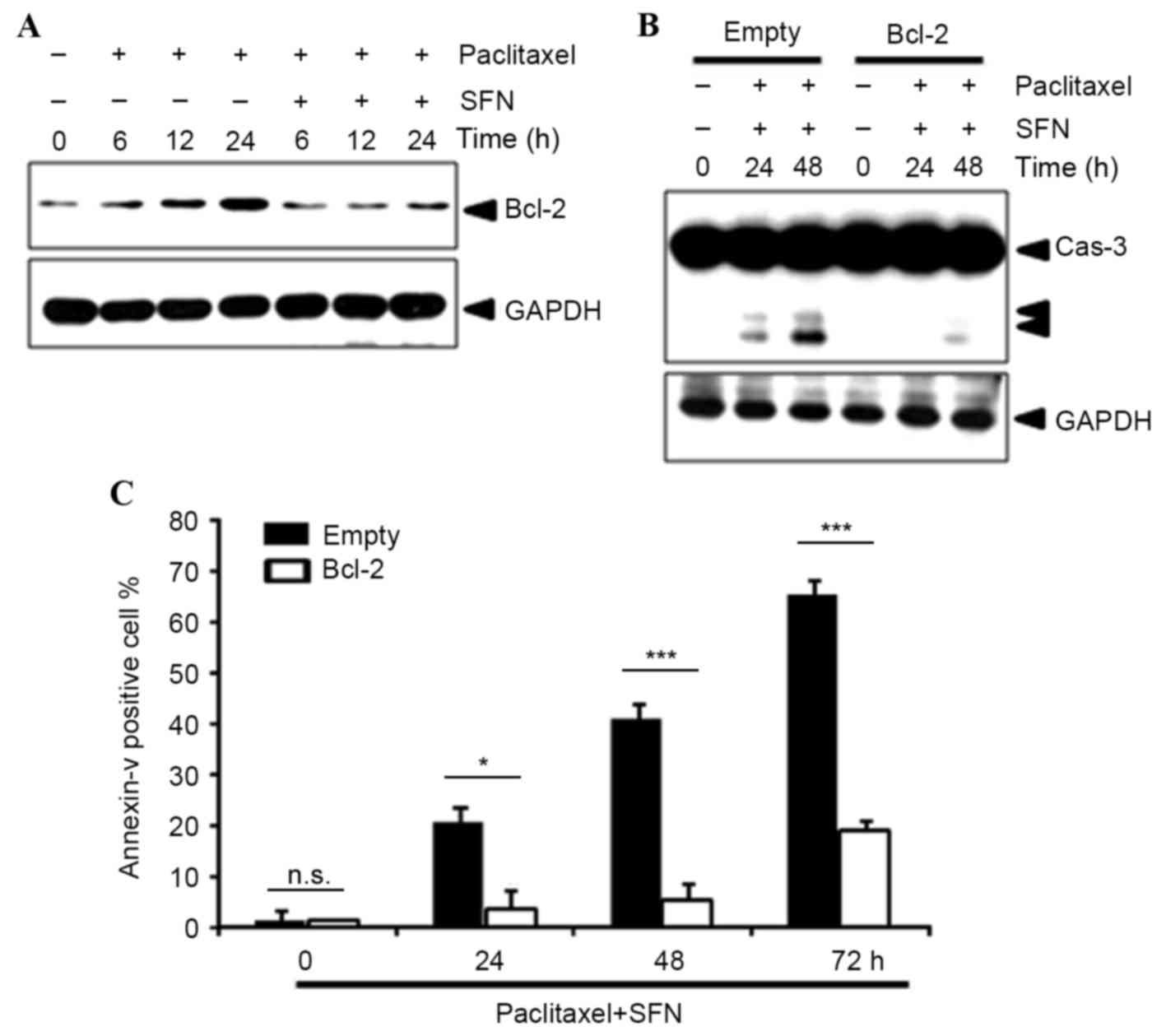
The level of protein expression of the
NF-κB-dependent survival gene Bcl-2 was examined using western
blotting. This demonstrated that pretreatment with SFN markedly
decreased Bcl-2 protein expression (Fig.
4A), suggesting that SFN-mediated NF-κB inhibition leads to
repression of Bcl-2 expression. In addition, overexpression of
Bcl-2 notably inhibited paclitaxel/SFN-induced caspase-3 activation
(Fig. 4B) and significantly inhibited
Annexin V staining (P=0.033, 0.0001, 0.0001, at 24, 48 and 72 h,
respectively; Fig. 4C).
Discussion
Paclitaxel, a microtubule stabilizer, is frequently
used as a chemotherapeutic agent in the treatment of various types
of human carcinoma, including breast cancer (2). However, paclitaxel frequently induces
drug resistance (2–5). The present study investigated the
synergistic anticancer effect of combined treatment with SFN and
paclitaxel in human breast cancer cells. Co-treatment with SFN
enhanced paclitaxel-induced apoptosis via increased activation of
caspase-3, −8 and −9. Additionally, co-treatment with SFN inhibited
paclitaxel-induced NF-κB activation, Bcl-2 expression and Akt
phosphorylation in MDA-MB-231 cells. The results of the present
study suggest that SFN and paclitaxel exhibit chemopreventive
activity by inducing breast cancer cell apoptosis.
NF-κB is a ubiquitously-expressed transcription
factor that regulates the expression of various genes associated
with cell proliferation, metastasis, cell cycle progression,
angiogenesis and apoptosis. Furthermore, the role of NF-κB in
chemoresistance is well-established (15,16).
Paclitaxel may activate NF-κB through activation of its primary
kinase, IKK (2,3). The results of the present study indicate
that paclitaxel activates NF-κB in human breast cancer cells
through the classic NF-κB activation signaling pathway, IKK
activation and IκBα degradation. Treatment of breast cancer cells
with SFN suppressed paclitaxel-induced IKK activation, leading to
suppression of NF-κB activation.
A previous study (17)
demonstrated that paclitaxel activates Akt, a serine/threonine
protein kinase, which is a downstream target of phosphoinositide
3-kinase. In the present study, SFN downregulated
paclitaxel-induced Akt activation in MDA-MB-231 cells. Similarly,
previous studies have shown that treatment with LY294002, a
specific inhibitor of phosphoinositide 3-kinase, resulted in
enhancement of paclitaxel-induced apoptosis through suppression of
NF-κB transcriptional activity (18,19).
Several previous studies have shown that NF-κB induces the
expression of cyclin D1, Bcl-2 and Bcl-xL (20,21). The
results of the present study demonstrated that the protein
expression levels of Bcl-2 induced by paclitaxel were decreased
following treatment with SFN due to inactivation of NF-κB
signaling. Furthermore, Bcl-2 overexpression reversed the effect of
combined treatment with SFN and paclitaxel on caspase-3 activity.
Therefore, the inhibition of breast cancer cell viability by SFN
may be mediated by the inhibition of Bcl-2. In conclusion, SFN
enhances the anti-tumorigenic activity of paclitaxel in breast
cancer cells by modulating NF-κB activation and Akt inhibition. The
results of the present study indicate that combined treatment with
paclitaxel and SFN is an effective clinical strategy for the
treatment of breast cancer.
Acknowledgements
The present study was supported by a Daegu
University 2012 Research Grant (Daegu University, Gyeongsan,
Korea).
References
|
1
|
Feuer EJ, Wun LM, Boring CC, Flanders WD,
Timmel MJ and Tong T: The life time risk of developing breast
cancer. J Natl Cancer Inst. 85:892–897. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Valero V and Hortobagyi GN: Are
anthracycline-taxane regimens the new standard of care in the
treatment of metastatic breast cancer? J Clin Oncol. 21:959–962.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wahl AF, Donaldson KL, Fairchild C, Lee
FY, Foster SA, Demers GW and Galloway DA: Loss of normal p53
function confers sensitization to Taxol by increasing G2/M arrest
and apoptosis. Nat Med. 2:72–79. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Haldar S, Chintapalli J and Croce CM:
Taxol induces bcl-2 phosphorylation and death of prostate cancer
cells. Cancer Res. 56:1253–1255. 1996.PubMed/NCBI
|
|
5
|
Yu D, Liu B, Tan M, Li J, Wang SS and Hung
MC: Overexpression of c-erbB-2/neu in breast cancer cells confers
increased resistance to Taxol via mdr-1-independent mechanisms.
Oncogene. 13:1359–1365. 1996.PubMed/NCBI
|
|
6
|
Pianetti S, Arsura M, Romieu-Mourez R,
Coffey RJ and Sonenshein GE: Her-2/neu overexpression induces
NF-kappaB via a PI3-kinase/Akt pathway involving calpain-mediated
degradation of IkappaB-alpha that can be inhibited by the tumor
suppressor PTEN. Oncogene. 20:1287–1299. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Aggarwal BB, Shishodia S, Takada Y,
Banerjee S, Newman RA, Bueso-Ramos CE and Price JE: Curcumin
suppresses the paclitaxel-induced nuclear factor-kappaB pathway in
breast cancer cells and inhibits lung metastasis of human breast
cancer in nude mice. Clin Cancer Res. 11:7490–7498. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pledgie-Tracy A, Sobolewski MD and
Davidson NE: Sulforaphane induces cell type-specific apoptosis in
human breast cancer cell lines. Mol Cancer Ther. 6:1013–1021. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cornblatt B, Ye L, Dinkova-Kostova A, Erb
M, Fahey JW, Singh NK, Chen MS, Stierer T, Garrett-Mayer E, Argani
P, et al: Preclinical and clinical evaluation of sulforaphane for
chemoprevention in the breast. Carcinogenesis. 28:1485–1490. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jackson SJ and Singletary KW: Sulforaphane
inhibits human MCF-7 mammary cancer cell mitotic progression and
tubulin polymerization. J Nutr. 134:2229–2236. 2004.PubMed/NCBI
|
|
11
|
Myzak MC, Hardin K, Wang R, Dashwood RH
and Ho E: Sulforaphane inhibits histone deacetylase activity in
BPH-1, LnCap and PC-3 prostate epithelial cells. Carcinogenesis.
27:811–819. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jackson SJ and Singletary KW:
Sulforaphane: A naturally occurring mammary carcinoma mitotic
inhibitor, which disrupts tubulin polymerization. Carcinogenesis.
25:219–227. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Benedict AL, Mountney A, Hurtado A, Bryan
KE, Schnaar RL, Dinkova-Kostova AT and Talalay P: Neuroprotective
effects of sulforaphane after contusive spinal cord injury. J
Neurotrauma. 29:2576–2586. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ozes ON, Mayo LD, Gustin JA, Pfeffer SR,
Pfeffer LM and Donner DB: NF-kappaB activation by tumour necrosis
factor requires the Akt serine-threonine kinase. Nature. 401:82–85.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang CY, Cusack JC Jr, Liu R and Baldwin
AS Jr: Control of inducible chemoresistance: Enhanced anti-tumor
therapy through increased apoptosis by inhibition of NF-kappaB. Nat
Med. 5:412–417. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang CY, Mayo MW, Korneluk RG, Goeddel DV
and Baldwin AS Jr: NF-kappaB antiapoptosis: Induction of TRAF1 and
TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation.
Science. 281:1680–1683. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mabuchi S, Ohmichi M, Kimura A, Hisamoto
K, Hayakawa J, Nishio Y, Adachi K, Takahashi K, Arimoto-Ishida E,
Nakatsuji Y, et al: Inhibition of phosphorylation of BAD and Raf-1
by Akt sensitizes human ovarian cancer cells to paclitaxel. J Biol
Chem. 277:33490–33500. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hu L, Hofmann J, Lu Y, Mills GB and Jaffe
RB: Inhibition of phosphatidylinositol 3′-kinase increases efficacy
of paclitaxel in in vitro and in vivo ovarian cancer models. Cancer
Res. 62:1087–1092. 2002.PubMed/NCBI
|
|
19
|
MacKeigan JP, Taxman DJ, Hunter D, Earp HS
III, Graves LM and Ting JP: Inactivation of the antiapoptotic
phosphatidylinositol 3-kinase-Akt pathway by the combined treatment
of taxol and mitogen-activated protein kinase kinase inhibition.
Clin Cancer Res. 8:2091–2099. 2002.PubMed/NCBI
|
|
20
|
Deeb D, Gao X, Dulchavsky SA and Gautam
SC: CDDO-Me inhibits proliferation, induces apoptosis,
down-regulates Akt, mTOR, NF-kappaB and NF-kappaB-regulated
antiapoptotic and proangiogenic proteins in TRAMP prostate cancer
cells. J Exp Ther Oncol. 7:31–39. 2008.PubMed/NCBI
|
|
21
|
Kim JH, Gupta SC, Park B, Yadav VR and
Aggarwal BB: Turmeric (Curcuma longa) inhibits inflammatory nuclear
factor (NF)-κB and NF-κB-regulated gene products and induces death
receptors leading to suppressed proliferation, induced
chemosensitization, and suppressed osteoclastogenesis. Mol Nutr
Food Res. 56:454–465. 2012. View Article : Google Scholar : PubMed/NCBI
|