Introduction
In Japan, lung cancer is the leading cause of
cancer-associated mortality (1).
Although surgical resection is the typical treatment for stage I
non-small cell lung cancer (NSCLC), the number of medically
inoperable cases treated with radiotherapy has increased (2). Furthermore, patients with operable NSCLC
may preferentially select radiotherapy in order to avoid surgery.
Radiotherapy, including stereotactic body radiotherapy (SBRT) and
particle beam therapy, serves an important role in the treatment of
stage I NSCLC (3,4).
Carbon ion radiotherapy (C-ion RT) provides an
improved dose distribution by the distal fall-off of the Bragg peak
and less lateral scatter compared with photon therapy. These
physical characteristics contribute to the delivery of high-dose
radiation to the tumor, while minimizing the dose to surrounding
normal tissue (5,6). Furthermore, C-ion RT exhibits a larger
mean linear energy transfer compared with other radiotherapy beams,
which causes a high rate of cancer cell death due to the formation
of double-stranded DNA breaks (7).
Considering these advantages, C-ion RT is expected to achieve high
local control rates without severe adverse events in several types
of tumor. For stage I NSCLC, a number of studies of C-ion RT
revealed that its efficacy and safety were comparable to SBRT
(8–10). Despite the high rate of local control
in C-ion RT for stage I NSCLC, regional lymph node or distant
metastasis are relatively common (8,9).
Identifying patients with a high risk of metastasis is important
and adjuvant chemotherapy may decrease the level of metastasis in
these patients. Therefore, the establishment of a pretreatment
prognostic factor for recurrence and survival in NSCLC is
required.
18F-fluorodeoxyglucose-positron emission
tomography (FDG-PET) has been revealed to be a useful technique for
tumor staging through its ability to detect of tumor extension or
metastasis (11–14). In several types of tumor, FDG-PET has
been demonstrated to predict survival and treatment response
(15–17). A meta-analysis of 13 studies on NSCLC
treated with surgery revealed that a high FDG-PET standardized
uptake value (SUV) of the primary tumor was a prognostic factor for
unfavorable survival (18). However,
the prognostic value of FDG-PET for stage I NLCLC treated with
radiotherapy remains unclear. The aim of the present study was to
evaluate whether the maximum SUV (SUVmax) of
pretreatment FDG-PET may predict the prognosis of stage I NSCLC
treated with C-ion RT.
Materials and methods
Study design and patients
Since 2010, patients with peripheral stage I (T1a-2a
N0 M0) NSCLC have been treated with C-ion RT at Gunma University
Heavy Ion Medical Center (Maebashi, Japan). The present study
analyzed the patients that were treated between June 2010 and June
2013 with a prospective protocol approved by the Institutional
Review Board of Gunma University Hospital (Maebashi, Japan). The
present study was registered at the University Hospital Medical
Information Network Center (http://www.umin.ac.jp; study no. UMIN000003797). Tumor
stage was determined through chest computed tomography (CT)
scanning, brain magnetic resonance imaging and PET/CT within 1
month prior to C-ion RT. Although the majority of patients
exhibited biopsy-proven NSCLC, several patients avoided biopsy due
to medical conditions. These patients were clinically diagnosed by
subsequent tumor growth on CT scans and/or by accumulation on
FDG-PET. Treatment options were based on discussions of the Cancer
Board of the hospital, including surgical and medical oncologists,
and a radiologist. All patients provided written informed consent
prior to treatment.
Treatment planning
C-ion beams of 290, 380 and 400 MeV were generated
by the heavy particle accelerator at Gunma University Heavy Ion
Medical Center. Details of the techniques for C-ion RT and
treatment planning have been previously described (19). CT simulation was performed following
immobilization of the patients on fixation cushions and
thermoplastic shells. Gross tumor volume was delineated as a
visible lesion on lung window CT images. The clinical target volume
margin was set at 5–8 mm to include subclinical disease extension.
The planning target volume margin included set-up and internal
margins, which were determined by tumor motion on four-dimensional
CT images. The dose of C-ion RT was expressed as Gy [relative
biological effectiveness (RBE)], which is defined as the physical
C-ion dose × RBE (3). Patients with
T1a-b and T2a were treated with 52.8 Gy (RBE) and 60.0 Gy (RBE),
respectively. All treatments were administered in four fractions
within a week.
PET scan acquisition
All patients underwent FDG-PET prior to C-ion RT.
Subsequent to fasting for 6 h, FDG-PET scans were performed 60 min
following intravenous FDG injection with a maximum activity of 400
Mbq. The SUVmax of the primary tumor was determined by
drawing the volume of interest on attenuation-corrected FDG-PET
reconstructed images. The SUVmax was automatically
calculated based on the maximum activity in the volume of interest,
injected FDG dose and patient weight (GE Healthcare Life Sciences,
Chalfont, UK; Discovery ST Elite).
Assessment and follow-up
Patient follow-up included a clinical examination
and CT scan every 3 months for the first year, and every 6 months
subsequently. Local recurrence was defined as progressive
abnormalities on CT images with accumulation on FDG-PET. The
majority of recurrent cases of NSCLC were pathologically confirmed
by biopsy, cytology or salvage surgery. Toxicities, including
pneumonitis, dermatitis, esophagitis and chest wall pain, were
scored according to the Common Terminology Criteria for Adverse
Events version 4.0 (20).
Statistical analysis
Local control, progression-free survival (PFS), and
overall survival (OS) were measured using the Kaplan-Meier
estimator method. The statistical significance of differences in
local control and survival was assessed using the log-rank test.
Local control, PFS and OS were calculated from the start date of
C-ion RT until the last available follow-up or until the events,
including recurrence, metastasis and death. Differences between two
groups were compared using a Student's t-test. All statistical
analyses were performed using SPSS software (version 21.0; SPSS
Inc., Armonk, NY, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Patient and tumor characteristics
A total of 45 patients were analyzed in the present
study and their clinicopathological characteristics are presented
in Table I. The median patient age
was 72 years (range, 47–85). There were 18 patients (40%) with T1a,
15 (33%) with T1b and 12 (27%) with T2a. Squamous cell carcinoma
was diagnosed in 16 patients (36%), adenocarcinoma in 24 (53%) and
clinically diagnosed lung cancer in 5 (11%). The number of patients
with operable NSCL was 30 (67%) and the number with inoperable
NSCLC was 15 (33%). The median follow-up time for all patients was
28.9 months (range, 7.9–56.7). In total, 2 patients (4%)
experienced grade 2 or 3 radiation pneumonitis; however, their
symptoms rapidly improved subsequent to conservative treatments. No
patients experienced any grade 4 or 5 toxicities.
 | Table I.Patient and tumor characteristics
(n=45). |
Table I.
Patient and tumor characteristics
(n=45).
| Characteristic | No. of
patients | Frequency (%) |
|---|
| Gender |
|
Male | 31 | 69 |
|
Female | 14 | 31 |
| ECOG performance
status |
| 0 | 19 | 42 |
| 1 | 24 | 53 |
| 2 | 2 | 4 |
| Operability |
|
Operable | 30 | 67 |
|
Inoperable | 15 | 33 |
| Histology |
|
Squamous cell carcinoma | 16 | 36 |
|
Adenocarcinoma | 24 | 53 |
|
Clinical lung cancer | 5 | 11 |
| T stage |
|
T1a | 18 | 40 |
|
T1b | 15 | 33 |
|
T2a | 12 | 27 |
| RT dose |
| 52.8 Gy
(RBE) | 33 | 73 |
| 60.0 Gy
(RBE) | 12 | 27 |
| FDG-PET |
|
SUVmax <5.5 | 29 | 64 |
|
SUVmax ≥5.5 | 16 | 36 |
Local control, progression-free
survival and overall survival
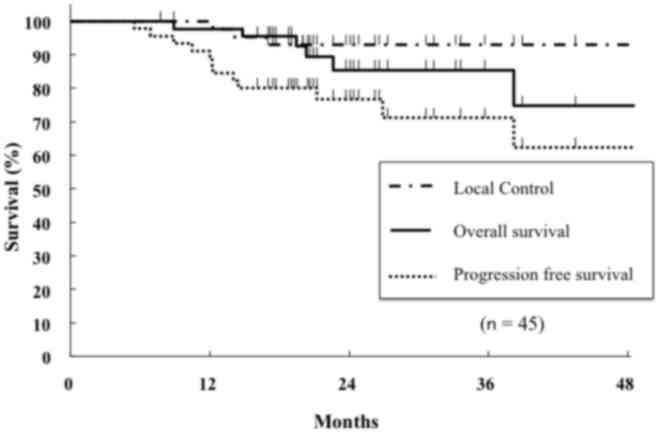
Fig. 1 shows the
curves of local control, PFS and OS for the entire cohort. The
2-year local control rate, PFS and OS were 93, 78 and 89%,
respectively. Of the 10 patients with recurrent NSCLC, 3 exhibited
local recurrences, 2 exhibited hilar lymph node metastasis and 5
exhibited distant metastasis (data not shown). At the time of
analysis, 8 patients had succumbed to lung cancer (n=4) or
comorbidities (n=4) (data not shown).
Outcomes according to the
SUVmax of FDG-PET
The mean SUVmax of primary tumors was 5.5
(range, 0.8–22.4), which was used as the cut-off to divide the
cohort into two groups. All patients were divided into a higher
(≥5.5) or lower (<5.5) SUVmax group. Patient and
tumor characteristics were not significantly associated with
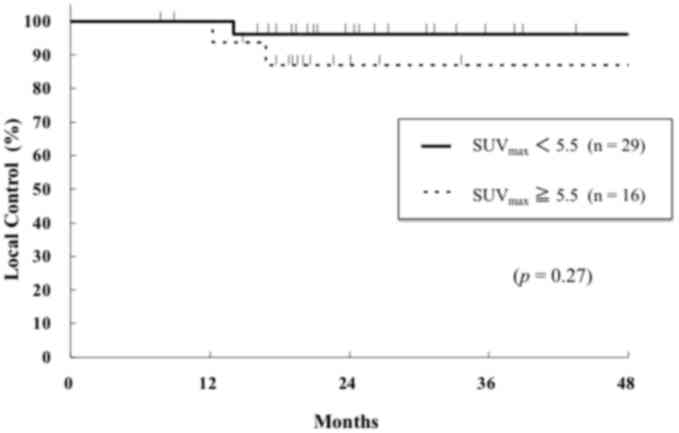
SUVmax groups. The 2-year local control rates for the
higher and lower SUVmax groups were 87 and 96%,
respectively, which was not significantly different (P=0.27;
Fig. 2). Patient clinicopathological
characteristics were not significantly associated with the local
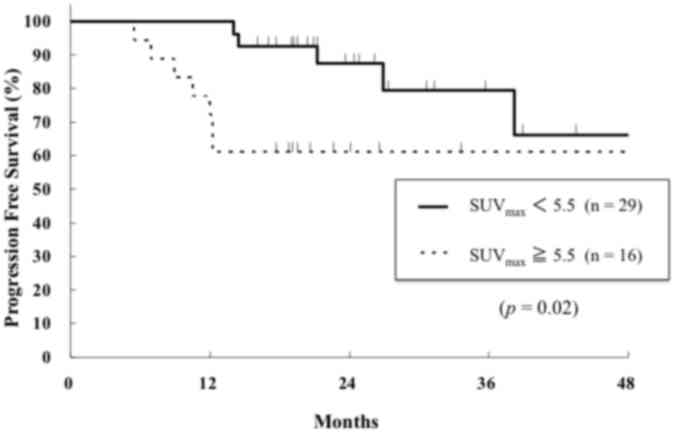
control rate (data not shown). The 2-year PFS rates for the higher
and lower SUVmax groups were 61 and 89%, respectively
(Fig. 3). The higher
SUVmax group exhibited a significantly worse PFS
compared with the lower SUVmax group (P=0.01; Fig. 3). In addition, operability (P<0.01)
and tumor stage (P=0.03) were significant prognostic factors for
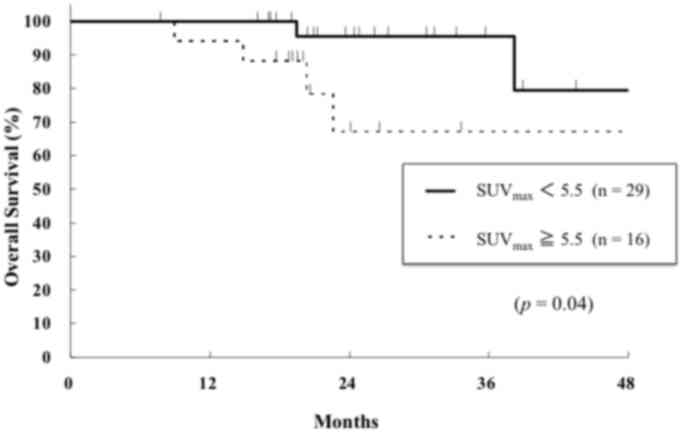
PFS (data not shown). The 2-year OS rates for the higher and lower
SUVmax groups were 76 and 96%, respectively, and the
difference betweem the two groups was statistically significant
(P=0.01, Fig. 4). Operability
(P<0.01) and performance status (P=0.03) were also significant
prognostic factors for OS (data not shown). Histological type was
not significant factor for PFS and OS. Multivariate analysis was
not performed due to the small number of patients in the present
study.
Discussion
Based on a 2-year follow-up period, the present
study revealed that C-ion RT produced 93% local control and 89% OS
for stage I NSCLC with minimal toxicity. Furthermore, a higher
SUVmax of the primary tumors on FDG-PET was
significantly associated with a worse PFS and OS compared with a
lower SUVmax. To the best of our knowledge, this is the
first report demonstrating that pretreatment SUVmax on
FDG-PET is a prognostic factor for stage I NSCLC treated with C-ion
RT.
In SBRT, the utility of SUVmax as a
prognostic factor for stage I NSCLC remains unclear; several
previous studies have identified no significant association between
SUVmax and patient outcomes (21–22).
Burdick et al (22) analyzed
pretreatment SUVmax in 72 patients with inoperable
T1-2N0M0 NSCLC treated with SBRT and concluded that pretreatment
SUVmax did not predict for local control, mediastinal
failure, distant metastases or OS. In Japan, Takeda et al
(23) revealed that a high
SUVmax of the primary tumor was associated with a
significantly worse local control rate in 95 patients with NSCLC
treated with SBRT. It was concluded that NSCLC tumors with a high
SUVmax may require dose escalation to improve local
control, but patient outcomes were not studied. Chang et al
(24) evaluated 130 patients with
stage I NSCLC who underwent SBRT with 50 Gy in four fractions and
concluded that a higher SUVmax was markedly associated
with worse PFS and OS, but not local control. In a study by Clarke
et al (25) on 82 patients
with inoperable stage I NSCLC treated with SBRT, it was concluded
that pretreatment SUVmax was associated with local
relapse and distant metastases.
A previous meta-analysis of 13 studies on NSCLC
treated with radiotherapy revealed that a high pre-treatment
SUVmax was significantly associated with unfavorable
local control in patient treated with SBRT (hazard ratio, 1.11; 95%
confidence interval, 1.06–1.18) (26). In the present study, the higher
SUVmax group did not exhibit worse local control
compared with the lower SUVmax group, possibly as the
local control rate of C-ion RT was high and the difference between
two groups was not significant. Regardless of FDG accumulation,
C-ion RT exhibits an advantage in the local control of primary
tumor.
Several surgical studies have evaluated the
prognostic value of pretreatment FDG-PET in NSCLC. Goodgame et
al (27) revealed that a
pretreatment SUVmax ≥5.5 predicted worse recurrence and
survival in 136 patients treated with surgery, and suggested that
stage I NSCLC with a high SUVmax should be considered
for adjuvant chemotherapy. In addition, Nair et al (28) systematically reviewed the association
between FDG uptake and prognosis in stage I NSCLC treated with
surgery. It was concluded that an increased tumor FDG uptake was
associated with worse patient survival and could potentially be
used as a biomarker for identifying high-risk patients with stage I
NSCLC. Similarly, the results of the present study demonstrated
that a high FDG accumulation was associated with worse PFS and
OS.
A high FDG uptake suggests that metabolically active
cancer cells take up glucose. Vesselle et al (29) reported that FDG uptake is associated
with tumor proliferation and a poorer differentiation in NSCLC. van
Baardwijk et al (30) revealed
that high SUVmax tumors exhibited significantly higher
hypoxic marker (hypoxia-inducible factor 1-α and glucose
transporter 1) expression compared with lower SUVmax
tumors. These results suggest that a higher FDG uptake is an
indicator of malignant and radioresistant characteristics, which
means that these tumors may progress. As the biological and
physiological advantages of C-ion RT produce a high local control
rate even for radioresistant tumors (for example, hypoxic cells),
FDG-PET was a significant prognostic factor for PFS and OS, but not
local control, in the present study.
The cut-off value for SUVmax remains
controversial. A previous study has indicated that an
SUVmax of 5–7 in tumors was important for predicting
outcomes (26). However, these values
depend on patient characteristics, treatments and radiation doses.
Additional studies are required to validate the findings of the
present study in this regard.
Although SUVmax has been the focus of
numerous studies, the usefulness of other PET parameters has also
been reported. Satoh et al (31) reported that metabolic tumor volume, in
addition to SUVmax, was significantly associated with
outcomes following SBRT in lung cancer. Generally, the
SUVmax of lung tumors is affected by the partial volume
effect due to its respiratory movement, and a previous study
(32) has attempted to correct this
effect by using four-dimensional PET/CT. Salavati et al
(32) demonstrated that
semi-automated correction for the partial volume effect improved
the accuracy of FDG quantification for malignant lesions of the
lung.
The present study revealed that C-ion RT is an
effective and safe treatment for stage I NSCLC. The 2-year local
control rate and OS were 93 and 89%, respectively, and the rate of
radiation pneumonitis that was above grade 2 was only 4%. These
results are comparable to that of a previous study of C-ion RT
(8). However, additional follow-up is
required to assess long-term outcomes and late adverse events
following C-ion RT.
In conclusion, patients with a pretreatment
SUVmax of ≥5.5 exhibited a worse 2-year PFS and OS
compared with those with an SUVmax of <5.5. These
results indicate that pretreatment SUVmax is a
prognostic marker that could be used to identify high-risk patients
with NSCLC. Based on the results of the present study, patients
with a higher SUVmax may benefit from adjuvant
chemotherapy following C-ion RT to decrease the risk of distant
metastasis. Additional studies are warranted to determine if
pretreatment SUVmax is associated with long-term
prognosis.
Acknowledgements
The abstract was presented at the ASTRO 56th annual
meeting Sep 14–17 2014 in San-Francisco, CA and published as
abstract no. 3007 in International Journal of Radiation Oncology,
Biology, Physics 90 (S607): 2014.
References
|
1
|
Nakamura K, Ukawa S, Okada E, Hirata M,
Nagai A, Yamagata Z, Ninomiya T, Muto K, Kiyohara Y, Matsuda K, et
al: Characteristics and prognosis of Japanese male and female lung
cancer patients: The BioBank Japan Project. J Epidemiol.
27:S49–S57. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Palma D, Visser O, Lagerwaard FJ,
Belderbos J, Slotman BJ and Senan S: Impact of introducing
stereotactic lung radiotherapy for elderly patients with stage I
non-small-cell lung cancer: A population-based time-trend analysis.
J Clin Oncol. 28:5153–5159. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Timmerman R, Paulus R, Galvin J, Michalski
J, Straube W, Bradley J, Fakiris A, Bezjak A, Videtic G, Johnstone
D, et al: Stereotactic body radiation therapy for inoperable early
stage lung cancer. JAMA. 303:1070–1076. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Onishi H, Shirato H, Nagata Y, Hiraoka M,
Fujino M, Gomi K, Karasawa K, Hayakawa K, Niibe Y, Takai Y, et al:
Stereotactic body radiotherapy (SBRT) for operable stage I
non-small-cell lung cancer: Can SBRT be comparable to surgery? Int
J Radiat Oncol Biol Phys. 81:1352–1358. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kanai T, Endo M, Minohara S, Miyahara N,
Koyama-ito H, Tomura H, Matsufuji N, Futami Y, Fukumura A, Hiraoka
T, et al: Biophysical characteristics of HIMAC clinical irradiation
system for heavy-ion radiation therapy. Int J Radiat Oncol Biol
Phys. 44:201–210. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Schulz-Ertner D and Tsujii H: Particle
radiation therapy using proton and heavier ion beams. J Clin Oncol.
25:953–964. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ando K and Kase Y: Biological
characteristics of carbon-ion therapy. Int J Radiat Biol.
85:715–728. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Miyamoto T, Baba M, Sugane T, Nakajima M,
Yashiro T, Kagei K, Hirasawa N, Sugawara T, Yamamoto N, Koto M, et
al: Carbon ion radiotherapy for stage I non-small cell lung cancer
using a regimen of four fractions during 1 week. J Thorac Oncol.
2:916–926. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Miyamoto T, Baba M, Yamamoto N, Koto M,
Sugawara T, Yashiro T, Kadono K, Ezawa H, Tsujii H, Mizoe JE, et
al: Curative treatment of Stage I non-small-cell lung cancer with
carbon ion beams using a hypofractionated regimen. Int J Radiat
Oncol Biol Phys. 67:750–758. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Iwata H, Murakami M, Demizu Y, Miyawaki D,
Terashima K, Niwa Y, Mima M, Akagi T, Hishikawa Y and Shibamoto Y:
High-dose proton therapy and carbon-ion therapy for stage I
non-small cell lung cancer. Cancer. 116:2476–2485. 2010.PubMed/NCBI
|
|
11
|
Shirai K, Nakagawa A, Abe T, Kawahara M,
Saitoh J, Ohno T and Nakano T: Use of FDG-PET in radiation
treatment planning for thoracic cancers. Int J Mol Imaging.
2012:6095452012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lardinois D, Weder W, Hany TF, Kamel EM,
Korom S, Seifert B, von Schulthess GK and Steinert HC: Staging of
non-small-cell lung cancer with integrated positron-emission
tomography and computed tomography. N Engl J Med. 348:2500–2507.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pieterman RM, van Putten JW, Meuzelaar JJ,
Mooyaart EL, Vaalburg W, Koëter GH, Fidler V, Pruim J and Groen HJ:
Preoperative staging of non-small-cell lung cancer with positron-
emission tomography. N Engl J Med. 343:254–261. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lammering G, de Ruysscher D, van Baardwijk
A, Baumert BG, Borger J, Lutgens L, van den Ende P, Ollers M and
Lambin P: The use of FDG-PET to target tumors by radiotherapy.
Strahlenther Onkol. 186:471–481. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dooms C, Verbeken E, Stroobants S,
Nackaerts K, De Leyn P and Vansteenkiste J: Prognostic
stratification of stage IIIA-N2 non-small-cell lung cancer after
induction chemotherapy: A model based on the combination of
morphometric-pathologic response in mediastinal nodes and primary
tumor response on serial 18-fluoro-2-deoxy-glucose positron
emission tomography. J Clin Oncol. 26:1128–1134. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mac Manus MP, Hicks RJ, Matthews JP, Wirth
A, Rischin D and Ball DL: Metabolic (FDG-PET) response after
radical radiotherapy/chemoradiotherapy for non-small cell lung
cancer correlates with patterns of failure. Lung Cancer. 49:95–108.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Huang W, Zhou T, Ma L, Sun H, Gong H, Wang
J, Yu J and Li B: Standard uptake value and metabolic tumor volume
of 18F-FDG PET/CT predict short-term outcome early in
the course of chemoradiotherapy in advanced non-small cell lung
cancer. Eur J Nucl Med Mol Imaging. 38:1628–1635. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Berghmans T, Dusart M, Paesmans M,
Hossein-Foucher C, Buvat I, Castaigne C, Scherpereel A, Mascaux C,
Moreau M, Roelandts M, et al: Primary tumor standardized uptake
value (SUVmax) measured on fluorodeoxyglucose positron emission
tomography (FDG-PET) is of prognostic value for survival in
non-small cell lung cancer (NSCLC): A systematic review and
meta-analysis (MA) by the European Lung Cancer Working Party for
the IASLC Lung Cancer Staging Project. J Thorac Oncol. 3:6–12.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tashiro M, Ishii T, Koya J, Okada R,
Kurosawa Y, Arai K, Abe S, Ohashi Y, Shimada H, Yusa K, et al:
Technical approach to individualized respiratory-gated carbon-ion
therapy for mobile organs. Radiol Phys Technol. 6:356–366. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
National Cancer Institute, . Common
Terminology Criteria for Adverse Events (CTCAE v4.0). https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htmlNovember
14–2016
|
|
21
|
Coon D, Gokhale AS, Burton SA, Heron DE,
Ozhasoglu C and Christie N: Fractionated stereotactic body
radiation therapy in the treatment of primary, recurrent, and
metastatic lung tumors: The role of positron emission
tomography/computed tomography-based treatment planning. Clin Lung
Cancer. 9:217–221. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Burdick MJ, Stephans KL, Reddy CA, Djemil
T, Srinivas SM and Videtic GM: Maximum standardized uptake value
from staging FDG-PET/CT does not predict treatment outcome for
early-stage non-small-cell lung cancer treated with stereotactic
body radiotherapy. Int J Radiat Oncol Biol Phys. 78:1033–1039.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Takeda A, Yokosuka N, Ohashi T, Kunieda E,
Fujii H, Aoki Y, Sanuki N, Koike N and Ozawa Y: The maximum
standardized uptake value (SUVmax) on FDG-PET is a strong predictor
of local recurrence for localized non-small-cell lung cancer after
stereotactic body radiotherapy (SBRT). Radiother Oncol.
101:291–297. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chang JY, Liu H, Balter P, Komaki R, Liao
Z, Welsh J, Mehran RJ, Roth JA and Swisher SG: Clinical outcome and
predictors of survival and pneumonitis after stereotactic ablative
radiotherapy for stage I non-small cell lung cancer. Radiat Oncol.
7:1522012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Clarke K, Taremi M, Dahele M, Freeman M,
Fung S, Franks K, Bezjak A, Brade A, Cho J, Hope A, et al:
Stereotactic body radiotherapy (SBRT) for non-small cell lung
cancer (NSCLC): Is FDG-PET a predictor of outcome? Radiother Oncol.
104:62–66. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Na F, Wang J, Li C, Deng L, Xue J and Lu
Y: Primary tumor standardized uptake value measured on
F18-Fluorodeoxyglucose positron emission tomography is of
prediction value for survival and local control in non-small-cell
lung cancer receiving radiotherapy: Meta-analysis. J Thorac Oncol.
9:834–842. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Goodgame B, Pillot GA, Yang Z, Shriki J,
Meyers BF, Zoole J, Gao F, Dehdashti F, Patterson A, Siegel BA and
Govindan R: Prognostic value of preoperative positron emission
tomography in resected stage I non-small cell lung cancer. J Thorac
Oncol. 3:130–134. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nair VS, Krupitskaya Y and Gould MK:
Positron emission tomography 18F-fluorodeoxyglucose uptake and
prognosis in patients with surgically treated, stage I non-small
cell lung cancer: A systematic review. J Thorac Oncol. 4:1473–1479.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Vesselle H, Schmidt RA, Pugsley JM, Li M,
Kohlmyer SG, Vallires E and Wood DE: Lung cancer proliferation
correlates with [F-18]fluorodeoxyglucose uptake by positron
emission tomography. Clin Cancer Res. 6:3837–3844. 2000.PubMed/NCBI
|
|
30
|
van Baardwijk A, Dooms C, van Suylen RJ,
Verbeken E, Hochstenbag M, Dehing-Oberije C, Rupa D, Pastorekova S,
Stroobants S, Buell U, et al: The maximum uptake of
(18)F-deoxyglucose on positron emission tomography scan correlates
with survival, hypoxia inducible factor-1alpha and GLUT-1 in
non-small cell lung cancer. Eur J Cancer. 43:1392–1398. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Satoh Y, Onishi H, Nambu A and Araki T:
Volume-based parameters measured by using FDG PET/CT in patients
with stage I NSCLC treated with stereotactic body radiation
therapy: Prognostic value. Radiology. 270:275–281. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Salavati A, Borofsky S, Boon-Keng TK,
Houshmand S, Khiewvan B, Saboury B, Codreanu I, Torigian DA, Zaidi
H and Alavi A: Application of partial volume effect correction and
4D PET in the quantification of FDG avid lung lesions. Mol Imaging
Biol. 17:140–148. 2015. View Article : Google Scholar : PubMed/NCBI
|