Introduction
Small supernumerary maker chromosome (sSMC) is
defined as a structurally abnormal chromosome that cannot be
characterized clearly by conventional cytogenetic banding. sSMCs
can be present in normal and abnormal karyotypes such as the Turner
syndrome karyotype (sSMCT) (1). The majority of patients with Tuner
syndrome exhibit a short stature. The birth heights of infants with
Turner syndrome are shorter than infants without the syndrome, and
the growth rate of infants with Turner syndrome is also lower
(2). Another feature of Turner
syndrome is the abnormal development of gonads, and mental
disabilities may also be present (3).
In the majority of cases, the size of sSMC is
smaller than chromosome 20 (4,5) and sSMCs
can be derived from autosomes and sex chromosomes (6). sSMCs may result from translocations,
with ~64% attributed to balanced translocations in the parent,
while the remaining ~36% arise de novo (7).
The sex-determining region on the Y chromosome (SRY)
gene isolated in 1990 was known as the testis-determining factor on
the Y chromosome (8,9). The SRY gene encodes a transcription
factor, which is a member of the high mobility group box family of
proteins and is important for the sex determination process
(10). Although birds and reptiles
lack the SRY gene, sex determination by the SRY gene is common in
mammals and it can be used as a potential marker for sex
determination in research (11).
Previous clinical studies reported great
heterogeneity in the origins of sSMCs (12). Therefore, it is important to
investigate a larger number of sSMC cases in order to expand the
current understanding of the origins of sSMCs and the correlation
between karyotype and phenotype, which may improve genetic
counseling. In the present study, the sSMCs in 17 patients with a
mos 45,X/46,X,+mar karyotype were characterized.
Materials and methods
Participants and physical
examination
Ethical approval was granted by Children's Hospital
of Soochow University (Suzhou, China). The informed consents were
obtained from all the participants. A total of 17 patients (1 male
and 16 females) were included in the present study. All patients
presented with a mos 45,X/46,X,+mar karyotype, a short stature and
abnormal development of the gonads. The average age of the patients
was 7.4 years, ranging from 2 months to 16 years. The stature of
the children was shorter than the children of the same age.
Physical examinations were performed in all 17
patients and the gonads were assessed by imaging. The Wechsler
Intelligence Scale for Children-V was employed to evaluate the
intellectual ability of the participants (13).
Cytogenetic analysis
Metaphase chromosomes were obtained from
phytohaemagglutinin stimulated lymphocyte cultures from biopsy
samples as previously described (14). The chromosomes were analyzed by Giemsa
banding and karyotyped according to the Use of the International
System for Human Cytogenetic Nomenclature (15). Karyotypes were based on the analysis
of 50 metaphases.
Fluorescence in situ hybridization
(FISH)
FISH was performed using commercially available
centromeric probes for chromosomes X and Y (Cytocell Ltd.,
Cambridge, UK) according to the manufacturer's protocol (16). The metaphase chromosomes of the
samples from the patients were analyzed. The DNA was counterstained
with DAPI. The chromosome X α-satellite (DXZ1) probe hybridized to
sequences located at Xp11.1-q11.1 and the chromosome Y satellite
III probe (DYZ3) hybridized to sequences located at Yp11.1-q11.1.
The image was analyzed using a FISH image acquisition system (LEICA
Q55OCW).
Polymerase chain reaction (PCR)
Samples from patients with sSMCs derived from the Y
chromosome were analyzed by PCR to detect the presence of the SRY
gene. Genomic DNA was extracted from the blood samples using the
QIAamp DNA Blood Mini Kit (Qiagen GmbH, Hilden, Germany) according
to the manufacturer's protocol. PCR reactions were performed in 10
µl reactions containing 20 mg genomic DNA, 0.4 µM/l primers, 60
µM/l deoxynucleotide triphosphates, 2 mM/l MgCl2, 0.5
units of DNA polymerase and 1 µl 10X Taq PCR MasterMix (HotStarTaq
Master Mix Kit, Qiagen GmbH).
PCR cycling conditions included, 95°C for 6 min,
followed by 35 cycles at 95°C for 45 sec, 58°C for 45 sec, an
extension step at 72°C for 60 sec and a final extension step at
72°C for 7 min. The sequence for the forward primer was
5′-GAATATTCCCGCTCTCCGG-3′ and for the reverse primer was
5′-ACAACCTGTTGTCCAGTTGC-3′. The agarose gel was visualized with a
BIO-RAD VersaDoc 3.0 (Bio-Rad Laboratories, Inc., Hercules, CA,
USA).
Results
Incidence rates of sSMC
The incidence of sSMC in males was markedly
decreased compared to the incidence in girls. Out of a total of 477
females presented with short stature and abnormal gonadal
development, sSMC was detected in 16 patients with Turner syndrome
(sSMCT) at a detection rate of ~3.4%. Out of a total of
349 males also presenting with short stature and abnormal gonadal
development, sSMC was detected in only 1 case, with a detection
rate of ~0.29%.
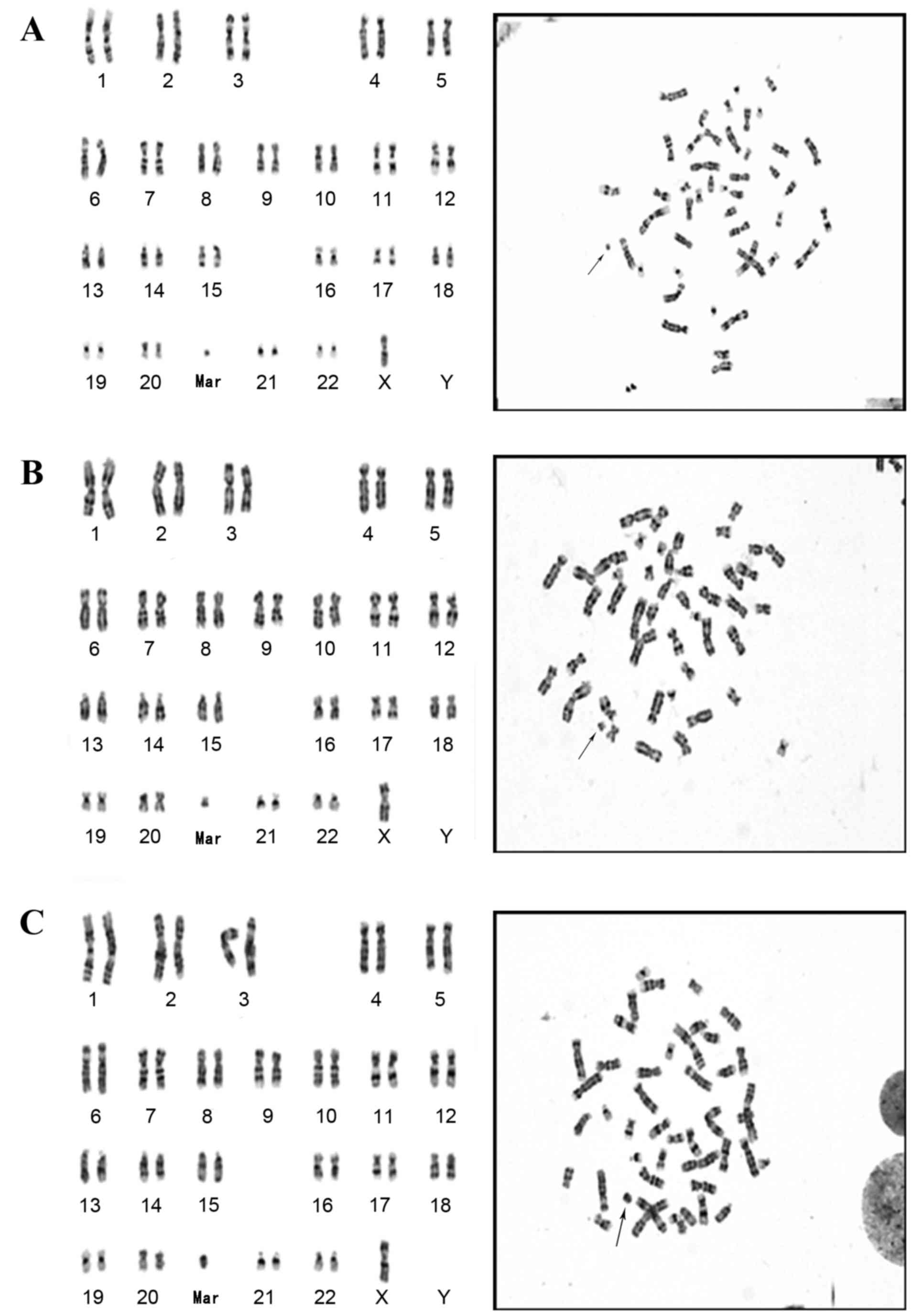
Origin and morphology of sSMC
Dual-color FISH analysis with DXZ1 (green) and DYZ3
(red) probes was employed to assess the origin of sSMCs (Fig. 1). Out of the 16 females detected with
SMCs, sSMCs in 14 cases were derived from the X chromosome and 2
cases were derived from the Y chromosome. The sSMC in the single
male was derived from the Y chromosome (Fig. 2).
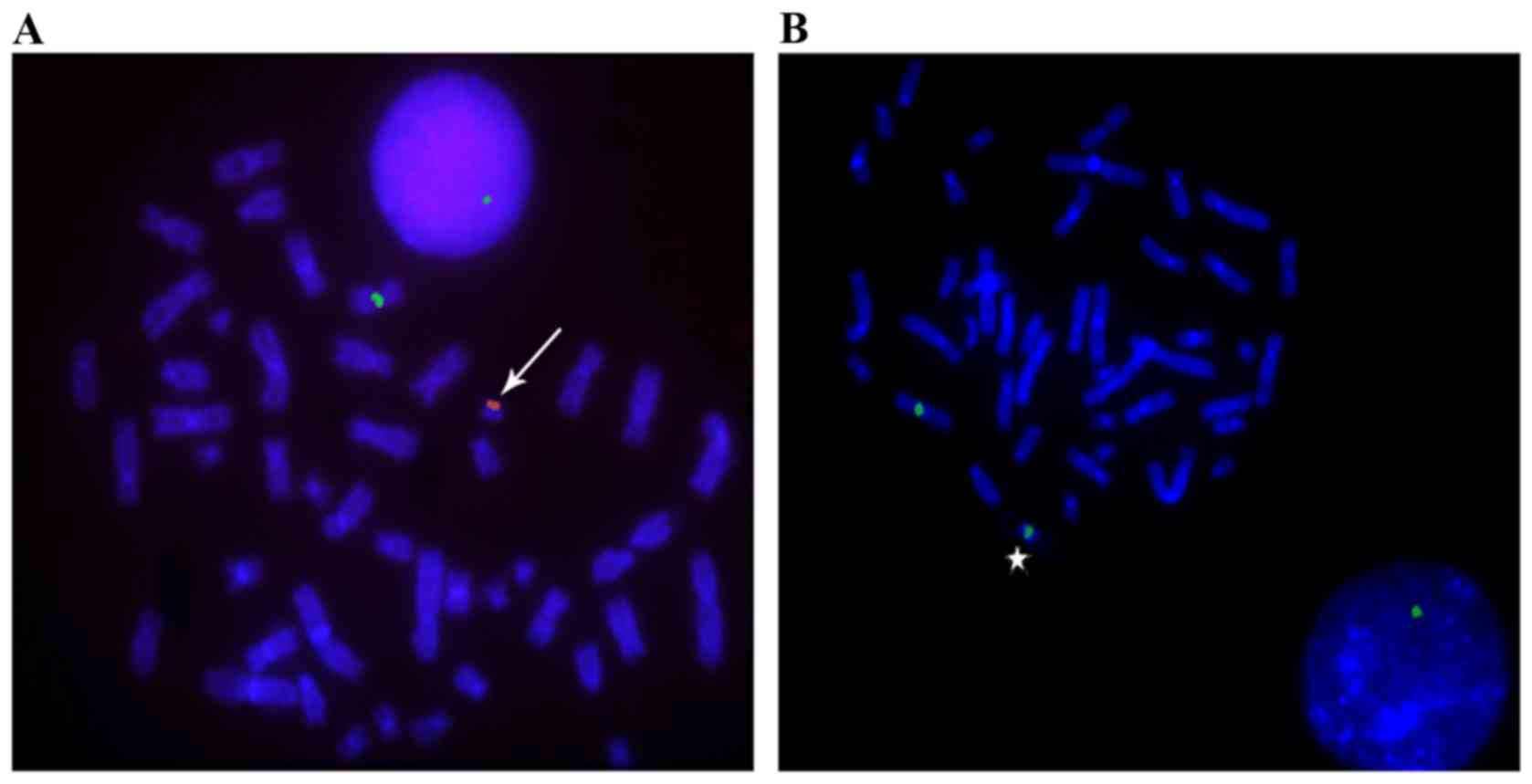 | Figure 1.Dual-color FISH of sSMC in patients
with 45,X/46,X,+mar mosaicism. The X chromosome centromere was
stained green, while the Y chromosome was stained red, using FISH
methods. (A) A chromosome spread from a patient, including an sSMC
derived from the Y chromosome (red, arrowhead). During metaphase,
the DAPI chromosome signal was 46,X,+mar; in interphase, it was
45,XO. (B) A chromosome spread from a patient with mosaicism,
including an sSMC derived from the X chromosome (green, star).
During metaphase, the DAPI chromosome signal was 46, X, +mar; in
interphase, it was 45,XO. Magnification, ×1,000. FISH, fluorescence
in situ hybridization; sSMC, small supernumerary maker
chromosome. |
Karyotype and FISH analyses indicated out of the 17
cases with sSMCs, 8 cases were ring-shaped and 9 were centric
minute-shaped sSMC. The results demonstrated that the
sSMCT cases were derived from the sex chromosome,
including predominantly from the X chromosome.
Detection of the SRY gene
The sSMCs in 2 females (cases 10 and 15) and 1 male
(case 14) were derived from the Y chromosome. PCR analysis
indicated that the SRY gene was detected in only cases 14 and 15
(Table I). Both individuals exhibited
feminine characteristics. Although the SRY gene was present in case
15, the gene may have been mutated, leading to a loss-of-function.
The Wechsler Intelligence Scale for Children-V test indicated that
3 (cases 3, 7 and 16) of the 17 children tested appeared to have
mental retardation (Table I).
 | Table I.Clinical feature of the small
supernumerary marker chromosome in children with mos 45,
X/46,X,+mar karyotype. |
Table I.
Clinical feature of the small
supernumerary marker chromosome in children with mos 45,
X/46,X,+mar karyotype.
| Case No. | Gender | Age of onset
(years) | Chromosomal origin of
sSMC | Shape of sSMC | SRY gane | Clinical
features | Mental
disability |
|---|
| 1 | F | 9 | X | min | ND | Turner | − |
| 2 | F | 7 | X | min | ND | Turner | − |
| 3 | F | 16 | X | r | ND | Turner | + |
| 4 | F | 6 | X | min | ND | Turner | − |
| 5 | F | 16 | X | r | ND | Turner | − |
| 6 | F | 9 | X | r | ND | Turner | − |
| 7 | F | 7 | X | min | ND | Turner | + |
| 8 | F | 3 | X | min | ND | Turner | − |
| 9 | F | 2 | X | r | ND | Turner | − |
| 10 | F | 5 | Y | min | − | Turner | − |
| 11 | F | 13 | X | r | ND | Turner | − |
| 12 | F | 6 | X | r | ND | Turner | − |
| 13 | F | 16 | X | r | ND | Turner | − |
| 14 | M | 6 | Y | min | + | Hypospadias, short
stature | − |
| 15 | F | 2 months | Y | min | + | Turner | − |
| 16 | F | 9 | X | r | ND | Turner | + |
| 17 | F | 1 | X | min | ND | Turner | − |
Discussion
The incidence rate of sSMC is relatively low, with a
rate of ~0.001% in newborn babies and a detection rate of ~0.004%
by antenatal diagnosis (1). In the
present study, the incidence of sSMC in males was markedly lower
compared to the incidence in girls, with ~0.29 vs. ~3.4%.
sSMCs can be derived from the autosomes and the sex
chromosomes (6). Liehr et al
(1) have previously tested the
origins of sSMCs in 512 cases, and it was reported that 72.6% were
derived from the Y chromosome, 27% from the X chromosome and 0.4%
from the autosomes. In the present study, 17 cases were analyzed
using dual-color FISH. It has been demonstrated that the sSMCs in
patients with a Turner syndrome karyotype were derived from the sex
chromosomes. In the 16 females, sSMCs in 14 cases were derived from
the X chromosome and sSMCs in 2 cases were derived from the Y
chromosome. The sSMC in the single male with hypospadias and a
short stature was derived from the Y chromosome.
It has been previously reported that the origin of
the sSMC in patients with Turner syndrome may impact on the risk of
malignant gonadal tumor; if the sSMC is derived from the Y
chromosome the risk may be increased by 30% (17). Therefore, it is important to establish
the origin of sSMCs.
45,X/46,XY is a disorder of sexual development
(DSD). Patients with DSDs present with abnormal sexual development
and short stature (18). Types of DSD
include Turner syndrome, mixed gonadal dysgenesis and genital tract
malformation (17,19,20). In
the present study, sSMCs in 3 patients (3 females and 1 male) were
derived from the Y chromosome. The SRY gene in case 15 may have
been mutated, leading to a loss-of-function. Notably, cases 10 and
15 were females, and whilst the SRY gene was absent in case 10, the
gene was present in case 15. Both females exhibited feminine
characteristics and clinical characteristics associated with Turner
syndrome.
In view of the short stature and abnormal sexual
development, patients with these features may be treated as Turner
syndrome patients. When treating 45,X/46,X,+mar karyotype patients,
the origin of sSMCs need to be assessed. To keep the basic social
gender and gonad function, the contradictory gonad should be
excised. During childhood, Turner syndrome patients may be treated
with recombinant human growth hormone to promote an increase in
stature. Patients would be treated with hormone to initiate puberty
~12 years old (21). In addition,
mental intervention is also necessary to treat the children.
In conclusion, patients with the mos 45,X/46,X,+mar
karyotype have varying origins of sSMC. A multidisciplinary
approach is necessary for early diagnosis and appropriate treatment
of these patients in order to reduce social and mental
consequences.
Acknowledgements
The authors thank the patients for participating in
the present study. The present study was supported by the grant of
Jiangsu province Association of Maternal and Child Health (grant
no. FYX201603), Jiangsu Provincial Medical Youth Talent (grant no.
QNRC2016238), Suzhou Science and Technology support program (grant
no. SS201647), and Suzhou Key Medical Center (grant no.
Szzx201505)
References
|
1
|
Liehr T, Mrasek K, Hinreiner S, Reich D,
Ewers E, Bartels I, Seidel J, Emmanuil N, Petesen M, Polityko A, et
al: Small supernumerary marker chromosomes (sSMC) in patients with
a 45,X/46,X,+mar karyotype-17 new cases and a review of the
literature. Sex Dev. 1:353–362. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Davenport ML, Punyasavatsut N, Stewart PW,
Gunther DF, Sävendahl L and Sybert VP: Growth failure in early
life: An important manifestation of turner syndrome. Horm Res.
57:157–164. 2002.PubMed/NCBI
|
|
3
|
Fjermestad KW, Naess EE, Bahr D and
Gravholt CH: A 6-year follow-up survey of health status in
middle-aged women with turner syndrome. Clin Endocrinol (Oxf).
85:423–429. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liehr T, Claussen U and Starke H: Small
supernumerary marker chromosomes (sSMC) in humans. Cytogenet Genome
Res. 107:55–67. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ou J, Wang W, Liehr T, Klein E, Hamid AB,
Wang F, Duan C and Li H: Characterization of three small
supernumerary marker chromosomes (sSMC) in humans. J Matern Fetal
Neonatal Med. 26:106–108. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Soheilipour F, Abed O, Behnam B,
Abdolhosseini M, Alibeigi P and Pazouki A: A rare case of mixed
gonadal dysgenesis with mosaicism 45, X/46, X, +mar. Int J Surg
Case Rep. 7C:35–38. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Trifonov V, Fluri S, Binkert F, Nandini A,
Anderson J, Rodriguez L, Gross M, Kosyakova N, Mkrtchyan H, Ewers
E, et al: Complex rearranged small supernumerary marker chromosomes
(sSMC), three new cases; evidence for an underestimated entity? Mol
Cytogenet. 1:62008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jäger RJ, Anvret M, Hall K and Scherer G:
A human XY female with a frame shift mutation in the candidate
testis-determining gene SRY. Nature. 348:452–454. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Iida T, Nakahori Y, Komaki R, Mori E,
Hayashi N, Tsutsumi O, Taketani Y and Nakagome Y: A novel nonsense
mutation in the HMG box of the SRY gene in a patient with XY sex
reversal. Hum Mol Genet. 3:1437–1438. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Moreira MA: SRY evolution in Cebidae
(Platyrrhini: Primates). J Mol Evol. 55:92–103. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wu QY, Li N, Li WW, Li TF, Zhang C, Cui
YX, Xia XY and Zhai JS: Clinical, molecular and cytogenetic
analysis of 46, XX testicular disorder of sex development with
SRY-positive. Bmc Urol. 14:702014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Melo JB, Matoso E, Polityko A, Saraiva J,
Backx L, Vermeesch JR, Kosyakova N, Ewers E, Liehr T and Carreira
IM: Molecular cytogenetic characterization of two cases with de
novo small mosaic supernumerary marker chromosomes derived from
chromosome 16: Towards a genotype/phenotype correlation. Cytogenet
Genome Res. 125:109–114. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Na SD and Burns TG: Wechsler intelligence
scale for children-V: Test review. Appl Neuropsychol Child.
5:156–160. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sheth F, Ewers E, Kosyakova N, Weise A,
Sheth J, Desai M, Andrieux J, Vermeesch J, Hamid AB, Ziegler M and
Liehr T: A small supernumerary marker chromosome present in a
Turner syndrome patient not derived from X- or Y-chromosome: A case
report. Mol Cytogenet. 2:222009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Garcia JR Gonzalez and Meza-Espinoza JP:
Use of the International system for human cytogenetic nomenclature
(ISCN). Blood. 108:3952–3953. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liehr T, Mrasek K, Weise A, Dufke A,
Rodríguez L, Martínez Guardia N, Sanchís A, Vermeesch JR, Ramel C,
Polityko A, et al: Small supernumerary marker chromosomes-progress
towards a genotype-phenotype correlation. Cytogenet Genome Res.
112:23–34. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Alvarez-Nava F and Puerta H: Y-chromosome
microdeletions in 45,X/46,XY patients. Am J Med Genet A.
140:1128–1130. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Layman LC, Tho SP, Clark AD, Kulharya A
and McDonough PG: Phenotypic spectrum of 45,X/46,XY males with a
ring Y chromosome and bilaterally descended testes. Fertil Steril.
91:791–797. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chang GY, Dong ZY, Wang W, Xiao Y, Chen
FS, Ni JH, Wang RF and Wang DF: Association of 45, X/46, XY
mosaicism with disorders of sex development: The clinical analysis
of 5 cases. Zhonghua Er Ke Za Zhi. 49:451–454. 2011.(In Chinese).
PubMed/NCBI
|
|
20
|
Hughes IA, Houk C, Ahmed SF and Lee PA;
Lawson Wilkins Pediatric Endocrine Society/European Society for
Paediatric Endocrinology Consensus Group, : Consensus statement on
management of intersex disorders. J Pediatr Urol. 2:148–162. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gravholt CH: Hormone replacement therapy
in turner syndrome is important-a new meta-analysis points at many
shortcomings in the available literature. Endocrine. 55:329–330.
2017. View Article : Google Scholar : PubMed/NCBI
|