Introduction
Approximately 10–15% of Caucasian patients with
advanced non-small cell lung cancer (NSCLC) have mutations in the
epidermal growth factor receptor (EGFR) gene. Several
clinical studies have demonstrated the efficacy of EGFR-tyrosine
kinase inhibitors (EGFR-TKIs) for treatment of NSCLC patients with
activating EGFR mutations (1–5).
Currently, three EGFR-TKIs (erlotinib, gefitinib, and afatinib)
have proven efficacy in the treatment of NSCLC in patients with
common activating EGFR mutations. Erlotinib and gefitinib
are reversible EGFR-TKIs, while afatinib is an irreversible
EGFR-TKIs. However, despite extensive knowledge about the mechanism
of action of EGFR-TKIs in NSCLC, some serious problems remain
unsolved.
First of all, few prospective and randomized studies
have directly compared the efficacy of the various classes of
EGFR-TKIs in patients with NSCLC harboring activating EGFR
mutations (6–8). There are the preliminary results of a
single-center randomized phase II trial comparing the efficacy of
gefitinib and erlotinib in second-line therapy of Asian NSCLC
patients with activating EGFR mutations (6), as well as the results of the global,
multi-center LUX-Lung 7 trial comparing the efficacy of afatinib
and gefitinib in first-line treatment of NSCLC patients with common
activating EGFR mutations (7).
The results of the multinational, randomized ARCHER 1009 trial
indicate that gefitinib and daconitinib have similar efficacy.
However, the ARCHER 1050 phase III trial comparing the efficacy of
these two drugs is ongoing (8). We
have preliminary results of phase III clinical studies comparing
the efficacy of third-generation inhibitors (i.e., osimertinib in
the FLAURA clinical trial, ASP8273 in the SOLAR clinical trial, and
rociletinib in the TIGER-1 clinical trial) and erlotinib or
gefitinib in first-line treatment of NSCLC patients with
EGFR mutations (see clinicaltrials.gov). In the meantime, our knowledge of
the effectiveness of various EGFR-TKIs comes from a limited number
of retrospective studies comparing gefitinib, erlotinib, and
afatinib.
The second serious problem related to EGFR-TKIs
administration is the lack of reliable knowledge about their
efficacy in patients with rare EGFR mutations. While
gefitinib, erlotinib, and afatinib have proven efficacy in patients
with the two major mutations in the EGFR gene (i.e., the
classical Glu746-Ala750 deletion in exon 19 and the common
p.Leu858Arg substitution in exon 21), their effectiveness in NSCLC
cases with rare EGFR mutations remains unclear. Molecular
tests based on real-time PCR techniques detect several rare
EGFR mutations, including: Different substitutions in codons
709 and 719 in exon 18, substitutions and insertions in exon 20, as
well as different substitutions in codons 858 and 861 in exon 21
(9). Such tests have confirmed that
rare EGFR mutations occur more frequently than previously
thought Results from the French National Cancer Institute network
(ERMETIC-IFCT) indicated that ~10% of EGFR-mutated NSCLC
patients may have rare EGFR gene mutations (10). Similarly, in our recent multicenter
study in Poland, we showed that 14.77% of patients with
EGFR-mutated NSCLC had rare mutations (11). Despite this, only a few retrospective
analyses have investigated the efficacy of EGFR-TKIs in patients
with rare EGFR mutations. Therefore, the predictive value of
rare EGFR mutations for deciding on the first-line treatment
option in patients with NSCLC remains unclear.
In this study, we conducted a retrospective analysis
of the effectiveness of different EGFR-TKIs in NSCLC patients with
common and rare EGFR mutations. To the best of our
knowledge, this is the first study worldwide to compare the
efficacy of erlotinib, gefitinib, and afatinib in patients with
rare and common EGFR mutations.
Materials and methods
Study population
This study was approved by the Local Bioethics
Committee of Medical University of Lublin. We retrospectively
analyzed clinical outcomes in 180 NSCLC patients (95% with
adenocarcinoma diagnosis) with different EGFR mutations, who
had received erlotinib (n=98), gefitinib (n=66), or afatinib (n=16)
therapy in four oncology centers in Poland (Warsaw, Lublin, Poznan,
and Lodz). All patients had clinically proven recurrent or locally
advanced or metastatic NSCLC. Patients with brain metastases
controlled with radiotherapy or neurosurgery without intensive
steroid therapy were included in the study. EGFR-TKIs were
administered orally at a daily dose of 150 mg for erlotinib, 250 mg
for gefitinib, and 40 mg for afatinib, and the cycle repeated every
28 days. The clinical parameters collected at the beginning of
EGFR-TKIs treatment included: Age, gender, smoking status
(including pack-years assessment), performance status (PS), stage
of disease, pathomorphological diagnosis, line of EGFR-TKIs
treatment, and information about prior surgical treatment.
Treatment was continued until progression or
unacceptable toxicity. After discontinuation of EGFR-TKIs
treatment, patients could receive chemotherapy or palliative
radiotherapy. During this study, third-generation of EGFR-TKIs
(e.g. osimertinib) have not been available in Poland. Also,
therapeutic programs in Poland did not allow any possibility of
switching the type of EGFR-TKIs in patients after progression on
EGFR-TKIs. Five patients (2.8%) had early, no treatment-related
toxicity of grade 4 and required discontinuation of EGFR-TKIs
treatment. They were not included in our survival analysis.
Response was assessed according to the Response
Evaluation Criteria in Solid Tumors (RECIST) guideline (version
1.1) and evaluation was performed by computed tomography every 2
months of EGFR-TKIs treatment. The treatment toxicity was assessed
by Common Toxicity Criteria (CTC) scale (version 4.0). Performance
status was evaluated according to Eastern Cooperative Oncology
Group (ECOG) scale.
EGFR gene mutations analysis
DNA was extracted from tumor tissue or tumor cells
obtained during routine diagnostic or therapeutic procedures
(bronchoscopy, endobronchial ultrasound-guided transbronchial
needle aspiration, mediastinoscopy, or surgical resection).
Formalin-fixed paraffin-embedded materials, or cytological slides
containing at least 10% of tumor cells, were used for molecular
examination. Mutations of the EGFR gene (NM_0,05228.3) were
tested using routine real-time PCR procedures and the EntroGen EGFR
Mutations Analysis kit (USA). The mutations in exons 18 to 21 were
examined (Table I). Non-classical
deletions in exon 19 were distinguished from the classical deletion
in exon 19 by a direct sequencing method.
 | Table I.Type of examined mutations in the
EGFR gene. |
Table I.
Type of examined mutations in the
EGFR gene.
| Exon | Type of
mutations |
|---|
| 18 | p.Gly719Ala
(c.2156G>C), p.Gly719Ser (c.2155G>A), p.Gly719Cys
(c.2155G>T) |
| 19 | c.2235–2249 del 15,
c.2235–2252>AAT del 18, c.2236–2253 del 18, c.2237–2251 del 15,
c.223c.7–2254 del 18, c.2237–2255>T del 19, c.2236–2250 del 15,
c.2238–2255 del 18, c.2238–2248>GC del 11, c.2238–2252>GCA
del 15, c.2239–2247 del 9, c.2239–2253 del 15, c.2239–2256 del 18,
c.2239–2248>C del 10, c.2239–2258>CA del 20, c.2240–2251 del
12, c.2240–2257 del 18, c.2240–2254 del 15, c.2239–2251>C del
13 |
| 20 | p.Thr790Met
(c.2369C>T), p.Ser768Ile (c.2303G>T), c.2307–2308 ins
GCCAGCGTG, c.2319–2320 ins CAC, c.2310–2311 ins GGT |
| 21 | p.Leu858Arg
(c.2573T>G), p.Leu861Gln (c.2582T>A) |
Statistical analysis
Treatment outcomes included response rate, disease
control rate, progression free survival (PFS), and overall survival
(OS). PFS and OS were defined as the time elapsed between the date
of EGFR-TKIs treatment beginning and the date of disease
progression or death, respectively. In the absence of information
about the progression or death, data were classified as censored
(time was calculated to the last observation). Statistical analysis
was performed using Statistica 10 (Statsoft, USA) and MedCalc 10
(MedCalc Software, Belgium). A P<0.05 was considered
statistically significant. Using the Fisher's exact test, we
assessed the associations between clinical factors and response
rate or disease control rate. The Kaplan-Meier log-rank test was
used to draw a comparison curve evaluating the survival probability
(PFS and OS). Cox regression model with a stepwise selection with
minimum AIC factor (Akaike Information Criterion) was used to
determine the influence of clinical and genetic factors on PFS and
OS.
Results
Patient characteristics
The groups of patients treated with different
EGFR-TKIs were comparable with respect to their demographic,
clinical, and molecular factors (Table
II). The median age of all patients was 67 years and 55%
patients were 67 years of age or older. 71% of EGFR-TKIs treated
patients were women, and 43%-non-smokers. Smokers with EGFR
mutations were rather heavy smokers, with a median pack-year
history of 20. Most patients had a diagnosis of adenocarcinoma
(95%) and distant metastases (81%), including brain metastases
(15%). NSCLC recurrence after surgery was found in 17% of patients.
All patients were in very good or good performance status.
EGFR-TKIs were used in the first (72%), second (24%), or third-line
(3.9%) of treatment. In progression after EGFR-TKIs therapy, 41% of
patients obtained palliative radiotherapy and 39% of patients
received one (30%) or more (9.4%) lines of chemotherapy.
 | Table II.Patients' characteristics. |
Table II.
Patients' characteristics.
| Characteristic | Total (n=180) | Erlotinib
(n=98) | Gefitinib
(n=66) | Afatinib
(n=16) |
|---|
| Age |
|
|
|
|
| Age
(years, median ± SD) | 67±11.8 | 67±11.09 | 69±13.06 | 62±9.84 |
| ≥67
years (n, %) | 99,55 | 52,53.1 | 42,63.6 | 5,31.25 |
| <67
years (n, %) | 81,45 | 46,46.9 | 24,36.4 | 11,68.75 |
| Gender |
|
|
|
|
| Female
(n, %) | 128,71.1 | 72,73.5 | 46,69.7 | 10,62.5 |
| Male
(n, %) | 52,28.9 | 26,26.4 | 20,30.3 | 6,37.5 |
| Histopathological
diagnosis |
|
|
|
|
|
Adenocarcinoma (n, %) | 171,95.0 | 92,93.9 | 64,97.0 | 15,94.0 |
|
Adenosquamous carcinoma (n,
%) | 3,1.7 | 2,2.0 | 1,1.5 | 0,0.0 |
| Large
cell carcinoma (n, %) | 2,1.1 | 1,1.0 | 0,0.0 | 1,6.0 |
| NOS (n,
%) | 4,2.2 | 3,3.1 | 1,1.5 | 0,0.0 |
| Performance status
(PS) |
|
|
|
|
| PS=0
(n, %) | 33,18.3 | 23,23,5 | 8,12.1 | 2,12.5 |
| PS≥1
(n, %) | 147,81.7 | 75,76.5 | 58,87.9 | 14,87.5 |
| Stage of
disease |
|
|
|
|
| IIIB
(n, %) | 33,18.3 | 22,22.4 | 8,12.1 | 3,18.8 |
| IV (n,
%) | 147,81.7 | 76,77.6 | 58,87.9 | 13,81.2 |
| CNS metastases |
|
|
|
|
| Yes (n,
%) | 28,15.6 | 16,16.3 | 12,18.2 | 0,0.0 |
| No (n,
%) | 152,84.4 | 82,83.7 | 54,81.8 | 16,100.0 |
| Prior surgical
treatment |
|
|
|
|
| Yes (n,
%) | 31,17.2 | 14,14.3 | 13,19.7 | 4,25 |
| No (n,
%) | 149,82.8 | 84,85.7 | 53,80.3 | 12,75 |
| Smoking
history |
|
|
|
|
| Yes (n,
%) | 59,32.8 | 30,30.6 | 23,34.8 | 6,37.5 |
| No (n,
%) | 78,43.3 | 47,48.0 | 22,33.3 | 9,56.25 |
| No data
(n, %) | 43,23.9 | 21,21.4 | 21,31.8 | 1,6.25 |
|
Pack-years (median ± SD) | 20±12.4 | 20±11.14 | 20±14.35 | 22.5±9.91 |
| Line of EGFR-TKI
therapy |
|
|
|
|
| I (n,
%) | 129,71.7 | 53,54.1 | 63,95.5 | 13,81.2 |
| II or
III (n, %) | 51,28.3 | 45,45.9 | 3,4.5 | 3,18.8 |
| Early EGFR-TKI
therapy discontinuation due to grade 3–4 toxicities |
|
|
|
|
| Yes (n,
%) | 175,97.2 | 96,98 | 64,97 | 15,94.0 |
| No (n,
%) | 5,2.8 | 2,2 | 2,3 | 1,6.0 |
| Chemotherapy after
EGFR-TKI treatment |
|
|
|
|
| Yes (n,
%) | 71,39.4 | 38,38.8 | 28,42.4 | 5,31.3 |
| No (n,
%) | 109,60.4 | 60,61.2 | 38,57.6 | 11,68.7 |
| Palliative
radiotherapy after EGFR-TKI treatment |
|
|
|
|
| Yes (n,
%) | 73,40.6 | 42,42.9 | 30,45.5 | 1,6.0 |
| No (n,
%) | 107,59.4 | 56,57.1 | 36,54.5 | 15,94.0 |
| EGFR
mutations status |
|
|
|
|
|
Deletion in exon 19 (n,
%) | 115,63.9 | 59,60.2 | 46,69.7 | 10,62.5 |
|
Substitution p.Leu858Arg (n,
%) | 52,28.9 | 32,32.7 | 16,24.2 | 4,25.0 |
| Rare
mutations (n, %) | 13,7.2 | 7,7.1 | 4,6.1 | 2,12.5 |
Classical exon 19 deletions were found in 64% of
patients, exon 21 p.Leu858Arg substitution was found in 29% of
patients, and a relatively large population of patients (7.2%) had
rare EGFR mutations. Among the rare EGFR mutations,
insertions in exon 20 were most frequently diagnosed (23% of rare
mutations), while double mutations of substitutions in codon 719
and 861 or 768 were found in two patients (15% of rare mutations).
Detailed characteristics of the 13 NSCLC patients with rare
EGFR gene mutations are outlined in Table II.
Response rates
Partial response (PR), complete response (CR), and
disease control was achieved in 55, 3.3, and 80.5% of patients,
respectively, while early progression occurred in 14.5% of patients
treated with EGFR-TKIs (Table III).
Demographic and clinical factors had no significant impact on the
risk of NSCLC progression (Table
III). Response to EGFR-TKIs was significantly (P<0.05) more
frequent in patients with a deletion in exon 19 than in patients
with rare mutations in EGFR gene. All patients with an
insertion in exon 20 of EGFR gene showed early disease
progression. All other patients with rare EGFR gene
mutations (including patients with p.Ser768Ile substitution in exon
20) responded to treatment, except for a female patient with
substitution at codon 747 of exon 19 and a female patient with
double mutations in codons 719 and 768 in exons 18 and 20, in whom
short stabilization of the disease occurred. The detailed
characteristics of the response to treatment in the 13 NSCLC
patients with rare EGFR gene mutations are provided in
Table III.
 | Table III.Response to EGFR-TKI treatment
according to demographic and clinical characteristics. |
Table III.
Response to EGFR-TKI treatment
according to demographic and clinical characteristics.
| Characteristic | Partial
response+complete response (n=105) | Stable disease
(n=49) | Progressive disease
(n=26) | p;
χ2 |
|---|
| Age |
|
|
|
|
| ≥67
years, n=99 (n, %) | 57,57.5 | 25,25.3 | 17,17.2 | 0.479;1.468 |
| <67
years, n=81 (n, %) | 48,59.3 | 24,29.6 | 9,11.1 |
| Gender |
|
|
|
|
| Female,
n=128 (n, %) | 78,60.9 | 35,27.3 | 15,11.7 | 0.247;2.796 |
| Male,
n=52 (n, %) | 27,51.9 | 14,26.9 | 11,21.2 |
| Histopathological
diagnosis |
|
|
|
|
|
Adenocarcinoma, n=171 (n,
%) | 99,57.9 | 47,27.5 | 25,14.6 | 0.872;0.273 |
| Other,
n=9 (n, %) | 6,66.7 | 2,22.2 | 1,11.1 |
|
| Performance status
(PS) |
|
|
|
|
| PS=0,
n=33 (n, %) | 24,72.7 | 7,21.2 | 2,6.1 | 0.14;3.938 |
| PS≥1,
n=147 (n, %) | 81,55.1 | 42,28.6 | 24,16.3 |
| Stage of
disease |
|
|
|
|
| IIIB,
n=33 (n, %) | 22,66.7 | 7,21.2 | 4,12.1 | 0.558;1.168 |
| IV,
n=147 (n, %) | 83,56.5 | 42,28.5 | 22,15.0 |
|
| CNS metastases |
|
|
|
|
| Yes,
n=28 (n, %) | 16 | 7 | 5 | 0.845;0.336 |
| No,
n=152 (n, %) | 89 | 42 | 21 |
|
| Prior surgical
treatment |
|
|
|
|
| Yes,
n=31 (n, %) | 21,67.7 | 6,19.4 | 4,12.9 | 0.477;1.481 |
| No,
n=149 (n, %) | 84,56.4 | 43,28.9 | 22,14.8 |
|
| Smoking
history |
|
|
|
|
| Yes,
n=59 (n, %) | 39,66.1 | 13,22.0 | 7,11.9 | 0.643;0.883 |
| No,
n=78 (n, %) | 46,59.0 | 19,24.4 | 13,16.6 |
|
|
|---|
| Line of EGFR-TKI
therapy |
|
|
|
|
| I,
n=129 (n, %) | 75,58.1 | 37,28.7 | 17,13.2 | 0.649;0.865 |
| II or
III, n=51 (n, %) | 30,58.8 | 12,23.5 | 9,17.6 |
| Type of EGFR-TKI
(reversible vs. irreversible) |
|
|
|
|
|
Erlotinib and gefitinib, n=164
(n, %) | 95,58.0 | 46,28.0 | 23,14.0 | 0.69;0.741 |
|
Afatinib, n=16 (n, %) | 10,62.5 | 3,18.75 | 3,18.75 |
|
| Type of EGFR-TKI
(only reversible) |
|
|
|
|
|
Erlotinib, n=98 (n, %) | 58,59.2 | 26,26.5 | 14,14.3 | 0.87;0.278 |
|
Gefitinib, n=66 (n, %) | 37,56.1 | 20,30.3 | 9,13.6 |
|
| Type of
EGFR-TKI (erlotinib vs. afatinib) |
|
|
|
|
|
Erlotinib, n=98 (n, %) | 58,59.2 | 26,26.5 | 14,14.3 | 0.764;0.537 |
|
Afatinib, n=16 (n, %) | 10,62.5 | 3,18.75 | 3,18.75 |
|
| Type of EGFR-TKI
(gefitinib vs. afatinib) |
|
|
|
|
|
Gefitinib, n=66 (n, %) | 37,56.1 | 20,30.3 | 9,13.6 | 0.626;0.936 |
|
Afatinib, n=16 (n, %) |
| 10,62.5 | 3,18.75 | 3,18.75 |
| EGFR mutations
status (only common) |
|
|
|
|
|
Deletion in exon 19, n=115 (n,
%) | 66,57.4 | 36,31.3 | 13,11.3 | 0.302;2.396 |
|
Substitution p.Leu858Arg, n=52
(n, %) | 32,61.5 | 11,21.2 | 9,17.3 |
| EGFR mutations
status (common vs. rare) |
|
|
|
|
| Common
mutations, n=167 (n, %) | 98,58.7 | 47,28.1 | 22,13.2 | 0.178;3.355 |
| Rare
mutations, n=13 (n, %) | 7,53.8 | 2,15.4 | 4,30.8 |
| EGFR mutations
status (deletion in exon 19 vs. rare) |
|
|
|
|
|
Deletion in exon 19, n=115 (n,
%) | 66,57.4 | 36,31.3 | 13,11.3 | 0.113;4.355 |
| Rare
mutations, n=13 (n, %) | 7,53.8 | 2,15.4 | 4,30.8 |
| EGFR
mutations status (substitution p.Leu858 arg vs. rare) |
|
|
|
|
|
Substitution p.Leu858Arg, n=52
(n, %); 1.218 | 32,61.5 | 11,21.2 | 9,17.3 | 0.544 |
| Rare mutations,
n=13 (n, %) | 7,53.8 | 2,15.4 | 4,30.8 |
Progression-free survival
The median PFS of patients receiving EGFR-TKIs
treatment was 10 months, and 34.3% had no disease progression
during observation. A comparison of the probability of PFS in NSCLC
patients with EGFR gene mutations treated with erlotinib,
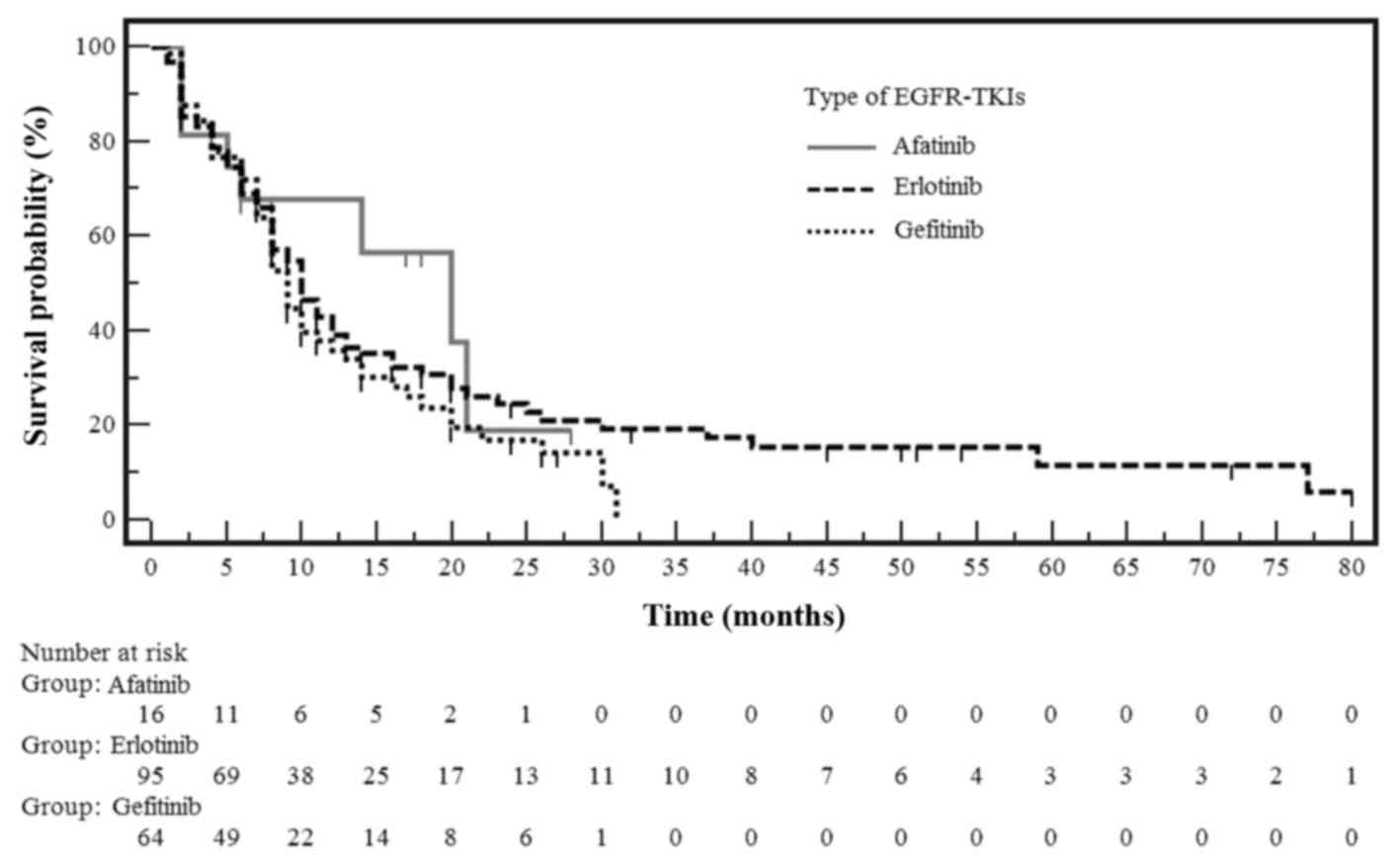
gefitinib or afatinib is shown in Fig.
1. The median PFS was 10 months in patients treated with
erlotinib, 9 months in patients treated with gefitinib, and 15
months in patients treated with afatinib (Table IV). Although patients treated with
afatinib showed the longest median PFS, it was not significantly
different from that observed with the other EGFR-TKIs.
 | Table IV.Progression-free survival (PFS) in
NSCLC patients treated with EGFR-TKIs. |
Table IV.
Progression-free survival (PFS) in
NSCLC patients treated with EGFR-TKIs.
|
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|
|---|
| Characteristic | Median PFS
(months) | P | HR (95% CI) | P | HR (95% CI) |
|---|
| Age |
|
|
|
|
|
| ≥67
years | 10 | 0.799 | 0.959
(0.679–1.679) | 0.664 | 0.921
(0.638–1.638) |
| <67 years | 10 |
|
|
|
|
| Gender |
|
|
|
|
|
|
Female | 10 | 0.094 | 1.345
(0.914–1.914) | 0.107 | 1.374
(0.935–2.935) |
|
Male | 8 |
|
|
|
|
| Histopathological
diagnosis |
|
|
|
|
|
|
Adenocarcinoma | 10 | 0.997 | 1.001
(0.468–2.468) | 0.532 | 1.294
(0.579–2.579) |
|
Other | 11 |
|
|
|
|
| Performance status
(PS) |
|
|
|
|
|
|
PS=0 | 10 | 0.399 | 1.208
(0.785–1.785) | 0.896 | 1.036
(0.611–1.611) |
|
PS≥1 | 10 |
|
|
|
|
| Stage of
disease |
|
|
|
|
|
|
IIIB | 13 | 0.191 | 1.343
(0.879–2.879) | 0.242 | 1.37
(0.811–2.811) |
| IV | 9 |
|
|
|
|
| CNS metastases |
|
|
|
|
|
|
Yes | 9 | 0.256 | 1.277
(0.79–2.79) | 0.421 | 1.214
(0.758–1.758) |
| No | 10 |
|
|
|
|
| Prior surgical
treatment |
|
|
|
|
|
|
Yes | 16 | 0.094 | 1.432
(0.956–2.956) | 0.285 | 1.307
(0.802–2.802) |
| No | 9 |
|
|
|
|
| Smoking
history |
|
|
|
|
|
|
Yes | 9 | 0.448 | 1.139
(0.806–1.806) | 0.859 | 1.035
(0.713–1.713) |
| No | 10 |
|
|
|
|
| Line of EGFR-TKI
therapy |
|
|
|
|
|
| I | 10 | 0.325 | 0.841
(0.588–1.588) | 0.23 | 0.787
(0.533–1.533) |
| II or
III | 10 |
|
|
|
|
| Type of EGFR-TKI
(reversible vs. irreversible) |
|
|
|
|
|
|
Erlotinib and gefitinib | 10 | 0.533 | 1.243
(0.648–2.648) | 0.454 | 1.333
(0.63–2.63) |
|
Afatinib | 18 |
|
|
|
|
| Type of EGFR-TKI
(only reversible) |
|
|
|
|
|
|
Erlotinib | 10 | 0.25 | 1.219
(0.847–1.847) |
|
|
|
Gefitinib | 9 |
|
|
|
|
| Type of EGFR-TKI
(erlotinib vs. afatinib) |
|
|
|
|
|
Erlotinib | 10 | 0.623 | 1.191
(0.586–2.586) |
|
|
|
Afatinib | 18 |
|
|
|
|
| Type of EGFR-TKI
(gefitinib vs. afatinib) |
|
|
|
|
|
Gefitinib | 9 | 0.431 | 1.329
(0.655–2.655) |
|
|
|
Afatinib | 18 |
|
|
|
|
| EGFR
mutations status (only common) |
|
|
|
|
|
Deletion in exon 19 | 11 | 0.095 | 1.361
(0.909–2.909) |
|
|
| Substitution
p.Leu858Arg | 8 |
|
|
|
|
| EGFR
mutations status (deletion in exon 19 vs. rare) |
|
|
|
|
|
Deletion in exon 19 | 11 | 0.0043 | 0.429
(0.099–0.099) |
|
|
| Rare
mutations | 5 |
|
|
|
|
| EGFR
mutations status (substitution p.Leu 858Arg vs. rare) |
|
|
|
|
|
Substitution p.Leu858Arg | 8 | 0.115 | 0.616
(0.236–1.236) |
|
|
| Rare
mutations | 5 |
|
|
|
|
| EGFR
mutations status (common vs. rare) |
|
|
|
|
| Common
mutations | 10 | 0.009 | 2.155
(0.907–5.907) | 0.008 | 2.437
(1.263–4.263) |
| Rare
mutations | 5 |
|
|
|
|
Demographic and clinical factors had no significant
impact on the PFS of patients treated with EGFR-TKIs. While,
molecular factors did influence the clinical outcomes of EGFR-TKIs
treatment (Table IV). Patients with
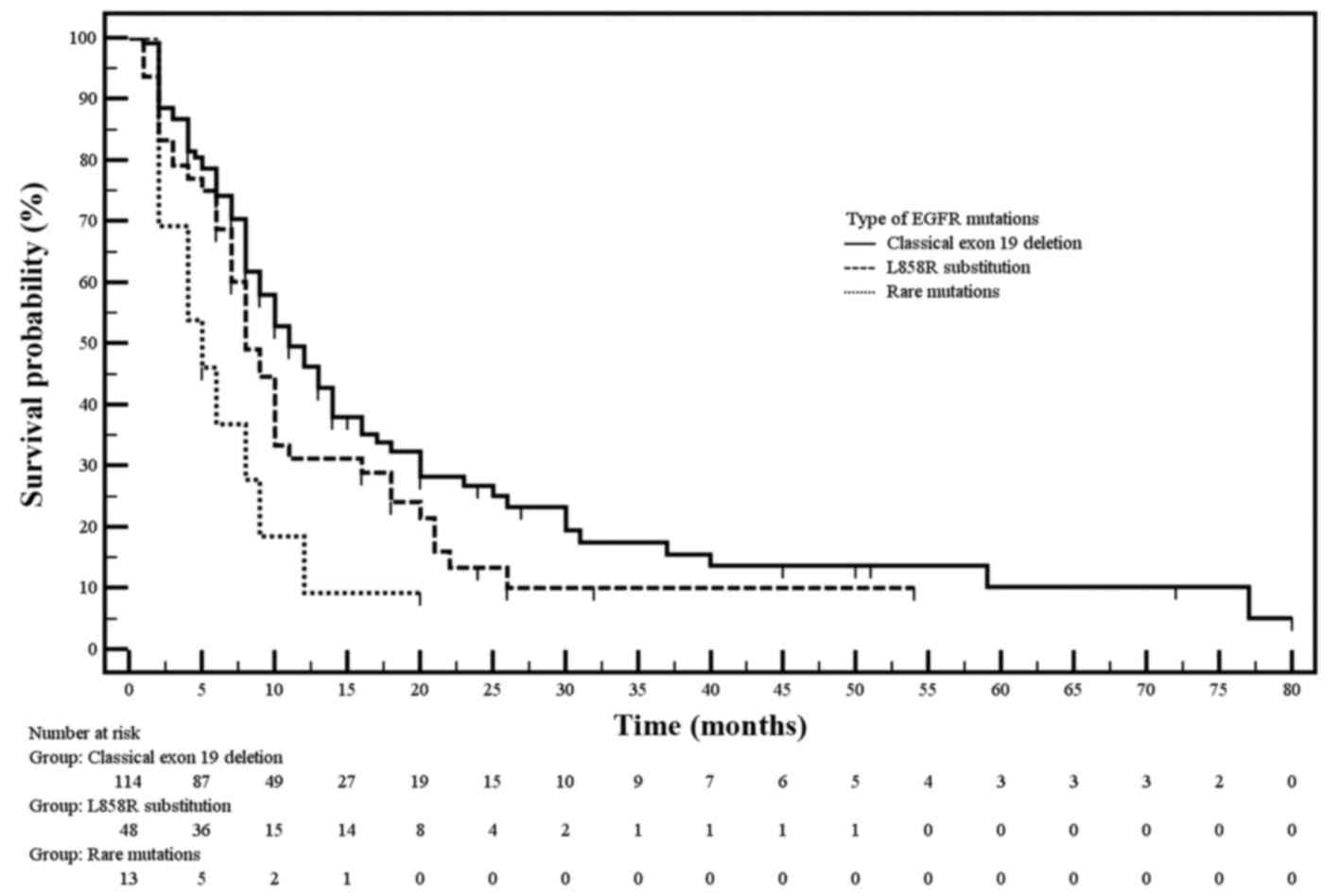
common EGFR mutations showed significantly longer median PFS
than patients with rare EGFR mutations (10 vs. 5 months;
P=0.009). The significant difference in median PFS occurred between
the group of patients with exon 19 deletion and group of patients
with rare EGFR mutations (P<0.005). The median PFS was
only slightly longer in patients with substitution p.Leu858Arg
compared to patients with rare EGFR mutations. Moreover,
insignificant (P=0.095) longer median PFS was observed in patients
with exon 19 deletion than in patients with p.Leu858Arg
substitution (Table IV, Fig. 2).
Overall survival
Demographic, clinical, and molecular factors did not
affect the median OS (27 months) in our study (Table V). One-year and two-years OS for
patients treated with EGFR-TKIs was 66.3 and 26.3%, respectively. A
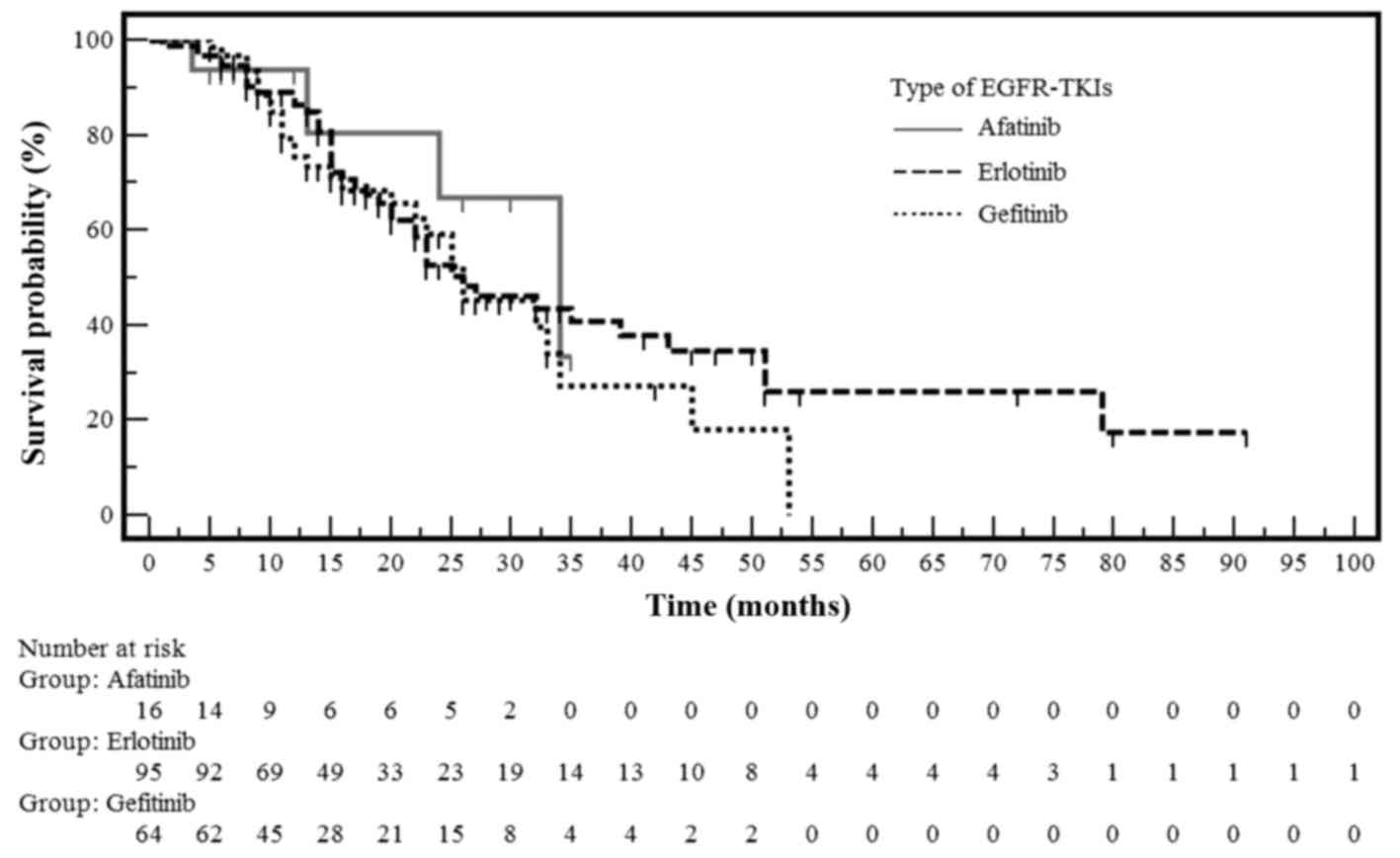
comparison of the probability of OS in NSCLC patients with
EGFR gene mutations treated with erlotinib, gefitinib or
afatinib is shown in Fig. 3. The
median OS was 26 months in the gefitinib and erlotinib groups,
whereas in the afatinib group, the median OS had not been reached
at the time of the analysis (Table
V). However, these differences in OS were not statistically
significant among the three treatment arms.
 | Table V.Overall survival (OS) in NSCLC
patients treated with EGFR-TKIs. |
Table V.
Overall survival (OS) in NSCLC
patients treated with EGFR-TKIs.
|
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|
|---|
| Characteristic | Median OS
(months) | P | HR (95% CI) | P | HR (95% CI) |
|---|
| Age |
| ≥67
years | 32 | 0.482 | 0.853
(0.536–1.536) | 0.373 | 0.797
(0.485–1.485) |
| <67
years | 25 |
|
|
|
|
| Gender |
|
Female | 32 | 0.25 | 1.322
(0.784–2.784) | 0.224 | 1.405
(0.814–2.814) |
|
Male | 23 |
|
|
|
|
| Histopathological
diagnosis |
|
Adenocarcinoma | 26 | 0.686 | 0.815
(0.27–2.27) | 0.896 | 0.929
(0.308–2.308) |
|
Other | 22 |
|
|
|
|
| Performance status
(PS) |
|
PS=0 | 32 | 0.447 | 1.263
(0.713–2.713) | 0.598 | 0.82
(0.394–1.394) |
|
PS≥1 | 26 |
|
|
|
|
| Stage of
disease |
|
IIIB | Not reached | 0.077 | 1.829
(1.038–3.038) | 0.092 | 2.013
(0.895–4.895) |
| IV | 26 |
|
|
|
|
| CNS metastases |
|
Yes | 23 | 0.405 | 1.282
(0.668–2.668) | 0.553 | 1.235
(0.617–2.617) |
| No | 26 |
|
|
|
|
| Prior surgical
treatment |
|
Yes | 51 | 0.181 | 1.477
(0.867–2.867) | 0.333 | 1.39
(0.716–2.716) |
| No | 25 |
|
|
|
|
| Smoking
history |
|
Yes | 26 | 0.706 | 1.089
(0.692–1.692) | 0.873 | 0.96
(0.581–1.581) |
| No | 26 |
|
|
|
|
| Line of EGFR-TKI
therapy |
| I | 26 | 0.841 | 0.955
(0.602–1.602) | 0.807 | 0.939
(0.568–1.568) |
| II or
III | 26 |
|
|
|
|
| Type of EGFR-TKI
(reversible vs. irreversible) |
|
Erlotinib and gefitinib | 26 | 0.408 | 1.77
(0.603–5.603) | 0.455 | 1.739
(0.41–7.41) |
|
Afatinib | Not reached |
|
|
|
|
| Type of
EGFR-TKI (only reversible) |
|
Erlotinib | 26 | 0.353 | 1.238
(0.768–1.768) |
|
|
|
Gefitinib | 26 |
|
|
|
|
| Type of
EGFR-TKI (erlotinib vs afatinib) |
|
Erlotinib | 26 | 0.418 | 1.754
(0.513–5.513) |
|
|
|
Afatinib | Not reached |
|
|
|
|
| Type of
EGFR-TKI (gefitinib vs afatinib) |
|
Gefitinib | 26 | 0.425 | 1.739
(0.497–5.497) |
|
|
|
Afatinib | Not reached |
|
|
|
|
| Chemotherapy after
EGFR-TKI treatment |
|
Yes | 26 | 0.677 | 0.909
(0.576–1.576) | 0.679 | 1.11
(0.679–1.679) |
| No | 26 |
| Palliative
radiotherapy after EGFR-TKI treatment |
|
Yes | 26 | 0.434 | 1.191
(0.757–1.757) | 0.8 | 0.931
(0.538–1.538) |
| No | 32 |
|
|
|
|
| EGFR
mutations status (only common) |
|
Deletion in exon 19 | 27 | 0.604 | 1.143
(0.669–1.669) |
|
|
|
Substitution p.Leu858Arg | 26 |
|
|
|
|
| EGFR
mutations status (deletion in exon 19 vs. rare) |
|
Deletion in exon 19 | 27 | 0.201 | 0.588
(0.166–1.166) |
|
|
| Rare
mutations | 22 |
|
|
|
|
| EGFR
mutations status (substitution p.Leu858 arg vs. rare) |
|
Substitution p.Leu858Arg | 26 | 0.799 | 0.885
(0.304–2.304) |
|
|
| Rare
mutations | 22 |
|
|
|
|
| EGFR
mutations status (common vs. rare) |
| Common
mutations | 26 | 0.251 | 1.605
(0.576–4.576) | 0.31 | 1.592
(0.652–3.652) |
| Rare
mutations | 22 |
Adverse events
Severe, no treatment-related toxicity (grade 4)
resulting in discontinuation of EGFR-TKIs treatment only occurred
in five patients in our study. Afatinib and erlotinib showed
significantly more frequent rash and other skin toxicities, as well
as diarrhea, compared to gefitinib. All patients treated with
afatinib showed mild diarrhea. Hepatotoxicity occurred only in
three patients treated with gefitinib.
Discussion
This study was the first to directly compare
EGFR-TKIs treatment efficacy in patients with NSCLC harboring
common and rare activating EGFR mutations. Erlotinib,
gefitinib, and afatinib had similar effectiveness in patients with
common and rare EGFR mutations, although patients treated
with afatinib had a slightly longer PFS. A relatively large
proportion of our patients (7.2%) had rare EGFR gene
mutations, and these patients had significantly poorer median PFS
than those with common EGFR mutations (P<0.05). Moreover,
patients with a rare insertion in exon 20 of EGFR gene
showed early disease progression. Therefore, detection of specific
EGFR mutations is important for EGFR-TKIs treatment
outcomes.
Similar to our results, a previous meta-analysis by
Liang et al indicated that erlotinib, gefitinib, and
afatinib have equivalent efficacy. This meta-analysis included
twelve phase III global clinical trials involving 1812 NSCLC
patients with activating EGFR gene mutations (12). Authors reported a 1-year PFS of 43%,
compared to 34% in our study. Moreover, 1-and 2-year OS rates were
79 and 50% in Liang study, compared to 66 and 26% in our study. The
slight improvement in EGFR-TKIs efficacy shown by Liang et
al (12) compared to us may be
due to the fact that the patients in the clinical trials were
closely matched. Moreover, most clinical trials enrolled an Asian
patients compared to the Caucasian population used in our study.
However, the nonrandomized design of our study did not allow a
reliable assessment of the efficacy of particular EGFR-TKIs.
Despite the lower efficacy of the EGFR-TKIs observed
in our study, the type and severity of adverse events was similar
to those described in previous clinical trials (12). For example, afatinib and erlotinib
resulted in a more severe rash and diarrhea in patients compared
with gefitinib. Therefore, our results in a Caucasian cohort
indicate that EGFR-TKIs are effective and show similar side effect
profiles to previous studies in Asian populations.
Similar to our findings, Lim et al showed no
difference in PFS between the erlotinib- and gefitinib-treated
groups (11.7 vs. 14.5 months; P=0.507) in a retrospective
case-control study of matched Asian patients (121 pairs) with NSCLC
(13). These patients were young
(median age 58 years), mostly non-smokers (64%), in very good or
good performance status (91%), and received EGFR-TKIs treatment
mainly in the second-line (74%) Patients showed excellent overall
response rates to erlotinib and gefitinib (77 and 74.5%,
respectively) (13). However, Kim
et al showed the drugs were not as efficient in a randomized
phase II study of 96 Asian patients with advanced NSCLC: In the
erlotinib- and gefitinib-treatment arms the response rates were 40
and 48%, and the median PFS was only 3.1 and 4.9 months,
respectively (6). The authors
concluded that the reason for treatment failure was including
patients with unknown EGFR gene mutations with at least two
out of three clinical factors associated with a higher incidence of
EGFR gene mutations (6).
Similarly, in a recent randomized phase III study of 562 pretreated
patients with lung adenocarcinoma (including 401 with EGFR
mutations), the response rates were 44 and 46% and the median PFS
was 7.5 and 6.5 months in erlotinib- and gefitinib-treatment arms,
respectively (14).
The first head-to-head comparison of afatinib and
gefitinib was recently reported in the prospective phase IIb
LUX-Lung 7 clinical trial (7). In
this trial, 319 Caucasian and Asian NSCLC patients with common
EGFR gene mutations were randomized to first-line therapy
with afatinib or gefitinib (7).
Afatinib showed significant improvement in PFS, with a median
duration of response of 10.1 months compared to 8.4 months with
gefitinib (HR=0.73; 95% CI, 0.57–0.95; P=0.0165) (7). Similarly, in our study, we found a
slight improvement in PFS with afatinib (18 months) compared to the
two reversible EGFR-TKIs (10 months) although this was not
statistically significant (HR=1.243; 95% CI, 0.648–2.382; P=0.533).
However, due to the small sample size (n=16) of the
afatinib-treated group, we cannot make any definitive conclusions
about the observed difference in PFS.
We found that demographic and clinical factors did
not affect the effectiveness of the EGFR-TKIs treatment of patients
harboring EGFR gene mutations. While some authors have
emphasized the impact of patients' performance status on the
effectiveness of EGFR-TKIs treatment. The differences in EGFR-TKIs
effectiveness in past studies were only found when groups of
patients in good and very good performance status (PS=0 or 1) were
compared with groups of patients with satisfactory performance
status (PS=2) (15). Such comparison
was not performed in the current study (only patients with PS=0 or
1 were included). Therefore, the impact of performance status on
EGFR-TKIs requires further investigation.
We found that patients with the common exon 19
deletion in EGFR had a slightly longer PFS after treatment
with EGFR-TKIs than patients with exon 21 p.Leu858Arg substitution
(11 vs. 8 months; P=0.095) or rare EGFR mutations (11 vs. 5
months; P<0.005). Urata et al found no significant
difference in the PFS among patients with the EGFR
p.Leu858Arg mutation (n=172), the EGFR exon 19 deletion
(n=192), or those with rare EGFR mutations (n=25) who were
treated with gefitinib and erlotinib (14). Zhang et al also showed that the
patients with the exon 19 deletion in EGFR receiving
first-line EGFR-TKIs had longer PFS than those with exon 21
substitution (16). Similarly, Urata
et al identified patients with the EGFR exon 19
deletion subgroup had slightly longer PFS when treated with
gefitinib and erlotinib than those with p.Leu858Arg mutation
(14). Furthermore, analysis of two
phase III trials, LUX-Lung 3 and LUX-Lung 6, indicated that the
first-line afatinib compared to chemotherapy improved OS for
patients with the EGFR exon 19 deletion but not for patients
with p.Leu858Arg substitution (17).
We found that the common EGFR mutations (exon 19 deletion
and p.Leu858Arg) did not impact the OS in this study. However, we
did not specifically investigate these differences in the
afatinib-treated group due to the small sample size (n=16).
Beau-Faller et al recently proved that rare
EGFR gene mutations could be associated with resistance to
EGFR-TKIs treatment (distal exon 20 insertions) or sensitivity to
EGFR-TKIs treatment (exon 18 substitution or complex EGFR
mutations) in Caucasian NSCLC patients (10). When investigating 50 NSCLC patients
with rare EGFR gene mutations treated with EGFR-TKIs, they
found that primary resistance to EGFR-TKIs was diagnosed in 54% of
patients with exon 20 mutations, in 66% of patients with exon 18
substitutions, and in 14% of patients with more complex EGFR
mutations (10). However, median OS
from EGFR-TKIs was better for patients with exon 18 (22 months)
than for patients with exon 20 mutations (9.5 months) (10). Our results fully agree with those of
Beau-Faller et al (10)
primarily finding that patients with exon 20 insertions of
EGFR failed to respond to EGFR-TKIs treatment. Resistance to
EGFR TKIs therapy has been associated with a Thr790Met substitution
in exon 20 of EGFR (18).
However, no patients with primary Thr790Met mutation were enrolled
in our study. By contrast, patients with non-classical exon 19
deletions (especially deletions of greater than 15 bp) and rare
substitutions (i.e., mutations in codon 858 and 861 in exon 21) had
good response to EGFR-TKIs treatment (19,20). Our
study confirms results indicating that rare EGFR mutations
are important for EGFR-TKIs treatment outcomes (21). However, further research is required
to build a database of all EGFR mutations and their
individual impact on the differing EGFR-TKIs treatments.
To combat treatment resistance, third-generation
EGFR-TKIs against the p.Thr790Met substitution in exon 20 of
EGFR have been developed, including osimertinib,
rociletinib, HM61713, ASP8273, EGF816, and PF-0,674,7775 (22). Recently, osimertinib has been
registered for treatment of p.Thr790Met positive patients after
failure of first- or second-generations EGFR-TKIs therapy. Clinical
trials on these third-generation EGFR-TKIs are currently underway.
However, further research is required to develop novel inhibitors
that combat resistance in some of the other rare EGFR
mutations.
Our study confirms that EGFR-TKIs treatment is
effective in NSCLC patients with EGFR gene mutations,
irrespective of demographic and clinical factors. We found no
significant differences in the effectiveness of erlotinib,
gefitinib, and afatinib among our Caucasian cohort of patients.
However, qualification of patients with rare EGFR gene
mutations, especially those with exon 20 insertions, to EGFR-TKIs
treatment requires special attention due to the varied
effectiveness of EGFR-TKIs treatment in this group of patients.
Acknowledgements
We thank all participating patients and their
families. We also thank Proper Medical Writing Sp. z o.o, Poland,
for editorial support and linguistic corrections.
References
|
1
|
Maemondo M, Inoue A, Kobayashi K, Sugawara
S, Oizumi S, Isobe H, Gemma A, Harada M, Yoshizawa H, Kinoshita I,
et al: Gefitinib or chemotherapy for non-small-cell lung cancer
with mutated EGFR. N Engl J Med. 362:2380–2388. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mok TS, Wu YL, Thongprasert S, Yang CH,
Chu DT, Saijo N, Sunpaweravong P, Han B, Margono B, Ichinose Y, et
al: Gefitinib or carboplatin-paclitaxel in pulmonary
adenocarcinoma. N Engl J Med. 361:947–957. 2010. View Article : Google Scholar
|
|
3
|
Rosell R, Carcereny E, Gervais R,
Vergnenegre A, Massuti B, Felip E, Palmero R, Garcia-Gomez R,
Pallares C, Sanchez JM, et al: Erlotinib versus standard
chemotherapy as first-line treatment for European patients with
advanced EGFR mutation-positive non-small-cell lung cancer
(EURTAC): A multicentre, open-label, randomised phase 3 trial.
Lancet Oncol. 13:239–246. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wu WS, Wu CH, Lai SL, Chiu CH, Shih JF,
Lee YC and Chen YM: Erlotinib salvage therapy in pulmonary
adenocarcinoma patients with disease progression after previous
EGFR-TKI treatment. Am J Clin Oncol. 39:556–562. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wu YL, Zhou C, Hu CP, Feng J, Lu S, Huang
Y, Li W, Hou M, Shi JH, Lee KY, et al: Afatinib versus cisplatin
plus gemcitabine for first-line treatment of Asian patients with
advanced non-small-cell lung cancer harbouring EGFR mutations
(LUX-Lung 6): An open-label, randomised phase 3 trial. Lancet
Oncol. 15:213–222. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kim ST, Uhm JE, Lee J, Sun JM, Sohn I, Kim
SW, Jung SH, Park YH, Ahn JS, Park K and Ahn MJ: Randomized phase
II study of gefitinib versus erlotinib in patients with advanced
non-small cell lung cancer who failed previous chemotherapy. Lung
Cancer. 75:82–88. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Park K, Tan E-H, Zhang L, Hirsh V, O'Byrne
K and Boyer M: LBA2_PR Afatinib (A) vs gefitinib (G) as first-line
treatment for patients (pts) with advanced non-small cell lung
cancer (NSCLC) harboring activating EGFR mutations: Results of the
global, randomized, open-label, Phase IIb trial LUX-Lung 7 (LL7).
Ann Oncol. 26 Suppl 9:ix161–ix162. 2015. View Article : Google Scholar
|
|
8
|
Ramalingam SS, O'Byrne K, Boyer M, Mok T,
Janne PA, Zhang H, Liang J, Taylor I, Sbar EI and Paz-Ares L:
Dacomitinib versus erlotinib in patients with EGFR-mutated advanced
nonsmall-cell lung cancer (NSCLC): Pooled subset analyses from two
randomized trials. Ann Oncol. 27:423–410. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Karachaliou N, Molina-Vila MA and Rosell
R: The impact of rare EGFR mutations on the treatment response of
patients with non-small cell lung cancer. Expert Rev Respir Med.
9:241–244. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Beau-Faller M, Prim N, Ruppert AM,
Nanni-Metéllus I, Lacave R, Lacroix L, Escande F, Lizard S, Pretet
JL, Rouquette I, et al: Rare EGFR exon 18 and exon 20 mutations in
non-small-cell lung cancer on 10 117 patients: A multicentre
observational study by the French ERMETIC-IFCT network. Ann Oncol.
25:126–131. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Krawczyk P, Reszka K, Ramlau R, PowrÓzek
T, Pankowski J, Wojas-Krawczyk K, Kalinka-Warzocha E, Szczęsna A,
Nicoś M, Jarosz B, et al: Prevalence of rare EGFR gene mutations in
nonsmall-cell lung cancer: A multicenter study on 3856 Polish
Caucasian patients. Ann Oncol. 27:358–359. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liang W, Wu X, Fang W, Zhao Y, Yang Y, Hu
Z, Xue C, Zhang J, Zhang J, Ma Y, et al: Network meta-analysis of
erlotinib, gefitinib, afatinib and icotinib in patients with
advanced non-small-cell lung cancer harboring EGFR mutations. PLoS
One. 9:e852452014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lim SH, Lee JY, Sun JM, Ahn JS, Park K and
Ahn MJ: Comparison of clinical outcomes following gefitinib and
erlotinib treatment in non-small-cell lung cancer patients
harboring an epidermal growth factor receptor mutation in either
exon 19 or 21. J Thorac Oncol. 9:506–511. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Urata Y, Katakami N, Morita S, Kaji R,
Yoshioka H, Seto T, Satouchi M, Iwamoto Y, Kanehara M, Fujimoto D,
et al: Randomized phase III study comparing gefitinib with
erlotinib in patients with previously treated advanced lung
adenocarcinoma: WJOG 5108L. J Clin Oncol. 34:3248–3257. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhou C and Yao LD: Strategies to improve
outcomes of patients with EGFR-mutant non-small cell lung cancer:
Review of the literature. J Thorac Oncol. 11:174–186. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang Y, Sheng J, Kang S, Fang W, Yan Y,
Hu Z, Hong S, Wu X, Qin T, Liang W and Zhang L: Patients with exon
19 deletion were associated with longer progression-free survival
compared to those with L858R mutation after first-line EGFR-TKIs
for advanced non-small cell lung cancer: A meta-analysis. PLoS One.
9:e1071612014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang JC, Wu YL, Schuler M, Sebastian M,
Popat S, Yamamoto N, Zhou C, Hu CP, O'Byrne K, Feng J, et al:
Afatinib versus cisplatin-based chemotherapy for EGFR
mutation-positive lung adenocarcinoma (LUX-Lung 3 and LUX-Lung 6):
Analysis of overall survival data from two randomised, phase 3
trials. Lancet Oncol. 16:141–151. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wu SG, Liu YN, Tsai MF, Chang YL, Yu CJ,
Yang PC, Yang JC, Wen YF and Shih JY: The mechanism of acquired
resistance to irreversible EGFR tyrosine kinase inhibitor-afatinib
in lung adenocarcinoma patients. Oncotarget. 7:12404–12413.
2016.PubMed/NCBI
|
|
19
|
de Pas T, Toffalorio F, Manzotti M,
Fumagalli C, Spitaleri G, Catania C, Delmonte A, Giovannini M,
Spaggiari L, de Braud F and Barberis M: Activity of epidermal
growth factor receptor-tyrosine kinase inhibitors in patients with
non-small cell lung cancer harboring rare epidermal growth factor
receptor mutations. J Thorac Oncol. 6:1895–1901. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lohinai Z, Hoda MA, Fabian K, Ostoros G,
Raso E, Barbai T, Timar J, Kovalszky I, Cserepes M, Rozsas A, et
al: Distinct epidemiology and clinical consequence of classic
versus rare EGFR mutations in lung adenocarcinoma. J Thorac Oncol.
10:738–746. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Klughammer B, Brugger W, Cappuzzo F,
Ciuleanu T, Mok T, Reck M, Tan EH, Delmar P, Klingelschmitt G, Yin
AY, et al: Examining treatment outcomes with erlotinib in patients
with advanced non-small cell lung cancer whose tumors harbor
uncommon EGFR mutations. J Thorac Oncol. 11:545–555. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang S, Cang S and Liu D: Third-generation
inhibitors targeting EGFR T790M mutation in advanced non-small cell
lung cancer. J Hematol Oncol. 9:342016. View Article : Google Scholar : PubMed/NCBI
|