Introduction
Disseminated carcinomatosis of the bone marrow
(DCBM) is a condition in which diffusely invading bone marrow (BM)
metastases are frequently accompanied by disseminated intravascular
coagulation (DIC) (1). DCBM of solid
tumors is typically recognized as incurable and fatal. Although
almost all types of malignancies may metastasize to the BM, the
most common non-hematological malignancies are prostate, lung,
breast and stomach, in addition to neuroblastoma (2,3).
DCBM of colorectal cancer is relatively rare. DCBM
of colorectal cancer has been observed with a frequency of 0–2%
among solid tumors (2,3). DCBM is not necessarily accompanied by
DIC (1). Therefore, DCBM with DIC of
colorectal cancer is rare in solid tumors. Only 7 cases of DCBM
with DIC of colorectal cancer have been reported previously in the
literature (Table I) (4–10).
 | Table I.Reported cases of DCBM with DIC
(including suspicious cases) of colorectal cancer with a BM biopsy
for definitive diagnosis. |
Table I.
Reported cases of DCBM with DIC
(including suspicious cases) of colorectal cancer with a BM biopsy
for definitive diagnosis.
| Author, year | Age (years),
gender | Primary site | Histology | PT, sec | INR | D-dimer, µ/ml | Fibrinogen,
mg/dl | FDP, µ/ml | Plt, ×104/µl | JMHLW score | Diagnosis alive | Postoperative
time | DIC recovery | Prognosis, days | Treatment | Refs. |
|---|
| Yoshioka et
al, 1992 | 62, male | Rectum | Mod | 13.3 |
| – | 142 | >40 | 7.1 | 11 | No | Synchronous | No | Succumbed, 12 | Anti-DIC | (4) |
| Huang et al,
2005 | 79, male | Rectum | Mod | 21.1 |
| >1,050 | 233.8 | >20 | 5.8 | >8 | Yes |
Synchronousa | Yes | Succumbed, 83 | Anti-DIC, 5-FU,
LV | (5) |
| Misawa et al,
2008 | 51, male | Ascending colon | Sig | – |
| 61.5 | 95.2 | 69.4 | 12.9 | >7 | No | Synchronous | No | Succumbed, 25 | Anti-DIC | (6) |
| Van B et al,
2014 | 65, female | Sigmoid colon | Sig | 19.2 |
| 14.45 | 50 | – | 12.7 | >6 | Yes | Synchronous | Yes | Succumbed, 210 | XELOX, FOLFIRI | (7) |
| Nakashima et
al, 2014 | 65, mail | Rectum | Muc | – | – | – | – | 246.7 | 7.9 | >7 | Yes | Synchronous | Yes | Succumbed, 128 | Anti-DIC,
mFOLFOX6 | (8) |
| Naito, 2014 | 61, mail | Transverse
colon | Sig | 21.8 | 1.98 | – | 51 | 57 | 8.6 | 10 | Yes | Synchronous | Yes | Alive, 118 | Anti-DIC, XELOX,
BV, denosumab | (9) |
| Lim DH, 2014 | 74, female | Right-sided
colon | Sig | 18.2 | 1.50 | – | – | – | 0.4 | >7 | No | 3 years | No | Succumbed, 10 | Anti-DIC | (10) |
| Present case,
2015 | 65, mail | Rectum | Mod | 15.8 |
| 152.1 | 124.8 | 225.3 | 3.4 | 9 | Yes | 8 months | Yes | Succumbed, 263 | Anti-DIC, denosumab
mFOLFOX6, |
To the best of our knowledge, there has been only
one report of DCBM with DIC of curatively resected colon cancer as
the first presentation of relapse, but none involving rectal cancer
(10). In addition, DCBM with DIC has
cancer emergency status, and a definitive diagnosis is sometimes
difficult to achieve whilst the patient is alive and able to
withstand chemotherapy. In the previous case of DCBM with DIC of
colon cancer, the diagnosis was made at postmortem (10). The present study describes a case of
DCBM with DIC of rectal cancer as the first presentation of
recurrence, which was successfully treated with chemotherapy and
resulted in a promising prognosis.
Case report
The patient was a 65-year-old male, who presented
with anal bleeding and was admitted to Nara Hospital, Faculty of
Medicine, Kinki University (Nara, Japan) in June 2014. Written
informed consent was obtained from the patient, and the study was
ethically approved by the Institutional Research Board of Kinki
University Nara Hospital (Nara, Japan). Colonoscopy demonstrated
the presence of a type 2 rectal tumor. Subsequent histopathological
examination of the biopsy from the lesion revealed the presence of
adenocarcinoma and the patient was finally diagnosed with rectal
cancer. A laparoscopic low anterior resection with ileostomy was
performed in July 2014 to prevent anastomotic leakage. The
histopathological stage was determined as pT3N2M0, stage IIIC. Two
months subsequent to the initial surgery, the patient underwent
additional surgery to close the ileostomy. Postoperatively, the
patient received adjuvant chemotherapy with 120 mg of oral S-1 for
consecutive 28 days followed by a 14-day rest period as 1 course.
However, owing to a grade 3 adverse event (vomiting) during the
second course, chemotherapy was discontinued. The patient was then
followed up regularly with no evidence of disease recurrence.
In February 2015, 8 months subsequent to the first
surgery, the patient experienced nasal bleeding and once more
consulted Nara Hospital. The patient was diagnosed with DIC based
on the DIC score calculated according to DIC diagnostic criteria
issued by Japan's Ministry of Health, Labour and Welfare (11). On the first day of admission, initial
laboratory data indicated severe thrombocytopenia with a platelet
count of 3.4×104/µl (normal range,
13–33×104/µl), decreased from a count of
33.4×104/µl measured 2 months previously. Initial
laboratory data exhibited a white blood cell count of
1.4×104/µl (normal range, 0.4–1.0×104/µl) and
a hemoglobin level of 12.9 g/dl (normal range, 12–16 g/dl). The
prothrombin time international normalized ratio (PT-INR) was 1.39
(normal range, 0.9–1.13 international normalized ratio), the
partial thromboplastin time was 34.0 sec (normal range, 28–40 sec),
the fibrinogen level was 124.8 mg/dl (normal range, 150–340 mg/dl),
the fibrin degradation product (FDP) level was 225.3 µg/ml (normal
range, 0–8 µg/ml) and the d-dimer level was 152.1 µg/ml (normal
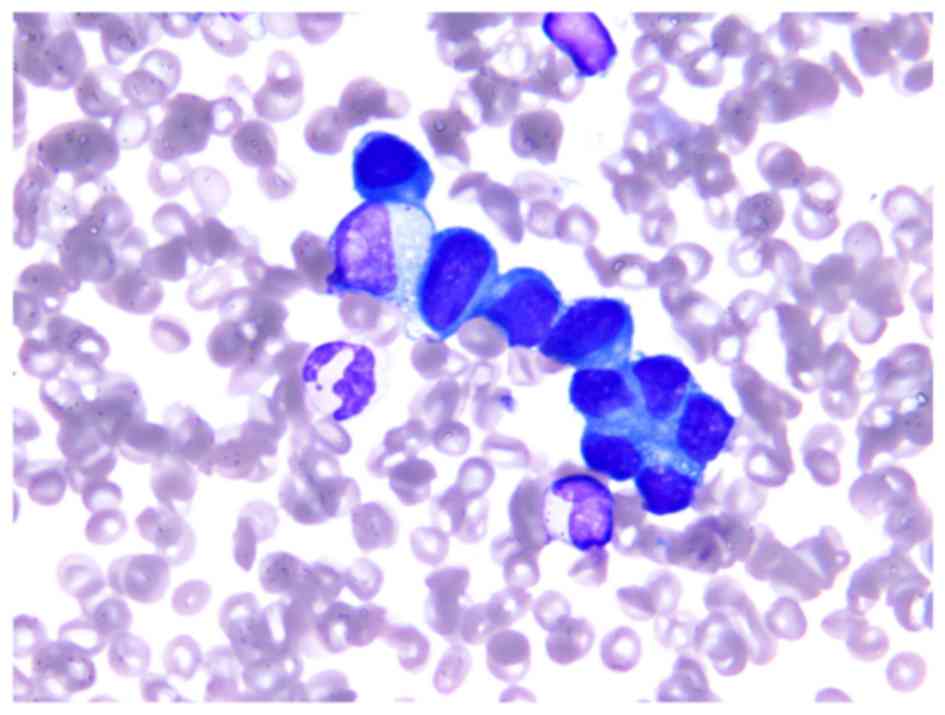
range, 0–1 µg/ml). On the second day of admission, DCBM with DIC
was suspected and a BM biopsy was performed to obtain a definitive
diagnosis. On the third day of admission, CT scans of the whole
body and bone scintigraphy revealed systemic bone metastasis and
multiple small lung metastases. On the fourth day of admission, the
pathological examination of BM demonstrated the existence of
carcinoma, and the patient was definitively diagnosed with DCBM
from curatively resected rectal cancer (Fig. 1). Soon after the definitive diagnosis
of DCBM, systemic chemotherapy with a modified folinic acid,
leucovorin (LV), 5-fluorouracil (5-FU), and oxaplatin (OX)
(mFOLFOX6) regimen was initiated. The following treatment was
repeated every 2 weeks: OX 85 mg/m, LV 200 mg/m2, 5-FU
bolus 400 mg/m2, 5-FU infusion 2,400 mg/m2
over 46 h. Performance Status was 2, and 80% of the regular dose
was administered. On the fifth day of admission, denosumab was
administered as a treatment for bone metastases. For treatment of
DIC and improvement of the systemic condition, a repeated
transfusion of platelet concentrates was performed and anti-DIC
treatment consisted of systemic recombinant human thrombomodulin
(rTM) and nafamostat mesilate (NM) administered intravenously.
Following the first cycle of mFOLFOX6, blood test
results exhibited a platelet cell count of 11.0×104/µl
and thrombocytopenia had improved. The blood test was performed
using the inclusion criteria of DIC. PT-INR, fibrinogen and FDP
levels, and platelet count had improved to 1.16, 333.0 mg/dl, 23.0
µg/ml and 15.1×104/µl, respectively. No significant
toxicities other than grade 1 diarrhea and anorexia were reported.
Following the first cycle of chemotherapy, the tumor marker
carcinoembryonic antigen (CEA) dramatically decreased from 346.6 to
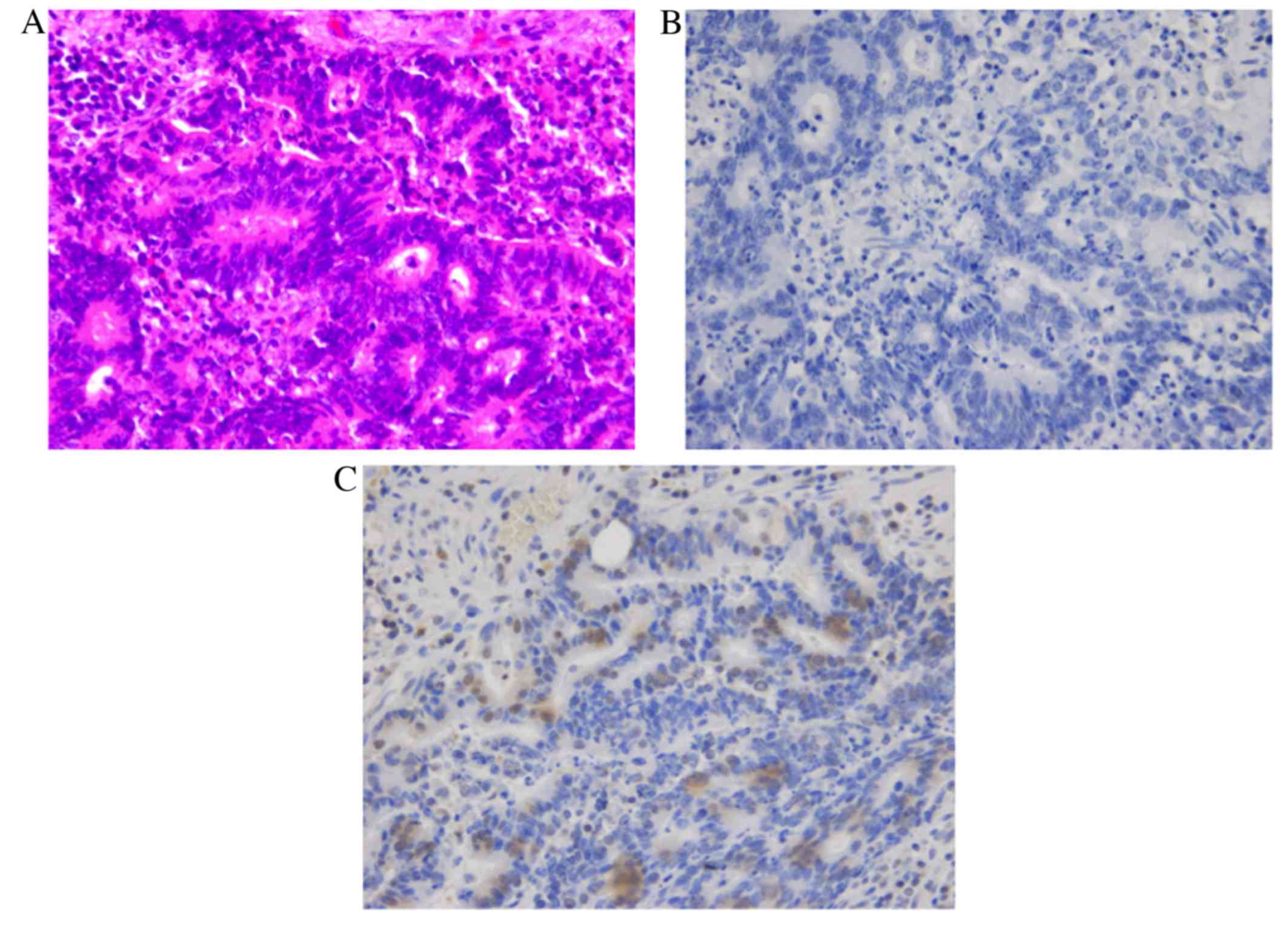
21.7 ng/ml. To predict the chemosensitivity of mFOLFOX6,
immunohistochemistry (IHC) was performed on the primary lesion for
excision repair cross-complementing 1 (ERCC1) and thymidylate
synthase (TS). IHC demonstrated no expression of TS and positive
expression of ERCC1 (Fig. 2).
After 2 cycles of chemotherapy, the patient was
discharged. The same treatment for a total of 12 cycles was
continued on an outpatient basis until September 2015, which was 7
months after initiation of chemotherapy. The patient was alive 263
days after the diagnosis of DIC, but succumbed to carcinomatous
meningitis in November 2015, which occurred as a result of disease
progression.
Discussion
To the best of our knowledge, the present case is
the first to document DCBM with DIC of resected rectal cancer as
the initial presentation of recurrence, which was successfully
treated with mFOLFOX6 and other anti-DIC therapies, and with a
longer prognosis than previous studies as can be observed in
Table I. Even when considering
including DCBM of colon cancer as the initial site of recurrence,
there is only one case that has been reported; however, DCBM was
only diagnosed postmortem (10). In
the present case, DCBM was diagnosed rapidly and DIC was treated
successfully with aggressive therapy, including chemotherapy.
Solid tumors in patients may be the cause of DIC
during their clinical course; a frequency of 1.6–6.8% has been
observed among patients with assorted solid tumors (12,13). In
addition, a frequency of 0–7.7% has been reported among patients
with colorectal cancer (12,13). The prognosis of patients with solid
tumors with DIC is much poorer than those without DIC (13). The exact mechanism resulting in DIC in
patients with solid tumors remains unclear (14). However, it is considered that all
pathways that contribute to the incidence of DIC are driven by
cytokines produced by tumor cells (15). The interactions between malignant
cells, monocytes and macrophages combine to generate tissue factors
and secretion cytokines, including tissue necrosis factor,
interleukin 1 and interleukin-6 (16). These cytokine-dependent modulators of
fibrinolysis and coagulation serve a role in cancer-related
DIC.
Cancer-related DIC could occur regardless of the
existence of DCBM, and DCBM is not necessarily accompanied by DIC
(1). Therefore, DCBM with DIC of
colorectal cancer is particularly rare and only seven cases have
been reported previously in the literature (Table I). In addition, DCBM has cancer
emergency status, and it is sometimes hard to diagnose when a
patient is alive and in a condition to withstand chemotherapy.
There are only five cases (including the present case) in which
chemotherapy was administered (Table
I) (5,7–9).
Treatments for DCBM with DIC conform to those for
cancer-related DIC. Immediate aggressive supportive treatment in
addition to systemic chemotherapy has been the only treatment to
improve prognosis thus far (1). When
treating DIC, it is important that the underlying disorder is
treated with chemotherapy. In fact, if the malignant disease is
able to be brought into remission, the DIC may typically disappear
simultaneously, as observed in the present case. While anti-DIC
treatments without chemotherapy may not improve DIC, all cases in
which recovery from DIC was successful were treated with
chemotherapy (Table I).
In the current case, mFOLFOX6 dramatically improved
DIC. To further evaluate the chemosensitivity of mFOLOFX6, IHC was
performed for TS and ERCC1. TS is a key enzyme in DNA and RNA
synthesis, and TS expression has been reported to be a useful
predictive marker of 5-FU-based chemotherapy (17,18). ERCC1
is implicated in the repair of damaged DNA, and ERCC1 expression
has been reported to be a useful predictive marker of
platinum-based chemotherapy, including OX (19). In the present case, the expression of
these two markers was evaluated in the primary lesion: TS was
negative and ERCC1 was positive. Certain previous studies have
reported that TS was a better predictive chemosensitivity marker
for OX and 5-FU chemotherapy than ERCC1 (20,21).
Results of the present study are in agreement with these previous
studies in that TS was observed to be a useful predictive
chemosensitivity marker of mFOLFOX6.
Supportive anti-DIC treatments consist of the
following anticoagulant treatment: rTM, heparin and anticoagulation
agents, including NM. Based on the hypothesis that extensive
activation of coagulation is characteristic of DIC, a rational
treatment approach may be anticoagulant treatment; therefore rTM
and NM were administered in the current case. However, the safety
and efficacy of these treatments for patients with cancer with DIC
has rarely been addressed in clinical studies (22). In the present case, nasal bleeding
required repeated coagulation treatment and heparin was not
administered to avoid worsening of the nasal bleeding.
Denosumab is a fully human monoclonal antibody
against the human receptor activator of nuclear factor-κB ligand
and inhibits osteoclast differentiation (23). Denosumab administration is a potential
novel treatment choice for the management of bone metastases
(24). Previous studies have
demonstrated that it is able to reduce tumor-induced bone
destruction and bone resorption (24–26).
Although zoledronic acid has also been used in the treatment of
bone metastasis (27), several
studies have recently reported that denosumab was superior to
zoledronic acid (24,28–30).
Denosumab has been recently included in the treatment in
combination with chemotherapy against disseminated carcinomatosis
of the BM (9). In the present case,
denosumab may also have served a role in adding to the aggressive
intensive therapy, resulting in remission of DIC.
In conclusion, in cancer patients with DIC,
clinicians should consider DCBM in the differential diagnosis and
should perform a BM biopsy without delay to obtain a definitive
diagnosis. Once DCBM with DIC is diagnosed, rapid and appropriate
treatment management should be performed. An early diagnosis of DIC
and the administration of systemic chemotherapy and aggressive
supporting anti-DIC therapy may offer certain patients the
possibility of recovery from DIC, as described in the current
case.
References
|
1
|
Kusumoto H, Haraguchi M, Nozuka Y, Oda Y,
Tsuneyoshi M and Iguchi H: Characteristic features of disseminated
carcinomatosis of the bone marrow due to gastric cancer: The
pathogenesis of bone destruction. Oncol Rep. 16:735–740.
2006.PubMed/NCBI
|
|
2
|
Anner RM and Drewinko B: Frequency and
significance of bone marrow involvement by metastatic solid tumors.
Cancer. 39:1337–1344. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jonsson U and Rundles RW: Tumor metastases
in bone marrow. Blood. 6:16–25. 1951.PubMed/NCBI
|
|
4
|
Yoshioka K, Shimizu H, Yokoo S and Andachi
H: Disseminated carcinomatosis of bone marrow from submucosal
carcinoma in adenoma of the rectum. Intern Med. 31:1056–1059. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Huang WT, Chang KC, Shan YS, Tsao CJ and
Lee JC: Successful initial treatment with weekly 24-hour infusion
of 5-fluorouracil and leucovorin in a rectal cancer patient with
acute disseminated intravascular coagulation.
Hepatogastroenterology. 52:1436–1439. 2005.PubMed/NCBI
|
|
6
|
Misawa R, Kobayashi M, Ito M, Kato M,
Uchikawa Y and Takagi S: Primary colonic signet ring cell carcinoma
presenting carcinocythemia: An autopsy case. Case Rep
Gastroenterol. 2:301–307. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
van Bunderen CC, de Weger VA and
Griffioen-Keijzer A: Disseminated intravascular coagulation as
clinical manifestation of colorectal cancer: A case report and
review of the literature. Neth J Med. 72:186–189. 2014.PubMed/NCBI
|
|
8
|
Nakashima Y, Takeishi K, Guntani A,
Tsujita E, Yoshinaga K, Matsuyama A, Hamatake M, Maeda T, Tsutsui
S, Matsuda H, et al: Rectal cancer with disseminated carcinomatosis
of the bone marrow: Report of a case. Int Surg. 99:518–522. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Naito M, Yoshida Y, Aisu N, Tanimura S,
Hoshino S, Tanaka T, Nimura S, Tamura K and Yamashita Y: A report
of disseminated carcinomatosis of the bone marrow originating from
transverse colon cancer successfully treated with chemotherapy
using XELOX plus bevacizumab. Case Rep Oncol. 7:426–434. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lim DH, Lee SI and Park KW: Bone marrow
metastasis of colon cancer as the first site of recurrence: A case
report. Oncol Lett. 8:2672–2674. 2014.PubMed/NCBI
|
|
11
|
Kobayashi N, Maekawa T, Takada M, Tanaka H
and Gonmori H: Criteria for diagnosis of DIC based on the analysis
of clinical and laboratory findings in 345 DIC patients collected
by the research committee on DIC in Japan. Bibl Heamatol. 265–275.
1983.
|
|
12
|
Pasquini E, Gianni L, Aitini E, Nicolini
M, Fattori PP, Cavazzini G, Desiderio F, Monti F, Forghieri ME and
Ravaioli A: Acute disseminated intravascular coagulation syndrome
in cancer patients. Oncology. 52:505–508. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sallah S, Wan JY, Nguyen NP, Hanrahan LR
and Sigounas G: Disseminated intravascular coagulation in solid
tumors: Clinical and pathologic study. Thromb Haemost. 86:828–833.
2001.PubMed/NCBI
|
|
14
|
Levi M and Ten Cate H: Disseminated
intravascular coagulation. N Engl J Med. 341:586–592. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Levi M: Disseminated intravascular
coagulation in cancer patients. Best Pract Res Clin Haematol.
22:129–136. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Esmon CT: Possible involvement of
cytokines in diffuse intravascular coagulation and thrombosis.
Baillieres Best Pract Res Clin Haematol. 12:343–359. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Koopman M, Venderbosch S, Nagtegaal ID,
van Krieken JH and Punt CJ: A review on the use of molecular
markers of cytotoxic therapy for colorectal cancer, what have we
learned? Eur J Cancer. 45:1935–1949. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kumamoto K, Kuwabara K, Tajima Y, Amano K,
Hatano S, Ohsawa T, Okada N, Ishibashi K, Haga N and Ishida H:
Thymidylate synthase and thymidine phosphorylase mRNA expression in
primary lesions using laser capture microdissection is useful for
prediction of the efficacy of FOLFOX treatment in colorectal cancer
patients with liver metastasis. Oncol Lett. 3:983–989.
2012.PubMed/NCBI
|
|
19
|
Altaha R, Liang X, Yu JJ and Reed E:
Excision repair cross complementing-group 1: Gene expression and
platinum resistance. Int J Mol Med. 14:959–970. 2004.PubMed/NCBI
|
|
20
|
Shirota Y, Stoehlmacher J, Brabender J,
Xiong YP, Uetake H, Danenberg KD, Groshen S, Tsao-Wei DD, Danenberg
PV and Lenz HJ: ERCC1 and thymidylate synthase mRNA levels predict
survival for colorectal cancer patients receiving combination
oxaliplatin and fluorouracil chemotherapy. J Clin Oncol.
19:4298–4304. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Arienti C, Tesei A, Verdecchia GM,
Framarini M, Virzi S, Grassi A, Scarpi E, Turci L, Silvestrini R,
Amadori D and Zoli W: Role of conventional chemosensitivity test
and tissue biomarker expression in predicting response to treatment
of peritoneal carcinomatosis from colon cancer. Clin Colorectal
Cancer. 12:122–127. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Blake P, Delicata R, Cross N, Sturgeon G
and Hargest R: Large bowel obstruction due to colorectal carcinoma
can be safely treated by colonic stent insertion-case series from a
UK district general hospital. Colorectal Dis. 14:1489–1492. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lacey DL, Timms E, Tan HL, Kelley ML,
Dunstan CR, Burgess T, Elliott R, Colombero A, Elliott G, Scully S,
et al: Osteoprotegerin ligand is a cytokine that regulates
osteoclast differentiation and activation. Cell. 93:165–176. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Stopeck AT, Lipton A, Body JJ, Steger GG,
Tonkin K, de Boer RH, Lichinitser M, Fujiwara Y, Yardley DA,
Viniegra M, et al: Denosumab compared with zoledronic acid for the
treatment of bone metastases in patients with advanced breast
cancer: A randomized, double-blind study. J Clin Oncol:.
28:5132–5139. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fizazi K, Lipton A, Mariette X, Body JJ,
Rahim Y, Gralow JR, Gao G, Wu L, Sohn W and Jun S: Randomized phase
II trial of denosumab in patients with bone metastases from
prostate cancer, breast cancer, or other neoplasms after
intravenous bisphosphonates. J Clin Oncol. 27:1564–1571. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Smith MR, Egerdie B, Hernández Toriz N,
Feldman R, Tammela TL, Saad F, Heracek J, Szwedowski M, Ke C, Kupic
A, et al: Denosumab in men receiving androgen-deprivation therapy
for prostate cacner. N Engl J Med. 361:745–755. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Misso G, Porru M, Stoppacciaro A,
Castellano M, de Cicco F, Leonetti C, Santini D and Caraglia M:
Evaluation of the in vitro and in vivo antiangiogenic effects of
denosumab and zoledronic acid. Cancer Biol Ther. 13:1491–1500.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Henry DH, Costa L, Goldwasser F, Hirsh V,
Hungria V, Prausova J, Scagliotti GV, Sleeboom H, Spencer A,
Vadhan-Raj S, et al: Randomized, double-blind study of denosumab
versus zoledronic acid in the treatment of bone metastases in
patients with advanced cancer (excluding breast and prostate
cancer) or multiple myeloma. J Clin Oncol. 29:1125–1132. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Scagliotti GV, Hirsh V, Siena S, Henry DH,
Woll PJ, Manegold C, Solal-Celigny P, Rodriguez G, Krzakowski M,
Mehta ND, et al: Overall survival improvement in patients with lung
cancer and bone metastases treated with denosumab versus zoledronic
acid: Subgroup analysis from a randomized phase 3 study. J Thorac
Oncol. 7:1823–1829. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fizazi K, Carducci M, Smith M, Damião R,
Brown J, Karsh L, Milecki P, Shore N, Rader M, Wang H, et al:
Denosumab versus zoledronic acid for treatment of bone metastases
in men with castration-resistant prostate cancer: A randomised,
double-blind study. Lancet. 377:813–822. 2011. View Article : Google Scholar : PubMed/NCBI
|