Introduction
Renal cell carcinoma (RCC) is the most common type
of renal cancer, accounting for ~3% of malignancies in adults
(1). The prognosis of advanced cancer
is reported to be poor, with a 5-year survival rate of 5–10%
(2,3).
RCC has no characteristic symptom, so ~30% of patients are
presented with metastatic disease at the time of diagnosis. Radical
nephrectomy is the primary treatment choice for patients with RCC,
due to the resistance of radiotherapy and chemotherapy (4,5). However,
20–40% of patients developed recurrence following the curative
nephrectomy (6). Thus, it is
important to find a biomarker of RCC for early diagnosis and
targeted therapy based on the mechanisms of RCC tumorigenesis.
MicroRNAs (miRNAs, miRs) are a class of small
non-coding RNA that regulates gene expression by targeting the 3′
untranslated region (UTR) of targeted mRNAs (7–9). Previous
studies indicated that the abnormal expression of miRNA was
involved in tumorigenesis with a tissue-specific expression pattern
(6,7,10). miRNAs
have been revealed to be a potential choice of biomarker for a
number of diseases, particularly tumors (10–12). For
example, miR-210 has been described as a potential biomarker for
clear cell RCC (ccRCC) (11). In
addition, miRNAs may also regulate cellular processes including
proliferation, apoptosis and differentiation (10). Therefore, it is essential to identify
the role of miRNAs in tumorigenesis.
Studies investigating miR-125b (also termed
miR-125b-5p) demonstrated that miR-125b was deregulated in numerous
types of tumor, including osteosarcoma (12), hepatocellular carcinoma (13) and breast cancer (14). The function of miR-125b in a number of
cancer subtypes, including osteosarcoma and gastric cancer, was
explored (15,16). In osteosarcoma, miR-125b was described
as a tumor suppressor (15), and in
gastric cancer, miR-125b functions as an oncogene (16). Fu et al (17) detected the expression of miR-125b in
ccRCC tissues by in situ hybridization (ISH) and revealed
that miR-125b was upregulated in ccRCC tissues, and the expression
level of miR-125b is associated with the recurrence and survival of
patients with ccRCC following nephrectomy. However, the function
and mechanism of miR-125b in RCC remain unclear. In the present
study, quantitative polymerase chain reaction (qPCR) was performed
to detect the expression of miR-125b in RCC tissues and paired
adjacent normal tissues. The function of miR-125b in RCC cell
proliferation, migration, invasion and apoptosis was explored.
Materials and methods
Sample collection
In total, 24 paired RCC and adjacent normal tissues
were collected from Peking University Shenzhen Hospital (Shenzhen,
China) between December 2012 and December 2014. Collection and the
use of the tissues were reviewed and approved by the Ethics
Committees of Peking University Shenzhen Hospital, and written
informed consent was obtained from all the patients. The tissues
were immersed in RNAlater (Qiagen GmbH, Hilden, Germany) for 30
min, and were then stored at −80°C for further study. The adjacent
normal tissues were 2 cm away from the visible RCC lesions.
Following collection, the tissues were reviewed and classified by
hematoxylin and eosin staining (Beyotime Institute of
Biotechnology, Shanghai, China). The clinical and pathological
characteristics of the patients are presented in Table I.
 | Table I.Clinicopathological features of RCC
patients. |
Table I.
Clinicopathological features of RCC
patients.
|
Characteristics | Number of
cases |
|---|
| Mean age range,
years | 50 (25–70) |
| Gender |
|
|
Male | 15 |
|
Female | 9 |
| Histological
type |
|
| Clear
cell | 20 |
|
Papillary | 4 |
| pT-stage |
|
| T1 | 13 |
| T2 | 7 |
|
T3+T4 | 4 |
| Fuhrman grade |
|
| I | 10 |
| II | 10 |
|
III+IV | 4 |
| AJCC clinical
stages |
|
| I | 12 |
| II | 7 |
|
III+IV | 5 |
Cell culture and transfection
Human embryo kidney 293T cells (Type Culture
Collection of the Chinese Academy of Medical Sciences, Shanghai,
China), RCC 786-O and ACHN cells (both American Type Culture
Collection, Manassas, VA, USA) were used in the present study.
Cells were cultured in Dulbecco's modified Eagle's medium (DMEM)
(Gibco; Thermo Fisher Scientific. Inc., Waltham, MA, USA)
supplemented with 10% fetal bovine serum (FBS) (Gibco; Thermo
Fisher Scientific, Inc.), 1% antibiotics (100 µl/ml penicillin and
100 mg/ml streptomycin sulfates) and 1% glutamine, at 37°C in a 5%
CO2 atmosphere. Synthesized miR-125b mimic or inhibitor
(GenePharma, Shanghai, China) was transfected into cells with
Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.)
mixed in Opti-MEM® I reduced serum medium (Gibco; Thermo
Fisher Scientific, Inc.) for upregulation or downregulation of
miR-125b. qPCR was performed to detect the expression of miR-125b
following transfection. Sequences are presented in Table II.
 | Table II.Sequences used in the present
study. |
Table II.
Sequences used in the present
study.
| Name | Sequence |
|---|
| miR-125b mimic |
|
|
Sense |
5′-UCCCUGAGACCCUAACUUGUGA-3′ |
|
Antisense |
5′-ACAAGUUAGGGUCUCAGGGAUU-3′ |
| Negative
control |
|
|
Sense |
5′-UUCUCCGAACGUGUCACGUTT-3′ |
|
Antisense |
5′-ACGUGACACGUUCGGAGAATT-3′ |
| miR-125b |
5′-UCACAAGUUAGGGUCUCAG |
| inhibitor | GGA-3′ |
| Inhibitor
negative |
5′-CAGUACUUUUGUGUAGUA |
| control | CAA-3′ |
| U6 primer |
| Forward |
5′-CTCGCTTCGGCAGCACA-3′ |
| Reverse |
5′-ACGCTTCACGAATTTGCGT-3′ miR-125b
primer |
| Forward |
5′-TCCCTGAGACCCTAACTTGTGA-3′ |
| Reverse | Universal primer
(miScript SYBR-Green PCR kit) |
RNA extraction, cDNA synthesis and
quantitative polymerase chain reaction (qPCR)
Total RNA was extracted from the tissues and cells
following transfection with TRIzol reagent (Invitrogen; Thermo
Fisher Scientific, Inc.) and purified with the RNeasy Maxi kit
(Qiagen GmbH), according to the manufacturer's protocol. NanoDrop
2000/2000c (Thermo Fisher Scientific, Inc.) was used to detect the
concentration of extracted RNA. RNA (1 µg) was used to perform
reverse transcription with the miScript reverse transcription kit
(Qiagen GmbH) to get cDNA. The following thermocycling conditions
were used: 37°C for 60 min and 95°C for 5 min. qPCR was then
performed with the miScript SYBR® Green PCR kit (Qiagen
GmbH) to detect the expression of miR-125b on the Roche Lightcycler
480 quantitative PCR system, following the manufacturer's protocol
(Roche Diagnostics, Basel, Switzerland) and performed in
triplicate. Primers used are presented in Table II. U6 was used as an internal
control. Universal primer provided by the miScript SYBR Green PCR
kit was used as a reverse primer of miR-125b. The following
thermocycling conditions were used in the qPCR reaction: 95°C for 1
min; 40 cycles of 95°C for 10 sec, 55°C for 30 sec and 70°C for 30
sec. The expression of miR-125b was analyzed using the
2−ΔΔCq method (18).
Cell proliferation assay
MTT and Cell Counting Kit-8 (CCK-8) assays were
performed to assess proliferative ability of 786-O and ACHN cells
following transfection. In each well of a 96-well plate, ~3,000
cells were seeded and 24 h later transfected with 5 pmol miR-125b
mimics, inhibitors, mimic negative control (NC) or inhibitor NC.
For the MTT assay, 20 µl MTT (5 mg/ml; Sigma-Aldrich; Merck
Millipore, Darmstadt, Germany) was added to the wells, which were
detected at 0, 24, 48 and 72 h following transfection, and then
incubated at 37°C for 4 h. Subsequently, the mixed medium was
replaced by 150 µl dimethylsulfoxide (DMSO; Sigma-Aldrich; Merck
Millipore) and then agitated at 0.04 × g for 15 min at room
temperature. The optical density (OD) value of each well was then
evaluated using the ELISA microplate reader (Bio-Rad Laboratories,
Inc., Hercules, CA, USA) at a wavelength of 490 nm. For the CCK-8
assay, 15 µl CCK-8 (Beyotime Institute of Biotechnology) was added
to the wells, which were detected at 0, 24, 48 and 72 h
post-transfection. The OD value of each well was measured at a
wavelength of 490 nm 1.5 h later. The experiments were performed in
triplicate and repeated at least three times.
Cell scratch assay
The cell scratch assay was performed to assess the
migratory ability of 786-O and ACHN cells following transfection.
In each well of a 6-well plate ~6×105 cells were seeded,
and 24 h later, the cells were transfected with 200 pmol miR-125b
mimics, inhibitors, mimic NC or inhibitor NC. A vertical horizontal
line was scratched with a sterile 200 µl pipette tip 6 h subsequent
to transfection. The cells were rinsed with PBS to remove the
floating cells. The images of the scratch were captured using a
digital camera system and a Leica DMIRB inverted fluorescence
microscope (Leica Microsystems GmbH, Wetzlar, Germany), at 0 and 24
h (magnification, ×100). The experiments were performed in
triplicate and repeated at least three times.
Transwell assay
A Transwell assay was performed to assess the
migratory and invasive ability of cells following transfection. In
the assay, Transwell chamber inserts (BD Biosciences, Franklin
Lakes, USA) with (for invasion) or without (for migration) Matrigel
(BD Biosciences) were used. Cells were transfected with miR-125b
mimics, inhibitors, mimic NC or inhibitor NC. A total of 6 h
following the transfection, 1×104 transfected cells in
200 µl serum-free DMEM medium were seeded in the upper chamber of
the insert. The bottoms of the inserts were incubated in the medium
with 10% FBS. Following 36 h (for migration) or 48 h (for
invasion), cells on the upper membrane were removed using a cotton
swab, and those that had migrated or invaded to the bottom of the
inserts were stained with crystal violet (1 mg/ml; Sigma-Aldrich;
Merck Millipore) for 15 min at room temperature and counted using a
Leica DMIRB inverted microscope (magnification, ×200). The
experiments were performed in triplicate and repeated at least
three times.
Flow cytometry assay
The apoptotic rate of cells was determined by
performing a flow cytometry assay with Dead Cell Apoptosis kit with
Annexin V fluorescein isothiocyanate (FITC) and propidium iodide
(PI) (all from Invitrogen; Thermo Fisher Scientific, Inc.). In each
well of a 6-well plate, ~3×105 786-O or ACHN cells were
seeded, and 24 h later, cells were transfected with miR-125b
mimics, inhibitor, mimic NC or inhibitor NC. At 48 h
post-transfection, all cells were harvested and washed twice with
cold PBS. Following re-suspension in 100 µl of binding buffer,
cells were stained with 5 µl Annexin V-FITC and 3 µl PI. Following
15 min, 400 µl of binding buffer was added to each tube. Flow
cytometry (EPICS, Xl-4; Beckman Coulter, Inc., Brea, CA, USA) was
then used to evaluate the apoptosis rate. The experiments were
performed in triplicate and repeated at least three times.
Statistical analysis
The paired t-test was used to compare the
expression levels of miR-125b in matched tissues. Student's
t-test was used to analyze assays for characterizing
phenotypes of cells. All statistical analyses were performed by
SPSS 19.0 (SPSS, Inc., Chicago, IL, USA). P<0.05 was considered
to indicate a statistically significant difference.
Results
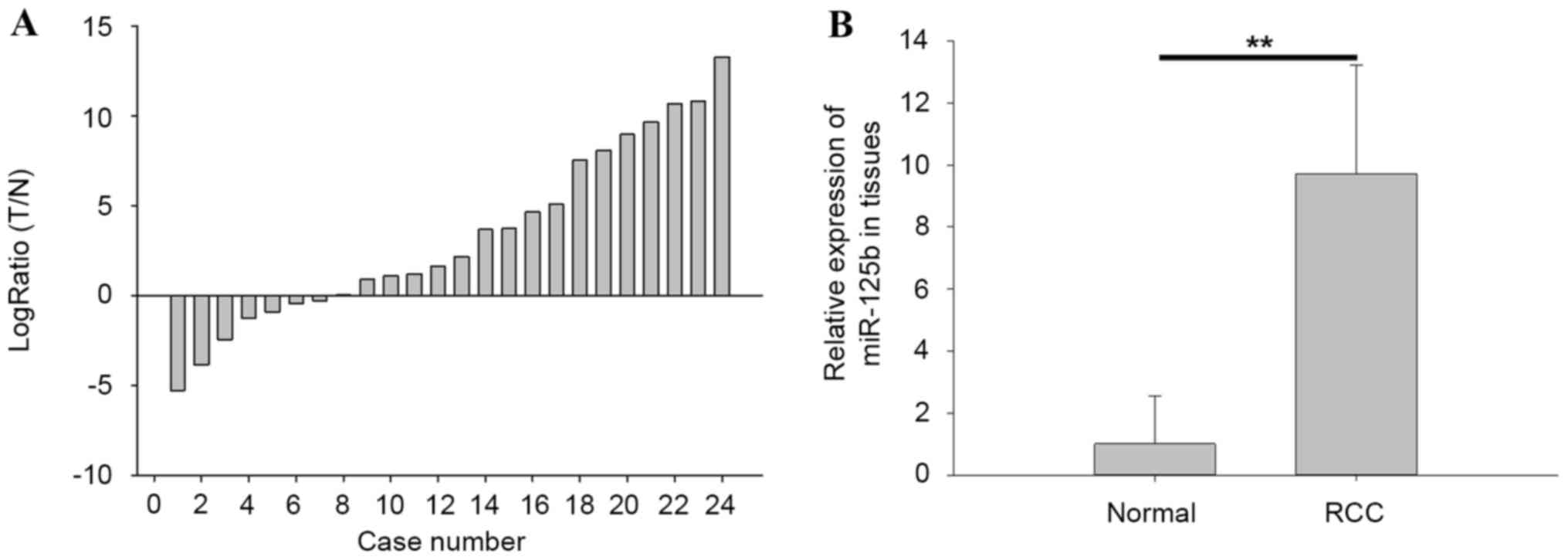
miR-125b was upregulated in renal cell
carcinoma (RCC) tissues compared with normal tissues
The expression of miR-125b in tissues was detected
by qPCR. The ratios of miR-125b expression in paired tissues
[log2Ratio tumor tissues (T)/normal adjacent tissues
(N)] are presented in Fig. 1A, in
which miR-125b was upregulated in 17 RCC tissues. The results of
qPCR also demonstrated that the expression level of miR-125b (with
mean relative expression 9.72) in RCC tissues was significantly
higher compared with in normal tissues (P<0.01), as demonstrated
in Fig. 1B.
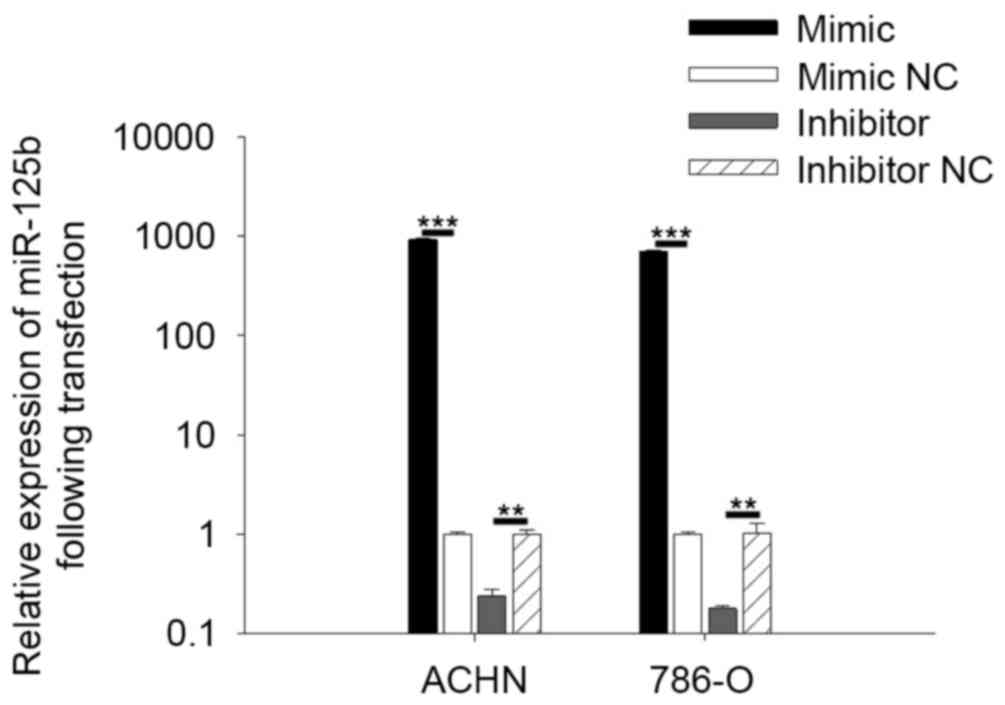
Validation of cell transfection
efficiency
To quantify the expression of miR-125b in RCC cells
following transfection, qPCR was performed. The results indicated
that expression of miR-125b in cells transfected with miR-125b
mimic was significantly 704.36 (786-O) and 926.44 (ACHN) times
higher compared with cells transfected with mimic NC (P<0.001).
Expression of miR-125b in cells transfected with miR-125b inhibitor
was 0.18 (786-O) and 0.24 (ACHN) times higher than the cells
transfected with inhibitor NC (P<0.01; Fig. 2).
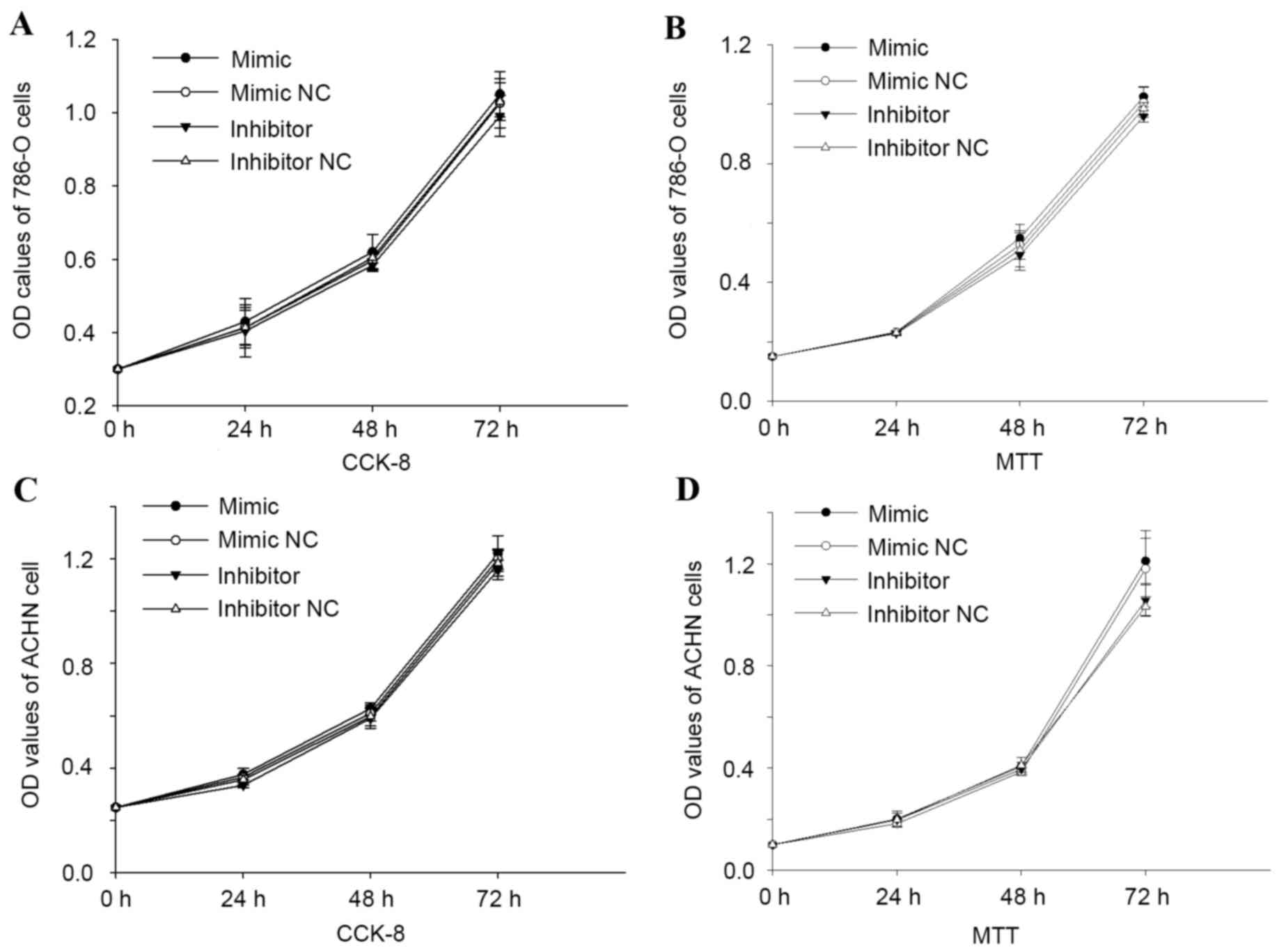
miR-125b has no marked effect on the
proliferation of renal cell carcinoma (RCC) cells
MTT and CCK-8 assays were performed to detect the
proliferation of cells. The two assays revealed that overexpression
or downregulation of miR-125b had no effect on RCC 786-O and ACHN
cell proliferation. The OD values of cells transfected with
miR-125b mimic were not significantly different from those of cells
transfected with mimic NC (P>0.05), and no significant
difference was observed between the inhibitor and inhibitor NC
group (P>0.05). The results are presented in Fig. 3.
miR-125b promoted cell mobility
Transwell and cell scratch assays were performed to
assess the impact of miR-125b on cell mobility. The results of the
cell scratch assay are demonstrated in Fig. 4. As presented in Fig. 4A, overexpression of miR-125b promoted
the 786-O cell migratory distances by 53.93% (P<0.01), while
downregulation of miR-125b reduced the migratory distances by
33.25% (P<0.01). In ACHN cells, overexpression of miR-125b
promoted migratory distances by 62.59% (P<0.01), and
downregulation of miR125b reduced cell migratory distances by
51.99% (P<0.01; Fig. 4B).
The results of the Transwell assay for 786-O cells
are shown in Fig. 5. In Fig. 5A, results of the Transwell invasive
assay revealed that upregulation of miR-125b promoted cell invasive
ability by 91.71% (P<0.01) and downregulation of miR-125b
reduced cell invasive ability by 51.43% (P<0.01). For the
Transwell migratory assay, the migratory cells that were
transfected with miR-125b mimic were 1.60 times higher compared
with cells transfected with mimic NC (P<0.01). Migratory cells
transfected with miR-125b inhibitor were 0.59 times higher compared
with cells transfected with inhibitor NC (P<0.01; Fig. 5B). In ACHN cells, the Transwell
invasion assay (Fig. 6A) showed that
invasive ability of cells transfected with miR-125b mimic was
promoted by 52.07% (P<0.01) and reduced by 23.88% (P<0.01)
for cells transfected with inhibitor compared with mimic NC or
inhibitor NC. The migratory ability of cells was promoted by 56.21%
(P<0.01) in the mimic group and reduced by 22.66% (P<0.01) in
the inhibitor group, compared with the mimic NC or inhibitor NC
group (Fig. 6B). The results of
Transwell and cell scratch assays revealed that miR-125b is
associated with RCC cell mobility.
miR-125b regulates cell apoptosis
Flow cytometry was performed to detect the apoptosis
rate of cells following transfection. As presented in Fig. 7, the apoptotic rate of 786-O cells
transfected with miR-125b inhibitor or inhibitor NC was 30.85% vs.
20.87% (P<0.01; Fig. 7A). The
apoptotic rate of cells transfected with miR-125b mimic or mimic NC
was 10.45 vs. 21.04 (P<0.01; Fig.
7B). The apoptotic rate of ACHN cells transfected with miR-125b
inhibitor was 18.30% with 7.39% for cells transfected with
inhibitor NC (P<0.01; Fig. 8A).
The apoptotic rate of cells transfected with miR-125b mimic or
mimic NC was 4.64 vs. 7.52 (P<0.01; Fig. 8B). The results showed that miR-125b
regulates RCC cell apoptosis.
Discussion
Deregulation of miRNAs has been detected in numerous
types of cancer, including RCC and gastric cancer (11,16), and
has been confirmed to be capable of promoting cancer initiation and
progression (11), a number of which
function as oncogenes or tumor suppressors, depending on the
differences of the targeted gene. The expression of miR-125b has
been revealed to be deregulated in a number of types of cancer,
including gastric cancer (16),
non-small-cell lung cancer (NSCLC) (19) and osteosarcoma (12). Numerous previous studies about gene
microarray in RCC revealed that miR-125b was downregulated in ccRCC
tissues (20–22). However, another study identified that
miR-125b was upregulated in ccRCC by performing ISH (17). In the present study, the expression of
miR-125b in RCC tissues and paired adjacent normal tissues was
detected and the results revealed that miR-125b was upregulated in
RCC tissues compared with adjacent normal tissues. Further study of
miR-125b indicated that overexpression of miR-125b could promote
cell migration, invasion and reduce cell apoptosis, while
downregulation of miR-125b inhibited cell migration, invasion and
induced cell apoptosis. The results of CCK-8 and MTT assays showed
that miR-125b had no effect on cell proliferation. It can be
inferred that the pathway of miR-125b in RCC is associated with
cell mobility and apoptosis.
Another study of miR-125b in ccRCC revealed that
miR-125b is under-expressed in metastatic tumors, based on the
microarray results of 18 patients (23). However, Fu et al (17) demonstrated that miR-125b was
upregulated in ccRCC based on 276 samples by ISH, and higher
expression of miR-125b predicted shorter survival time and a higher
rate of recurrence. The two studies about miR-125b in ccRCC,
including the present study, are limited, since the cases were
collected from a single institute or area, which could not exclude
the bias of area.
A number of studies about miR-125b indicated that
miR-125b acted as a tumor suppressor in osteosarcoma, ovarian
cancer, hepatocellular carcinoma (HCC) and gastric cancer (15,24–26). In
ovarian cancer, miR-125a and miR-125b could inhibit ovarian cancer
cell migration, invasion and proliferation by regulating
EIF4E-binding protein 1 (24). A
study on HCC revealed that the tumor suppressive miR-125b could
negatively regulate the expression of long non-coding RNA HOTTIP
(25), and low expression of miR-125b
was a prognostic marker for poor disease status and outcome
(13). It was also revealed that
miR-125b could suppress gastric cancer proliferation and invasion
by targeting MCL1, and the low expression of miR-125b was
associated with advanced clinical stage, lymph node metastases and
poor clinical outcomes. Thus, miR-125b may be used as a biomarker
for gastric cancer (26). Wang et
al (15) revealed that tumor
suppressive miR-125b could negatively regulate osteosarcoma cell
proliferation, invasion and migration, and that overexpression of
miR-125b enhanced the chemosensitivity of osteosarcoma cells to
cisplatin by targeting Bcl-2. Another study revealed that low
expression of miR-125b in osteosarcoma predicted a poor prognosis
(12). miR-125b was also
downregulated in human papillomavirus-16 (HPV-16) E6 positive
esophageal cancer tissues (27).
miR-125b has the potential to be used as biomarker for diagnosis,
targeted therapy or predicting prognosis.
In NSCLC, miR-125b was identified as an oncogene. Li
et al (19) revealed
overexpression of miR-125b promoted human NSCLC metastasis by
targeting TP53INP1. Another study about miR-125b in lung cancer
revealed that knockdown of miR-125b could induce apoptosis and G1/S
phase arrest and inhibit the invasive ability of lung cancer cells
(28). miR-125b could also regulate
kinesin light chain-2, which was upregulated in elderly patients
with NSCLC, and could predict a poor clinical outcome of elderly
patients with NSCLC (29). Studies of
miR-125b in breast cancer revealed that miR-125b was decreased in
pre-invasive breast cancer (30) and
high expression of miR-125b was associated with poor prognosis in
breast cancer (31). In colorectal
cancer (CRC), the expression of miR-125b was downregulated
(32), while in patients with early
colorectal neoplasia (including precancerous lesions and early
CRCs) the expression of serum miR-125b was also upregulated
compared with healthy controls. Therefore, miR-125b may be used as
biomarker for early detection of colorectal neoplasia (33). Thus, the expression of miR-125b in
different stages of tumors varies, indicating that the role of
miR-125b is constantly changing during the tumorigenesis.
miR-125b has also been shown to serve a role in
numerous non-tumor diseases. For example, miR-125b was
overexpressed in HPV infected cells and patients and was decreased
in patients with invasive cervical carcinoma (34). miR-125b could also protect the
ethanol-induced apoptosis and embryotoxicity (35). Due to incomplete binding with the
3′-untranslated region of target mRNA, miR-125b may serve various
roles in different diseases or processes (36,37). miRNA
may regulate different genes and serve different functions.
In conclusion, the results of the present study
revealed that miR-125b was upregulated in RCC tissues and regulated
RCC cell migration, invasion and apoptosis, indicating that
miR-125b served a significant role in RCC. Furthermore, the pathway
of miR-125b would be identified and the study for miR-125b as a
biomarker would be performed.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81101922), the
Guangdong Natural Science Foundation (grant no. 2015A030313889),
the Science and Technology Development Fund Project of Shenzhen
(grant nos. JCYJ20120616144352139, JCYJ20130402114702124,
JCYJ20140415162542975 and JCYJ20150403091443329) and the Fund of
Guangdong Key Medical Subject.
References
|
1
|
Tostain J, Li G, Gentil-Perret A and
Gigante M: Carbonic anhydrase 9 in clear cell renal cell carcinoma:
A marker for diagnosis, prognosis and treatment. Eur J Cancer.
46:3141–3148. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hadoux J, Vignot S and De La Motte Rouge
T: Renal cell carcinoma: Focus on safety and efficacy of
temsirolimus. Clin Med Insights Oncol. 4:143–154. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Su Z, Chen D, Zhang E, Li Y, Yu Z, Shi M,
Jiang Z, Ni L, Yang S, Gui Y, et al: MicroRNA-509-3p inhibits
cancer cell proliferation and migration by targeting the
mitogen-activated protein kinase kinase kinase 8 oncogene in renal
cell carcinoma. Mol Med Rep. 12:1535–1543. 2015.PubMed/NCBI
|
|
4
|
Alt AL, Boorjian SA, Lohse CM, Costello
BA, Leibovich BC and Blute ML: Survival after complete surgical
resection of multiple metastases from renal cell carcinoma. Cancer.
117:2873–2882. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rasmussen F: Metastatic renal cell cancer.
Cancer Imaging. 13:374–380. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zou X, Zhong J, Li J, Su Z, Chen Y, Deng
W, Li Y, Lu S, Lin Y, Luo L, et al: miR-362-3p targets nemo-like
kinase and functions as a tumor suppressor in renal cancer cells.
Mol Med Rep. 13:994–1002. 2016.PubMed/NCBI
|
|
7
|
Jiang JX, Gao S, Pan YZ, Yu C and Sun CY:
Overexpression of microRNA-125b sensitizes human hepatocellular
carcinoma cells to 5-fluorouracil through inhibition of glycolysis
by targeting hexokinase II. Mol Med Rep. 10:995–1002.
2014.PubMed/NCBI
|
|
8
|
Akman HB, Selcuklu SD, Donoghue MT,
Akhavantabasi S, Sapmaz A, Spillane C, Yakicier MC and Erson-Bensan
AE: ALCAM is indirectly modulated by miR-125b in MCF7 cells. Tumour
Biol. 36:3511–3520. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yang Q, Wang Y, Lu X, Zhao Z, Zhu L, Chen
S, Wu Q, Chen C and Wang Z: MiR-125b regulates
epithelial-mesenchymal transition via targeting Sema4C in
paclitaxel-resistant breast cancer cells. Oncotarget. 6:3268–3279.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wei X, Chen D, Lv T, Li G and Qu S: Serum
microRNA-125b as a potential biomarker for glioma diagnosis. Mol
Neurobiol. 53:163–170. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Iwamoto H, Kanda Y, Sejima T, Osaki M,
Okada F and Takenaka A: Serum miR-210 as a potential biomarker of
early clear cell renal cell carcinoma. Int J Oncol. 44:53–58.
2014.PubMed/NCBI
|
|
12
|
Karbasy SH, Taheriazam A, Mirghasemi A,
Sedaghati F, Shakeri M, Yahaghi E and Bahador R: RETRACTED ARTICLE:
Upregulation of miR-300 and downregulation of miR-125b act as
potential predictor biomarkers in progression, metastasis, and poor
prognosis of osteosarcoma. Tumour Biol. Sep 2–2015.(Epub ahead of
print). PubMed/NCBI
|
|
13
|
Tsang FH, Au V, Lu WJ, Shek FH, Liu AM,
Luk JM, Fan ST, Poon RT and Lee NP: Prognostic marker microRNA-125b
inhibits tumorigenic properties of hepatocellular carcinoma cells
via suppressing tumorigenic molecule eIF5A2. Dig Dis Sci.
59:2477–2487. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
He H, Xu F, Huang W, Luo SY, Lin YT, Zhang
GH, Du Q and Duan RH: miR-125a-5p expression is associated with the
age of breast cancer patients. Genet Mol Res. 14:17927–17933. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang F, Yu D, Liu Z, Wang R, Xu Y, Cui H
and Zhao T: miR-125b functions as a tumor suppressor and enhances
chemosensitivity to cisplatin in osteosarcoma. Technol Cancer Res
Treat. 15:NP105–NP112. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wu JG, Wang JJ, Jiang X, Lan JP, He XJ,
Wang HJ, Ma YY, Xia YJ, Ru GQ, Ma J, et al: MiR-125b promotes cell
migration and invasion by targeting PPP1CA-Rb signal pathways in
gastric cancer, resulting in a poor prognosis. Gastric Cancer.
18:729–739. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fu Q, Liu Z, Pan D, Zhang W, Xu L, Zhu Y,
Liu H and Xu J: Tumor miR-125b predicts recurrence and survival of
patients with clear-cell renal cell carcinoma after surgical
resection. Cancer Sci. 105:1427–1434. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li Q, Han Y, Wang C, Shan S, Wang Y, Zhang
J and Ren T: MicroRNA-125b promotes tumor metastasis through
targeting tumor protein 53-induced nuclear protein 1 in patients
with non-small-cell lung cancer. Cancer Cell Int. 15:842015.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu H, Brannon AR, Reddy AR, Alexe G,
Seiler MW, Arreola A, Oza JH, Yao M, Juan D, Liou LS, et al:
Identifying mRNA targets of microRNA dysregulated in cancer: With
application to clear cell renal cell carcinoma. BMC Sys Biol.
4:512010. View Article : Google Scholar
|
|
21
|
He H, Wang L, Zhou W, Zhang Z, Wang L, Xu
S, Wang D, Dong J, Tang C, Tang H, et al: MicroRNA expression
profiling in clear cell renal cell carcinoma: Identification and
functional validation of key miRNAs. PLoS One. 10:e01256722015.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Juan D, Alexe G, Antes T, Liu H,
Madabhushi A, Delisi C, Ganesan S, Bhanot G and Liou LS:
Identification of a microRNA panel for clear-cell kidney cancer.
Urology. 75:835–841. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Heinzelmann J, Henning B, Sanjmyatav J,
Posorski N, Steiner T, Wunderlich H, Gajda MR and Junker K:
Specific miRNA signatures are associated with metastasis and poor
prognosis in clear cell renal cell carcinoma. World J Urol.
29:367–373. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lee M, Kim EJ and Jeon MJ: MicroRNAs 125a
and 125b inhibit ovarian cancer cells through post-transcriptional
inactivation of EIF4EBP1. Oncotarget. 7:8726–8742. 2016.PubMed/NCBI
|
|
25
|
Tsang FH, Au SL, Wei L, Fan DN, Lee JM,
Wong CC, Ng IO and Wong CM: Long non-coding RNA HOTTIP is
frequently up-regulated in hepatocellular carcinoma and is targeted
by tumour suppressive miR-125b. Liver Int. 35:1597–1606. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wu S, Liu F, Xie L, Peng Y, Lv X, Zhu Y,
Zhang Z and He X: miR-125b suppresses proliferation and invasion by
targeting MCL1 in gastric cancer. Biomed Res Int. 2015:3652732015.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zang B, Huang G, Wang X and Zheng S:
HPV-16 E6 promotes cell growth of esophageal cancer via
downregulation of miR-125b and activation of Wnt/β-catenin
signaling pathway. Int J Clin Exp Pathol. 8:13687–13694.
2015.PubMed/NCBI
|
|
28
|
Wang X, Zhang Y, Fu Y, Zhang J, Yin L, Pu
Y and Liang G: MicroRNA-125b may function as an oncogene in lung
cancer cells. Mol Med Rep. 11:3880–3887. 2015.PubMed/NCBI
|
|
29
|
Wang M, Zhu X, Sha Z, Li N, Li D and Chen
L: High expression of kinesin light chain-2, a novel target of
miR-125b, is associated with poor clinical outcome of elderly
non-small-cell lung cancer patients. Br J Cancer. 112:874–882.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lehmann TP, Korski K, Gryczka R, Ibbs M,
Thieleman A, Grodecka-Gazdecka S and Jagodziński PP: Relative
levels of let-7a, miR-17, miR-27b, miR-125a, miR-125b and miR-206
as potential molecular markers to evaluate grade, receptor status
and molecular type in breast cancer. Mol Med Rep. 12:4692–4702.
2015.PubMed/NCBI
|
|
31
|
Vilquin P, Donini CF, Villedieu M, Grisard
E, Corbo L, Bachelot T, Vendrell JA and Cohen PA: MicroRNA-125b
upregulation confers aromatase inhibitor resistance and is a novel
marker of poor prognosis in breast cancer. Breast Cancer Res.
17:132015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chen H and Xu Z:
Hypermethylation-associated silencing of miR-125a and miR-125b: A
potential marker in colorectal cancer. Dis Markers.
2015:3450802015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yamada A, Horimatsu T, Okugawa Y, Nishida
N, Honjo H, Ida H, Kou T, Kusaka T, Sasaki Y, Yagi M, et al: Serum
miR-21, miR-29a, and miR-125b are promising biomarkers for the
early detection of colorectal neoplasia. Clin Cancer Res.
21:4234–4242. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ribeiro J, Marinho-Dias J, Monteiro P,
Loureiro J, Baldaque I, Medeiros R and Sousa H: miR-34a and
miR-125b expression in HPV infection and cervical cancer
development. Biomed Res Int. 2015:3045842015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chen X, Liu J, Feng WK, Wu X and Chen SY:
MiR-125b protects against ethanol-induced apoptosis in neural crest
cells and mouse embryos by targeting Bak 1 and PUMA. Exp Neurol.
271:104–111. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Xie X, Hu Y, Xu L, Fu Y, Tu J, Zhao H,
Zhang S, Hong R and Gu X: The role of
miR-125b-mitochondria-caspase-3 pathway in doxorubicin resistance
and therapy in human breast cancer. Tumour Biol. 36:7185–7194.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Morelli E, Leone E, Cantafio ME, Di
Martino MT, Amodio N, Biamonte L, Gullàà A, Foresta U, Pitari MR,
Botta C, et al: Selective targeting of IRF4 by synthetic
microRNA-125b-5p mimics induces anti-multiple myeloma activity in
vitro and in vivo. Leukemia. 29:2173–2183. 2015. View Article : Google Scholar : PubMed/NCBI
|