Introduction
Gastric cancer (GC) is a leading cause of
cancer-related mortality globally (1). Prognosis is poor in patients with
advanced GC (2). Endoscopic surgical
resection is currently used for the treatment of early-stage GC
only (3). The lack of non-surgical
alternatives in treatment approaches has resulted in a requirement
to develop novel and targeted therapies to treat GC.
CCAAT/enhancer-binding proteins (C/EBPs) are
transcription factors that belong to the family of basic leucine
zipper proteins, and regulate gene expression in cell viability and
inflammation (4,5). C/EBPα, C/EBPβ and C/EBPδ are involved in
terminal differentiation of cells and inflammatory processes
(6–8).
Furthermore, the expression levels of C/EBPα and C/EBPβ are altered
in cancerous tissue compared with in surrounding healthy tissues
(9). Although it has been
demonstrated previously that C/EBPδ is involved in GC (10), its specific role remains unknown. The
specific roles of C/EBPα, C/EBPβ and C/EBPδ in GC remain
incompletely understood.
Therefore, the expression levels of C/EBPα, C/EBPβ
and C/EBPδ were investigated in GC cells as a potential treatment
method to decrease the viability of GC cells. Experiments were
conducted using the Epstein-Barr virus (EBV) episomal vector. EBV
episomal vector containing the latent origin of replication,
oriP, and nuclear antigen are able to efficiently transfect
host cells, and stably express and amplify genes of interest in
daughter cells following division (11).
Materials and methods
Cell culture
The GC cell lines MKN45 and MKN74 were purchased
from RIKEN BioResource Center Cell Bank (Tsukuba, Japan). Cells
were cultured in 10 cm dishes (Asahi Techno Glass Corporation,
Funabashi, Japan) in RPMI-1640 medium (Sigma; Merck KGaA,
Darmstadt, Germany) supplemented with 10% fetal bovine serum (FBS;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) at 37°C, in a
humidified chamber containing 5% CO2.
Plasmid construction and
transfection
A C/EBPα fragment, digested with
NruI-EcoRV from pG28B5.0 plasmid (kindly provided by
Dr Kleanthis G. Xanthopoulos, IRRAS AB, Stockholm, Sweden), was
subcloned into EcoRV sites of the episomal vector
pEBMulti-Neo (Wako Pure Chemical Industries, Ltd., Osaka, Japan) to
generate pEB/C/EBPα-Neo (12). A
C/EBPδ fragment digested with EcoRI from IRCB003O22 (RIKEN
BioResource Center DNA Bank, Tsukuba, Japan) was subcloned into
pBluescript2SK(−) (Agilent Technologies, Inc., Santa Clara, CA,
USA) to construct pBlue/C/EBPδ. An
EcoRV-BamHI-digested fragment of pBlue/C/EBPδ was
subcloned into the EcoRV-BamHI site of pEBMulti-Neo
to generate pEB/C/EBPδ-Neo. Episomal plasmid vector constructs were
transfected into GC cells and normal gastric mucosa cells.
pEB/C/EBPα-Neo and pEB/C/EBPδ-Neo were transfected using
Lipofectamine® LTX (Thermo Fisher Scientific, Inc.) into
the cell cultures at concentrations of 10 or 100 ng/well in 96-well
plates and at 2 µg/well in 6-well plates, according to the
manufacturer's protocol. Mock transfection was carried out without
nucleic acid material.
Cell viability analysis
Cells were trypsinized, harvested, centrifuged at
100 × g for 3 min at 4°C and seeded in 96-well flat-bottom plates
(Asahi Techno Glass Corporation) at a density of 1,000 cells/well,
and incubated for 24 h in Dulbecco's modified Eagle's medium
(Sigma; Merck KGaA) supplemented with 10% FBS. Following incubation
for 24 h, the cells were transfected with pEB/C/EBPα-Neo or
pEB/C/EBPδ-Neo. Following incubation for 72 h, cell viability was
analyzed using a
3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium
inner salt (MTS) assay (Promega Corporation, Madison, WI, USA),
according to the manufacturer's protocol. MTS is bioreduced by
viable cells into a colored formazan product with an absorbance at
490 nm. Absorbance at 490 nm was analyzed using an iMark Microplate
Absorbance Reader (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Mock-transfected cells were used as a control.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
GC and normal gastric mucosa cells were cultured in
6-well plates (Asahi Techno Glass Corporation). When the cells
reached 70% confluence, they were transfected with 0, 0.25, 0.75 or
2.5 mg pEB/C/EBPα-Neo or pEB/C/EBPδ-Neo. Total RNA was isolated
using Isogen (Nippon Gene Co., Ltd., Tokyo, Japan) and 5 µg RNA was
used for first-strand cDNA synthesis, using the SuperScript III
First-Strand Synthesis system (Thermo Fisher Scientific, Inc.),
according to the manufacturer's protocol. qPCR was performed using
Fast SYBR Green Master Mix (Thermo Fisher Scientific, Inc.) for 40
cycles with 5 sec of denaturation at 95°C and 5 sec of
annealing/extension at 60°C. PCR primers and amplicon lengths for
qPCR are presented in Table I.
Ribosomal protein L19 (RPL19), a constitutively expressed
housekeeping gene (13), was used as
an endogenous control to monitor the amount of mRNA. The gene
expression levels were automatically analyzed using the MiniOpticon
system (Bio-Rad Laboratories, Inc.), based on the ΔΔCq method
(14). The relative expression levels
were calculated as the expression level of a specific gene divided
by that of RPL19. Human whole stomach RNA was purchased from
Clontech Laboratories, Inc. (Mountain View, CA, USA) and used as a
healthy control.
 | Table I.Primer sequences and conditions for
the quantitative polymerase chain reaction. |
Table I.
Primer sequences and conditions for
the quantitative polymerase chain reaction.
| Primer name | Sequence | Description | Product size, kb | GenBank®
accession no. |
|---|
| OMC321 |
5′-CGAATGCCAGAGAAGGTCAC-3′ | RPL19, forward | 157 | BC095445 |
| OMC322 |
5′-CCATGAGAATCCGCTTGTTT-3′ | RPL19, reverse |
|
|
| OMC351 |
5′-CGGACTTGGTGCGTCTAAGATG-3′ | C/EBPα, forward | 148 | U34070 |
| OMC352 |
5′-GCATTGGAGCGGTGAGTTTG-3′ | C/EBPα, reverse |
|
|
| OMC355 |
5′-AGAGGCGGAGGAGAACAAACAG-3′ | Cyclin D1,
forward | 180 | NM_053056 |
| OMC356 |
5′-AGGCGGTAGTAGGACAGGAAGTTG-3′ | Cyclin D1,
reverse |
|
|
| OMC569 |
5′-AAGCACAGCGACGAGTACAA-3′ | C/EBPβ, forward | 155 | BC007538 |
| OMC570 |
5′-AGCTGCTCCACCTTCTTCTG-3′ | C/EBPβ, reverse |
|
|
| OMC571 |
5′-AGAAGTTGGTGGAGCTGTCG-3′ | C/EBPδ, forward | 101 | BC105109 |
| OMC572 |
5′-CAGCTGCTTGAAGAACTGCC-3′ | C/EBPδ, reverse |
|
|
Statistical analysis
One-way analysis of variance was calculated using
JMP software (version 10.0.2; SAS Institute, Cary, NC, USA) to
evaluate statistical significance. P<0.05 was considered to
indicate a statistically significant difference.
Results
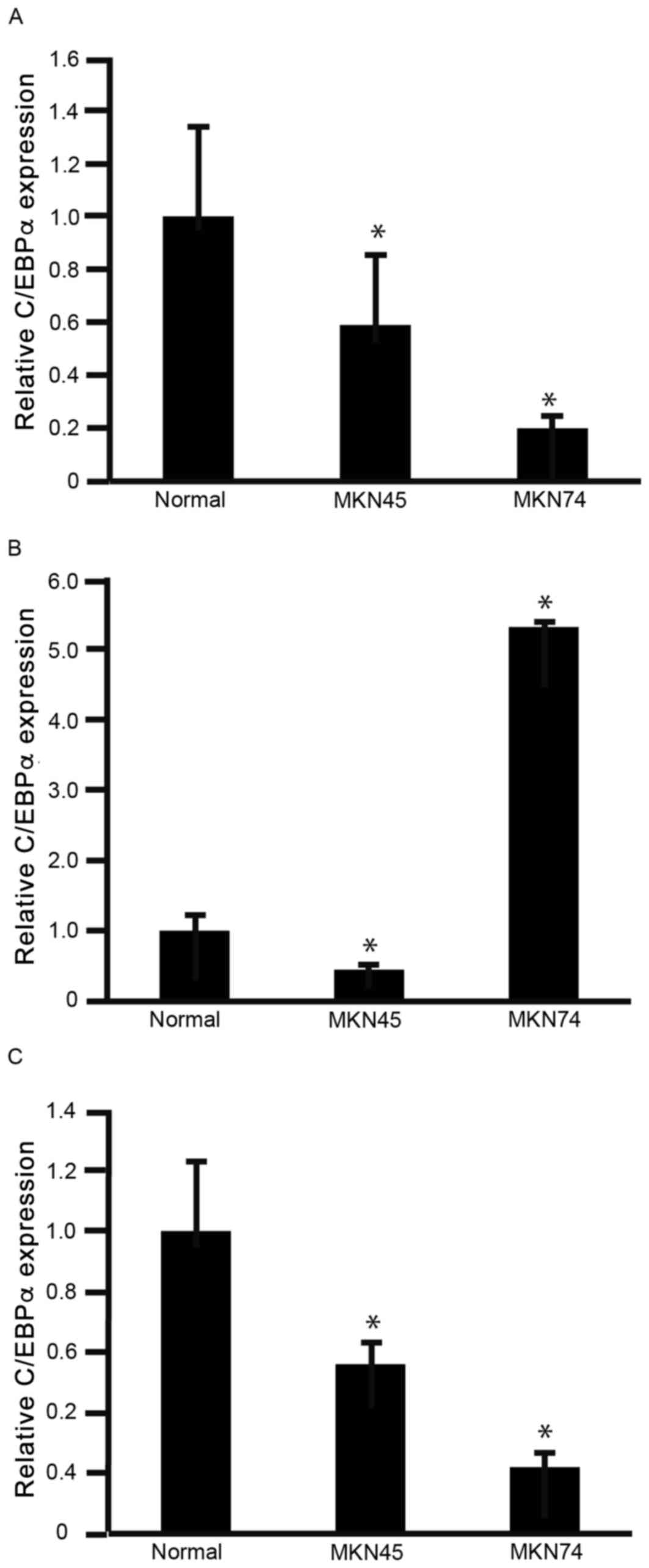
To determine the expression levels of C/EBPα
(Fig. 1A), C/EBPβ (Fig. 1B) and C/EBPδ (Fig. 1C) in GC cells, RNA was isolated from
MKN45 and MKN74 cells for analysis using RT-qPCR. The expression
levels of C/EBPα and C/EBPδ were significantly decreased in MKN45
and MKN74 cells, compared with normal stomach (gastric) mucosa
(P<0.05; Fig. 1A and C). The
expression levels of C/EBPβ were significantly decreased in MKN45
cells, but significantly increased in MKN74 cells, compared with
normal stomach mucosa (P<0.05; Fig.
1B). These results indicated that C/EBPα and C/EBPδ warranted
further investigation for their potential involvement in decreasing
GC cell viability. However, the biological significance of C/EBPβ
in GC cells was not clear from these results.
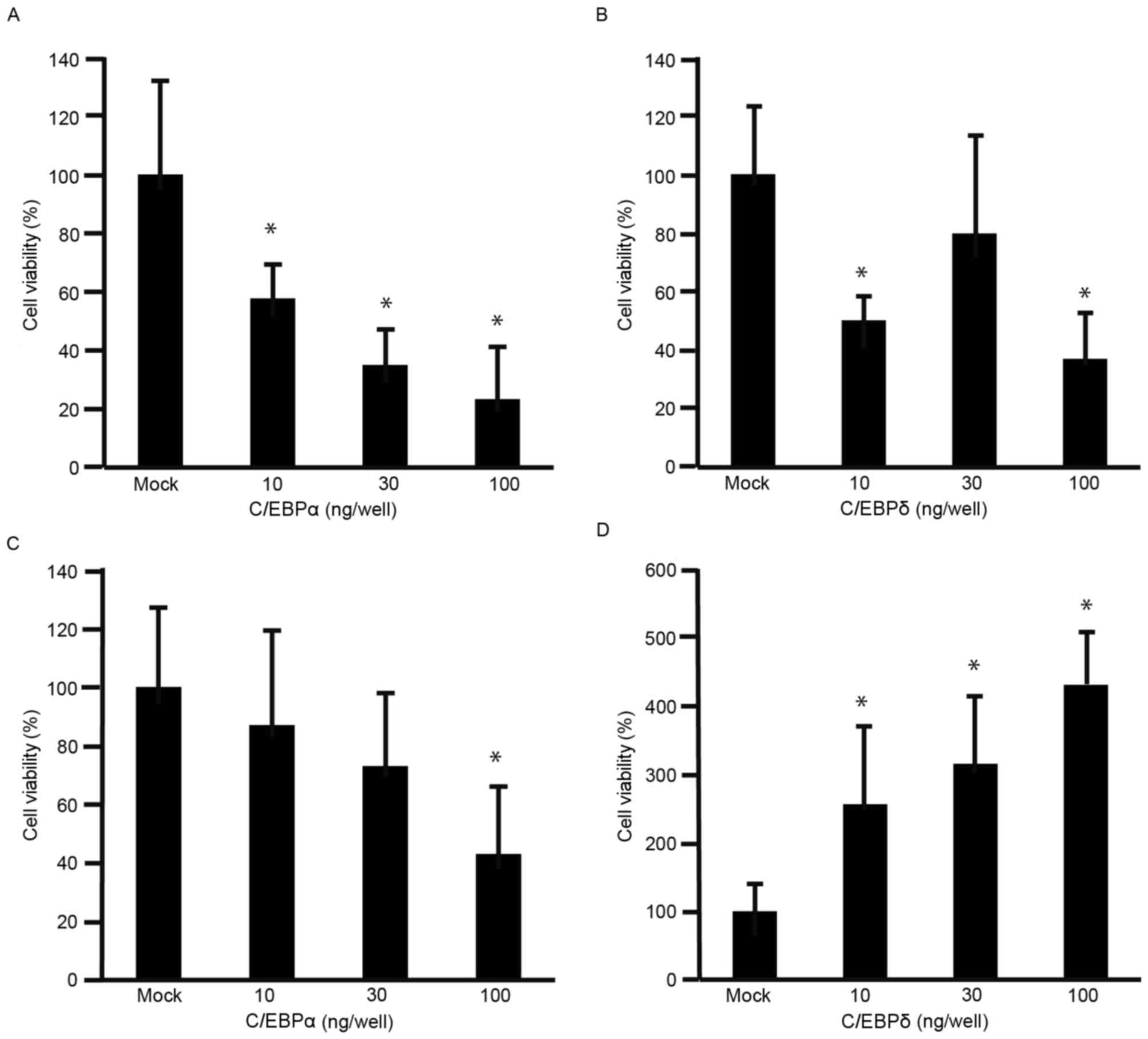
The viability of MKN45 cells was decreased by C/EBPα
(Fig. 2A) and C/EBPδ (Fig. 2B), compared with that of
mock-transfected cells (P<0.05). Although the viability of MKN74
cells was significantly decreased by C/EBPα at 100 ng/well
(P<0.05; Fig. 2C), it was
significantly increased by C/EBPδ (P<0.05; Fig. 2D). These results indicated that C/EBPα
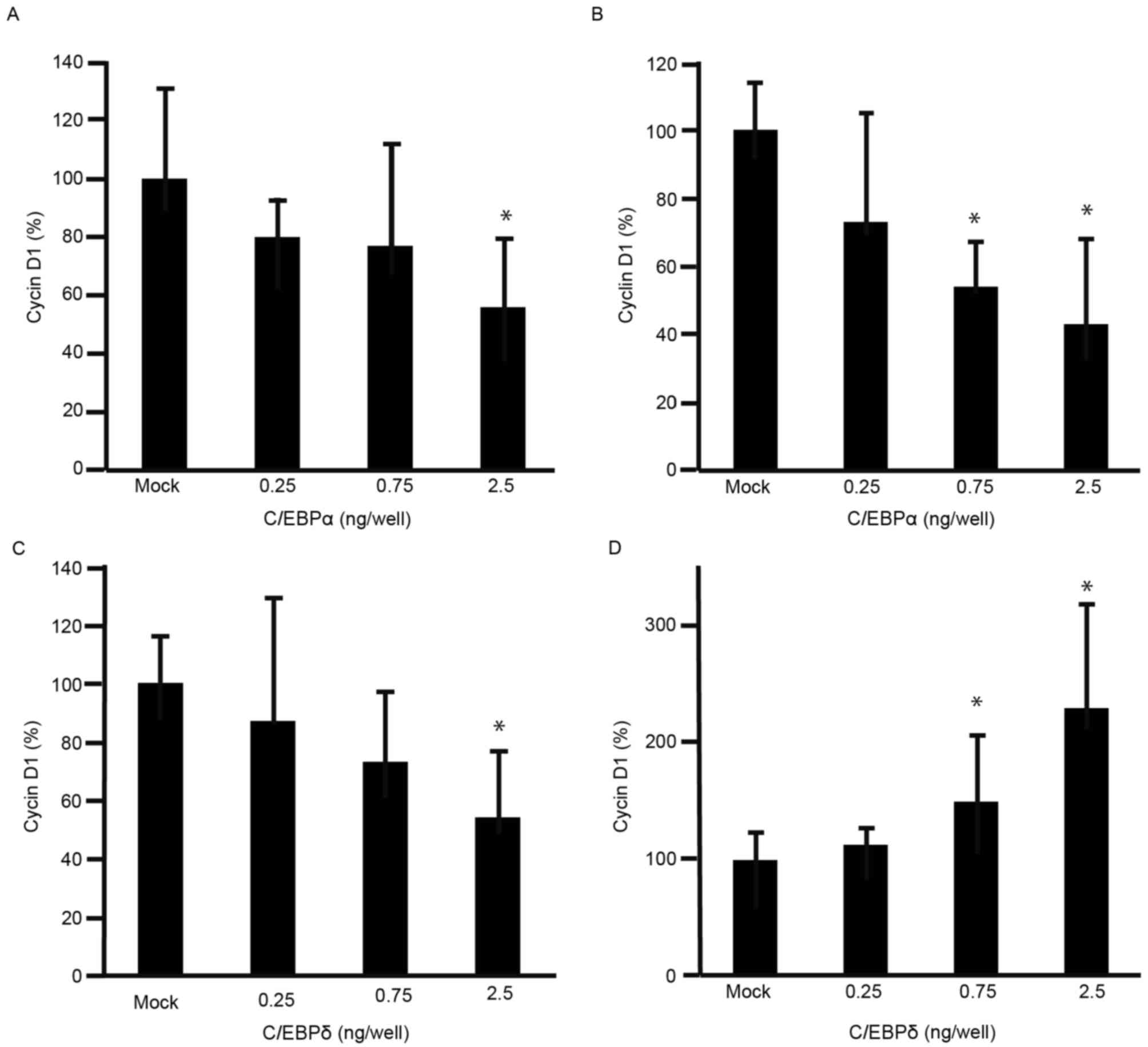
decreased the viability of GC cells. To further understand the
effects of C/EBPα and C/EBPδ on cell viability, expression levels
of cyclin D1 were analyzed using RT-qPCR. The expression levels of
cyclin D1 were decreased in MKN45 and MKN74 cells transfected with
C/EBPα (Fig. 3A and B). The
expression levels of cyclin D1 were decreased in MKN45 cells
transfected with C/EBPδ (Fig. 3C),
but increased in MKN74 cells transfected with C/EBPδ (Fig. 3D).
Discussion
C/EBPα is expressed in normal gastric epithelium and
downregulated in GC (15). Previous
studies have also demonstrated that C/EBPα decreased the viability
of hepatocellular carcinoma cells (16,17). In
the present study, the expression levels of C/EBPα were identified
to be decreased in GC cells. The results indicated that C/EBPα is
able to facilitate tumor cell suppression in GC. C/EBPα was able to
decrease metastasis of GC by upregulating microRNA-100 (18). The results of the present study
indicated that expressing C/EBPα is potentially useful in the
targeted treatment of GC.
Previous studies have demonstrated that, during
inflammation, C/EBPδ is recruited to the promoter region of
cyclooxygenase-2, although its expression levels remained constant
(19). Currently, to the best of our
knowledge, no conclusive study of the expression levels of C/EBPδ
in GC cells has been performed. In liver cancer, C/EBPδ acts as a
tumor suppressor (20). In the
present study, C/EBPδ was downregulated in GC cells, indicating
that C/EBPδ acted as a tumor suppressor. However, C/EBPδ decreased
the viability of MKN45 cells, but increased the viability of MKN74
cells. These results indicated that C/EBPδ may serve an ambiguous
role as a tumor suppressor or promoter, but it was not possible to
determine this conclusively. Further studies may assist in our
understanding of the functional role of C/EBPδ in GC. In the
present study, the expression levels of C/EBPβ were decreased in
MKN45 cells. It was assumed that not all GC cells exhibited
increased expression of C/EBPβ; however, it was not possible to
determine the percentage of GC cells that did. The expression level
of C/EBPβ in GC is generally increased compared with that in
healthy gastric epithelium (21). In
further studies, immunohistological and flow cytometric analysis of
cell cycle should be performed to improve our understanding of the
expression of C/EBPα, C/EBPβ and C/EBPδ.
The expression levels of C/EBPα and C/EBPδ were
decreased in GC cells compared with in healthy gastric epithelium.
Expression of C/EBPα decreased the viability of GC cells, and,
therefore, C/EBPα may potentially be used in novel GC
therapies.
Acknowledgements
The present study was supported by a Grant-in-Aid
for Scientific Research (C) from the Japan Society for the
Promotion of Science (grant no. 15K09032).
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
de Vita F, Di Martino N, Fabozzi A,
Laterza MM, Ventriglia J, Savastano B, Petrillo A, Gambardella V,
Sforza V, Marano L, et al: Clinical management of advanced gastric
cancer: The role of new molecular drugs. World J Gastroenterol.
20:14537–14558. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kim MY, Cho JH and Cho JY: Ever-changing
endoscopic treatment for early gastric cancer:
Yesterday-today-tomorrow. World J Gastroenterol. 20:13273–13283.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tsukada J, Yoshida Y, Kominato Y and Auron
PE: The CCAAT/enhancer (C/EBP) family of basic-leucine zipper
(bZIP) transcription factors is a multifaceted highly-regulated
system for gene regulation. Cytokine. 54:6–19. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kheolamai P and Dickson AJ: Liver-enriched
transcription factors are critical for the expression of hepatocyte
marker genes in mES-derived hepatocyte-lineage cells. BMC Mol Biol.
10:352009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tomizawa M, Garfield S, Factor V and
Xanthopoulos KG: Hepatocytes deficient in CCAAT/enhancer binding
protein alpha (C/EBP alpha) exhibit both hepatocyte and biliary
epithelial cell character. Biochem Biophys Res Commun. 249:1–5.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yamasaki H, Sada A, Iwata T, Niwa T,
Tomizawa M, Xanthopoulos KG, Koike T and Shiojiri N: Suppression of
C/EBPalpha expression in periportal hepatoblasts may stimulate
biliary cell differentiation through increased Hnf6 and Hnf1b
expression. Development. 133:4233–4243. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Akira S, Isshiki H, Sugita T, Tanabe O,
Kinoshita S, Nishio Y, Nakajima T, Hirano T and Kishimoto T: A
nuclear factor for IL-6 expression (NF-IL6) is a member of a C/EBP
family. EMBO J. 9:1897–1906. 1990.PubMed/NCBI
|
|
9
|
Tomizawa M, Horie H, Yamamoto H, Matsunaga
T, Sasaki F, Hashizume K, Hiyama E, Kaneko M, Suita S, Ando H, et
al: Reciprocal expression of CCAAT/enhancer binding proteins α and
β in hepatoblastomas and its prognostic significance. Oncol Rep.
17:341–344. 2007.PubMed/NCBI
|
|
10
|
Balamurugan K and Sterneck E: The many
faces of C/EBPδ and their relevance for inflammation and cancer.
Int J Biol Sci. 9:917–933. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yates JL, Warren N and Sugden B: Stable
replication of plasmids derived from Epstein-Barr virus in various
mammalian cells. Nature. 313:812–815. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Antonson P and Xanthopoulos KG: Molecular
cloning, sequence, and expression patterns of the human gene
encoding CCAAT/enhancer binding protein alpha (C/EBP alpha).
Biochem Biophys Res Commun. 215:106–113. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Davies B and Fried M: The L19 ribosomal
protein gene (RPL19): Gene organization, chromosomal mapping, and
novel promoter region. Genomics. 25:372–380. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tam S, Clavijo A, Engelhard EK and
Thurmond MC: Fluorescence-based multiplex real-time RT-PCR arrays
for the detection and serotype determination of foot-and-mouth
disease virus. J Virol Methods. 161:183–191. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Regalo G, Resende C, Wen X, Gomes B,
Durães C, Seruca R, Carneiro F and Machado JC: C/EBP alpha
expression is associated with homeostasis of the gastric epithelium
and with gastric carcinogenesis. Lab Invest. 90:1132–1139. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tomizawa M, Wang YQ, Ebara M, Saisho H,
Watanabe K, Nakagawara A and Tagawa M: Decreased expression of the
CCAAT/enhancer binding protein α gene involved in hepatocyte
proliferation in human hepatocellular carcinomas. Int J Mol Med.
9:597–600. 2002.PubMed/NCBI
|
|
17
|
Tomizawa M, Watanabe K, Saisho H,
Nakagawara A and Tagawa M: Down-regulated expression of the
CCAAT/enhancer binding protein alpha and beta genes in human
hepatocellular carcinoma: A possible prognostic marker. Anticancer
Res. 23:351–354. 2003.PubMed/NCBI
|
|
18
|
Shi DB, Wang YW, Xing AY, Gao JW, Zhang H,
Guo XY and Gao P: C/EBPα-induced miR-100 expression suppresses
tumor metastasis and growth by targeting ZBTB7A in gastric cancer.
Cancer Lett. 369:376–385. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen JJ, Huang WC and Chen CC:
Transcriptional regulation of cyclooxygenase-2 in response to
proteasome inhibitors involves reactive oxygen species-mediated
signaling pathway and recruitment of CCAAT/enhancer-binding protein
delta and CREB-binding protein. Mol Biol Cell. 16:5579–5591. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li CF, Tsai HH, Ko CY, Pan YC, Yen CJ, Lai
HY, Yuh CH, Wu WC and Wang JM: HMDB and 5-AzadC combination
reverses tumor suppressor CCAAT/enhancer-binding protein delta to
strengthen the death of liver cancer cells. Mol Cancer Ther.
14:2623–2633. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Regalo G, Canedo P, Suriano G, Resende C,
Campos ML, Oliveira MJ, Figueiredo C, Rodrigues-Pereira P, Blin N,
Seruca R, et al: C/EBPbeta is over-expressed in gastric
carcinogenesis and is associated with COX-2 expression. J Pathol.
210:398–404. 2006. View Article : Google Scholar : PubMed/NCBI
|